Abstract
Purpose
Patients receiving long-term glucocorticoid (GC) treatment are at risk of osteoporosis, while bone effects of substitution doses in Addison’s disease (AD) remain equivocal. The project was aimed to evaluate serum bone turnover markers (BTMs): osteocalcin, type I procollagen N-terminal propeptide (PINP), collagen C-terminal telopeptide (CTX), sclerostin, DKK-1 protein, and alkaline phosphatase (ALP) in relation to bone mineral density (BMD) during GC replacement.
Methods
Serum BTMs and hormones were assessed in 80 patients with AD (22 males, 25 pre- and 33 postmenopausal females) on hydrocortisone (HC) substitution for ≥3 years. Densitometry with dual-energy X-ray absorptiometry covered the lumbar spine (LS) and femoral neck (FN).
Results
Among BTMs, only PINP levels were altered in AD. BMD Z-scores remained negative except for FN in males. Considering T-scores, osteopenia was found in LS in 45.5% males, 24% young and 42.4% postmenopausal females, while osteoporosis in 9.0%, 4.0% and 21.1%, respectively. Lumbar BMD correlated positively with body mass (p = 0.0001) and serum DHEA-S (p = 9.899 × 10−6). Negative correlation was detected with HC dose/day/kg (p = 0.0320), cumulative HC dose (p = 0.0030), patient’s age (p = 1.038 × 10−5), disease duration (p = 0.0004), ALP activity (p = 0.0041) and CTX level (p = 0.0105). However, only age, body mass, ALP, serum CTX, and sclerostin remained independent predictors of LS BMD.
Conclusion
Standard HC substitution does not considerably accelerate BMD loss in AD patients and their serum BTMs: CTX, osteocalcin, sclerostin, DKK-1, and ALP activity remain within the reference ranges. Independent predictors of low lumbar spine BMD, especially ALP activity, serum CTX and sclerostin, might be monitored during GC substitution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with primary adrenal insufficiency (Addison’s disease, AD) require lifelong glucocorticoid (GC) replacement for their survival. According to the current recommendations, monitoring of steroid replacement is primarily based upon regular clinical assessment [1]. Still, adequate dosage of the exogenous steroids remains challenging, since no reliable markers to guide this treatment have been established to date. Therefore, subjects with adrenal failure are at risk of steroid over-replacement and subsequent complications [2, 3]. Long-lasting GC excess produces serious adverse effects, including negative impact on bone metabolism and decreased bone mineral density (BMD) [3,4,5,6]. Glucocorticoid-induced osteoporosis (GIO) affects primarily the trabecular bone and results mainly from impaired bone synthesis [7, 8].
It is well known that pharmacological doses of GCs display a detrimental effect on bone, which is dose- and duration-dependent [7, 9]. The risk of osteoporosis and fractures is evident for daily steroid doses exceeding 30 mg hydrocortisone (HC) or equivalent, and seems particularly elevated for synthetic GC, prednisone [10,11,12]. Based upon the results of the seminal study by Estaban et al. published in 1991, GC replacement in adrenal failure requires lower steroid dosages than those recommended in the past, i.e. just 15–25 mg HC per day [1, 13]. Still, literature data on bone health in AD are often inconsistent due to methodological reasons (small groups, different ages, various treatment regimens). Some studies demonstrate lack of considerable adverse effect [10, 14,15,16], while other data support impaired bone health in AD patients receiving conventional steroid substitution regimens [4, 5, 17]. Moreover, BMD results may not fully reflect bone fragility, as proportion of patients with osteoporotic fractures present BMD values above the WHO definition of osteoporosis [18]. Therefore, more sensitive, early indicators of the ongoing bone loss, would be strongly appreciated, with regard to potential optimization of the GC replacement.
Bone turnover makers (BTMs) are molecules released from the active osteoblasts and osteoclasts or collagen breakdown products, which are relatively easily measured in blood or urines [19]. Changes in their concentrations or excretion provide an insight into the dynamics of the bone remodelling and some of them proved to be clinically useful in the monitoring of treatment of osteoporosis [19, 20]. Major bone formation markers comprise propeptides of type I collagen – C-terminal (PICP) and N-terminal (PINP), osteoblast enzymes: alkaline phosphatase (ALP) and its bone-specific isozyme, and a matrix protein, osteocalcin [19, 20]. Bone resorption markers include, collagen degradation products: telopeptides of type I collagen (C-terminal, CTX and N-terminal, NTX), hydroxyproline, deoxypiridinoline, and osteoclastic enzymes, tartrate-resistant acid phosphatase 5b (TRAP5b) and cathepsin K [19, 21]. Additionally, there are some recently identified markers: RANKL, osteoprotegerin, Dickkopf-related protein 1 (DKK-1), and sclerostin. Altered circulating BTMs, such as CTX, osteocalcin, or increased ALP activity, typically reflect enhanced bone turnover, deterioration of its microarchitecture, and increased fracture risk, independent of BMD measurements [20, 22].
In patients exposed to GC excess, decreased concentrations of bone-specific ALP, PINP, osteocalcin, TRAP5b, and sclerostin, while increased CTX levels were reported [23, 24]. However, not all former analyses revealed consistent results, especially concerning bone resorption markers [25]. Even less data is available with regard to GC replacement doses. In several studies circulating BTMs remained within their reference ranges, or similar compared to the healthy controls [15, 16, 26]. On the other hand, longitudinal analyses conducted in patients treated for primary and secondary adrenal failure demonstrated significant fluctuations in serum osteocalcin in response to changes in GC dosages [27, 28]. Furthermore, treatment with dual-release HC formulation, considered more physiological, revealed higher serum osteocalcin compared to individuals receiving conventional HC tablets [3]. Only one former study evaluated circulating sclerostin in patients treated for AD, and found its elevated levels, but failed to demonstrate any correlation with the steroid dosage [29].
The purpose of this analysis was to investigate the relationship between serum BMTs and BMD in patients receiving long-term GC substitution at currently recommended doses, lower than those used in the past. We also hoped to explore whether BTM levels could potentially provide some additional guidance for optimal steroid replacement in Addison’s disease.
Patients and methods
Subjects
The study comprised 80 patients (mean age 51.6 ± 13.1 years) suffering from AD who remain under regular follow-up in the inpatient and outpatient endocrine clinics at Poznan University of Medical Sciences. Since their complete medical records were available, the diagnosis of primary adrenal insufficiency was well documented. It was based upon clinical symptoms, low morning serum cortisol together with elevated plasma ACTH, or confirmed by lack of rise in serum cortisol in response to intravenous stimulation with synthetic ACTH1–24 [1]. In order to minimize the group heterogeneity, the study was limited to patients with autoimmune origin of the disease, the major reason of primary adrenal failure nowadays. To be eligible for the study, patients had to be treated for at least 3 years, with stable GC dose during the last year. History of an episode such as acute infection requiring transiently increased steroid dosage was acceptable only if occurred no later than 3 months prior to the study.
The exclusion criteria comprised secondary adrenal insufficiency, other than autoimmune reasons for primary adrenal failure, congenital adrenal hyperplasia, type 1 diabetes, and current thyroid hormone imbalance according to serum TSH level. Patients with AD and hypothyroidism on stable thyroid replacement, as well as those who remained euthyroid after efficient treatment of hyperthyroidism were enroled. Individuals suffering from type 1 autoimmune polyendocrine syndrome were excluded to avoid interfering influence of the hypoparathyroidism.
Biochemical analyses
The measurements comprised routine evaluation of the fasting plasma glucose, serum sodium, potassium, and calcium levels, ALP activity, concentrations of TSH, PTH, 25-hydroxycholecalciferol (vitamin D3, 25[OH]D3), and dehyroepiandrosterone sulphate (DHEA-S). These parameters were determined by electrochemiluminescent (ECLIA) method using Modular Analytics E170 and relevant commercial kits from Roche Diagnostics. Additionally, 10 ml blood were collected, centrifuged to separate serum and frozen in 50ul aliquots at −20 °C until analysed. Evaluation of the BTMs comprised osteocalcin (using automated ECLIA from Roche Diagnostics; inter-assay CV ≤ 1.6%; intra-assay CV ≤ 0.8%), and commercially available ELISA assays for Procollagen Type 1 N-Terminal Propeptide (PINP, cat. E0957h, EIAab Science Inc., Wuhan, China; inter-assay CV ≤ 6.3%; intra-assay CV ≤ 8.5%), Collagen Type-1 C-terminal Telopeptide (CTX, cat. E0665h, EIAab Science Inc., Wuhan, China; inter-assay CV ≤ 5.5%; intra-assay CV ≤ 9.2%), bioactive Sclerostin (cat. BI-20472, Biomedica Medizinprodukte GmbH, Wien, Austria; inter-assay CV ≤ 1.0%; intra-assay CV ≤ 5.0%), and Dickkopf-related protein-1 (DKK-1, cat. BI-20413, Biomedica Medizinprodukte GmbH, Wien, Austria; inter-assay CV ≤ 3.0%; intra-assay CV ≤ 3.0%). The absorbance from ELISA assays was analysed on ELISA Ledetect 96 Microplate Reader (BioConcept, Allschwil, Switzerland) using MicroWin Software. All bone markers were evaluated in duplicates, in one batch.
Bone mineral density
Bone mineral density (g/cm2) at the lumbar spine and femoral neck was measured by dual-energy X-ray absorptiometry (DXA). DXA scans were taken using Lunar DPX (GE Healthcare, Madison, WI, US). Calibration of the scanner using a phantom was performed prior to each session according to the manufacturer’s protocol. Lunar database was used as a reference for calculating T- and Z-score values from the BMD measurements.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0c (GraphPad Software, San Diego, CA, US). Normality of the continuous variables was verified with Shapiro–Wilk test. Normally distributed variables were compared by means of the t-student test, while those with non-Gaussian distribution were analysed by the nonparametric Mann–Whitney test. Biochemical data of patients stratified with regard to their BMD status were compared using one-way ANOVA or Kruskal-Wallis test, depending on data distribution. Statistical correlations were assessed by Pearson’s or Spearman’s rank correlation coefficient, according to the data distribution. The multiple regression model was then developed to predict BMD in AD patients. Two-tailed p values < 0.05 were considered significant.
Results
The studied cohort comprised 22 males and 58 females (25 pre- and 33 postmenopausal). Their mean age at AD diagnosis was 36.1 ± 12.6 years, and was significantly higher in females compared to affected males (38.1 ± 12.6 vs. 31.0 ± 11.1 years; p = 0.023) (Table 1). Mean disease and replacement therapy duration was 15.0 ± 10.7 years, similar in both genders (p = 0.994).
In terms of GC replacement, all patients were receiving HC (mean daily dose 24.1 ± 3.4 mg). Fludrocortisone was substituted in 65 individuals, and 25 patients were taking DHEA supplements as well. Additionally, 64 subjects were on levothyroxine (LT4) replacement due to primary hypothyroidism, either autoimmune or iatrogenic. Nobody from the studied cohort was treated for osteoporosis at the time of the study, and only 9 individuals were supplementing calcium or vitamin D3, taken occasionally as OTC preparations. DHEA dosages seemed higher in males, but statistical significance appeared borderline (p = 0.058). The only gender-related difference was detected with regard to daily HC doses, which were higher in males (26.4 ± 3.6 mg vs. 24.1 ± 3.9 mg in females; p = 0.009) however, statistical significance vanished after correction for the body mass (p = 0.083). In line, approximate cumulative HC dose, calculated as a product of daily HC dose x years of treatment x 365 days, did not reveal sex differences (140 ± 101 g vs. 133 ± 99 g; p = 0.594).
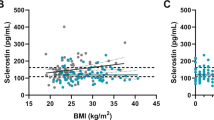
Biochemical data of the studied patients, including serum BTMs, are presented in Table 2. Mean concentrations of 25(OH)D3 were low, with no gender differences (p = 0.779). Hormonal measurements revealed normal serum TSH and PTH levels, since these were among the enrolment conditions, and, in accordance with AD characteristics, low serum DHEA-S. Among the studied hormones only this latter displayed sex-related differences, with higher levels detectable in males (p = 0.0003). Only patients replacing DHEA were able to achieve their DHEA-S levels within the reference limits. With regard to the studied BTMs, all values apart from PINP, remained within the reference ranges (Table 2). Osteocalcin levels were significantly higher in males compared to females (mean 33.1 ± 9.1 ng/mL vs. 22.9 ± 7.0 ng/mL, p < 0.0001). Serum sclerostin was not altered, although significantly higher in women than in men (44.9 ± 27.4 pmol/L vs. 31.4 ± 16.1 pmol/L, p = 0.030).
Since no control cohort was available in our study, Z-scores were used to estimate BMD in our patients compared to the reference population from the DXA device. Mean Z-score values were negative in the whole cohort, as well as in subgroups of males, pre- and postmenopausal females, except for the femoral neck in males, where mean Z-score remained positive. T-scores were applied to classify patients with normal BMD, osteopenia and osteoporosis, according to the WHO criteria [30, 31]. Lumbar osteopenia was found in 45.5% males, 24% young females, and 42.4% postmenopausal females, while osteoporosis was diagnosed in 9.0%, 4.0% and 21.1% patients, respectively. At the femoral neck, osteopenia was found in 22.7% men, 32% premenopausal women, and more than half of women post menopause (54.5%). Cases of femoral osteoporosis were detected only in this latter subgroup.
Stratification of patients by their BMD status – normal, osteopenia or osteoporosis – revealed significant differences in their mean age (the highest in patients with osteoporosis), disease duration (the longest in patients with osteoporosis), and body mass (the lowest in patients with osteoporosis) (Table 3). No differences were found in three subgroups with regard to the HC dosage, also when adjusted for body mass (p values > 0.05). However, mean cumulative HC dose was the lowest among subjects with normal BMD, whereas those with osteoporosis have received the highest overall HC amount. Statistical significance was also observed for DHEA-S concentrations - the highest levels were found in individuals with the best BMD status. Significant differences concerning BTMs in the AD patients stratified by their BMD were found only for ALP activity, serum CTX and sclerostin levels. The lowest ALP activities and CTX concentrations were detected in subjects with normal BMD, while opposite relationship was found for circulating sclerostin, with the lowest levels in patients suffering from osteoporosis.
Furthermore, we searched for plausible correlations between BMD in the lumbar spine, a location typically affected by GC excess, and other parameters evaluated in patients with AD (Table 4). Lumbar BMD correlated positively with BMD at the femoral neck, body mass and serum DHEA-S level. Negative correlation was detected between lumbar spine BMD and daily HC dose per kg body mass, cumulative HC dose, patient’s age and disease duration, as well as ALP activity and CTX concentration. On the contrary, no correlation of BMD was found with calcium homoeostasis parameters, osteocalcin, bone formation markers (except for ALP), and DKK-1, while the expected positive relationship with sclerostin level did not reach statistical significance level.
Eventually, in multiple linear regression only age, body mass, ALP activity, serum CTX, and circulating sclerostin remained independent predictors of BMD at the lumbar spine (Table 5), whereas disease duration, HC dose and DHEA-S concentration lost their significance. The adjusted R2 coefficient of determination for this model was 54.2%.
Discussion
The project was designed to evaluate BMD during long-lasting GC substitution and correlate these values with the circulating BTMs. The rationale was to determine if serum marker concentrations were altered by conventional steroid replacement and might plausibly provide some additional guidance for routine AD treatment. Our results indicate that, in addition to rising age and lower body mass, ALP activity, serum CTX and sclerostin levels are independent predictors of lower BMD in AD patients. These parameters might allow to identify individuals, who require particularly cautious glucocorticoid dosages and early preventive measures.
Daily HC dosages in our study remained in line with current recommendations for GC replacement [1]. Given long history of disease duration (mean 15.0 ± 10.7 years), elevated cumulative steroid doses, exceeding on average 130 g HC per person, seem to be mainly due to the long-lasting replacement. Correct results of serum electrolytes and fasting plasma glucose further support proper daily HC dosages. Among biochemical analyses, only vitamin D3 levels were seriously impaired, although this might have been expected at our latitude, with samples collected at various seasons of the year. Furthermore, autoimmune diseases, including AD, typically feature decreased 25(OH)D3 concentrations [32]. Nonetheless, although reduced, 25(OH)D3 levels in our cohort were not sufficiently low to enhance PTH synthesis.
Likewise, most of the BTMs in this study displayed normal levels in patients with AD and only PINP concentrations were decreased. Serum PINP is considered a reference marker of the bone formation, rising in response to bone fractures but also during efficient treatment of osteoporosis [19, 20]. Reduced PINP was formerly observed in endogenous hypercortisolism and as a result of prednisone intake [33, 34]. With this in mind, low PINP levels in our cohort might reflect genuine impairment of the osteogenesis due to steroid overtreatment. Nevertheless, other bone formation markers, including osteocalcin, which reduced levels used to be consistently associated with GC excess, did not display alterations in the current study [24]. In line, previous analyses in patients with adrenal failure did not reveal diminished serum PINP, although numbers of participants were limited [26, 35].
In densitometric evaluation, Z-scores in our study appeared negative in all subgroups and both locations, apart from the femoral neck in males. On the other hand, none of the mean Z-scores revealed lower than −2.0, i.e. below the age-adjusted expectations defined by International Society for Clinical Densitometry [36]. Therefore, although BMD in patients treated for AD may be decreased versus peak bone mass, the reduction is not substantially accelerated compared to that taking place in the general population. Assessed upon T-scores, only half of the studied cohort presented with normal BMD at the lumbar spine, and 53.8% at the femoral neck. However, when evaluated in subgroups, considerable differences in frequencies of osteopenia/osteoporosis were observed. The highest proportions of reduced BMD were found in postmenopausal women, in whom advanced age, and in consequence, longest disease duration, was combined with lack of protective effect of the estrogens. Altogether, osteopenia or osteoporosis was found in the lumbar spine of 63.5% and in the femoral neck of 72.6% postmenopausal women. On the contrary, in the premenopausal cohort a unique case of osteoporosis in the lumbar spine and no femoral neck osteoporosis were detected, suggesting that with sufficient estrogen supply, female patients on GC substitution do not present considerable deterioration of BMD. Similar observations were reported in series of AD patients from Spain, Germany and Belgium [15, 26, 37]. Overall, in the studied cohort, osteoporosis was more common within the lumbar spine, a location typically sensitive to steroid influence [7].
In order to further dissect the consequences of GC substitution, patients were divided into three subgroups according to their BMD (normal, osteopenia, osteoporosis). No differences in daily HC dosages were detected, while mean cumulative HC dose appeared the highest in individuals with osteoporosis, although disease duration was equally the longest in this subgroup. Previous analyses remain equivocal – some studies demonstrated direct relationship between BMD and steroid replacement dose [4, 5, 17] whereas others failed to confirm it [13, 15, 38]. Nevertheless, disease duration is also dependent upon patient’s age, and indeed subjects with osteoporosis were the eldest. Furthermore, these individuals displayed the lowest body mass compared to patients with osteopenia and normal BMD. Increasing age, lower body mass, longer time of GC administration, all well-established risk factors for osteoporosis, appeared correlated with the BMD value. A relationship between AD duration (treatment time) and BMD was formerly reported in some studies [13, 34] but was not consistent in other analyses [13, 16, 26]. In fact, when multiple regression model was developed, only age and body mass remained significant predictors of the lumbar BMD, whereas HC dose and disease duration lost their significance.
With regard to biochemical results, comparison of patients with normal BMD, osteopenia and osteoporosis revealed significantly elevated ALP activity and CTX concentrations, while decreased sclerostin and DHEA-S in subjects suffering from osteoporosis. These observations were further confirmed by negative correlations between ALP, CTX and BMD values. On the contrary, serum DHEA-S positively correlated with BMD, whereas correlation with sclerostin did not reach statistical significance (p = 0.0799). When it came to multiple regression modelling, only ALP, CTX, and sclerostin levels survived as independent BMD predictors, while DHEA-S was rejected from the model. Indeed, despite apparently positive influence of adrenal androgens, effects of DHEA replacement on bone mineral status in AD were equivocal in previous reports [15, 39]. ALP activity, although not fully specific for bone, remains widely available parameter, which can be easily followed in most medical settings, after excluding liver disease. All ALP values in our cohort remained within the reference ranges, therefore the utility of its evaluation would rely upon consecutive measurements to detect increased bone turnover and BMD loss [40]. Increased CTX level is another marker of deteriorating bone status, indicator of osteoporosis and fracture risk [20]. Elevated serum CTX and negative correlation between CTX and BMD were described in patients with Cushing’s syndrome [33].
Finally, sclerostin levels were unaltered in our cohort, however, lower circulating sclerostin appeared associated with deteriorating BMD. This observation was further supported in the multiple regression model, which pinpointed sclerostin as one of independent predictors of BMD in the lumbar spine. Another recent study in a smaller AD cohort did not reveal correlation between circulating sclerostin and trabecular bone BMD, although sclerostin was significantly higher among patients versus healthy controls [29]. On the contrary, patients with chronic endogenous hypercortisolism presented with decreased serum sclerostin, which was then rising after successful treatment [41]. Of note, in autoimmune disorders, systemic lupus erythematosus and Crohn disease, a positive correlation was found between serum sclerostin and the lumbar spine Z-score independent of steroid therapy [42]. Overall, data on circulating sclerostin in the context of GC excess, and its correlation with BMD remain contradictory.
The major limitation of the current study is lack of trabecular bone score (TBS) evaluation, which has recently emerged as a novel tool to assess bone microarchitecture and predict skeletal fragility [43, 44]. For instance, TBS allows to reliably compare bone impact of conventional HC replacement versus dual-release HC, showing that the use of the later formulations is associated with higher BMD and TBS scores, even though no differences were found with regard to circulating BTMs [44]. Unfortunately, no information about vertebral fractures, which often remain asymptomatic, was available in our cohort. Additionally, a control group composed of the sex- and age-matched healthy subjects with normal adrenocortical function would be valuable to more reliably assess and compare BTM levels. However, given the multitude of factors implicated in the BMD status, a very large cohort would be required to reflect the whole population. Therefore, we assumed that Z-scores calculated based upon the Lunar database could be used as a reference. Finally, BTMs measurements are not routinely available in most centres, and their practical usage is additionally limited by high variability, insufficient data for comparison of treatments by the same marker and inadequate quality control [20, 21]. Nonetheless, studies like ours may broaden the experience of BTM application in clinical practice and, with the technical progress in their evaluation, support incorporation of some BTMs into clinical follow-up in future.
In conclusion, despite its limitations, our study reveals that in patients with AD who receive standard substitution doses of HC, BMD decline is not significantly accelerated relative to the reference values for gender and age, reflected by Z-scores. Serum concentrations of the bone turnover markers: CTX, osteocalcin, sclerostin and DKK-1, as well as ALP activity remain within their reference ranges. In addition to increasing age, independent predictors of low BMD in the lumbar spine in AD patients are lower body mass, higher ALP activity, elevated serum CTX and lower sclerostin concentrations. These factors might be monitored during GC substitution to identify subjects at particular risk for BMD decline. Nonetheless, confirmation of their real usefulness in routine clinical practice requires further prospective observations.
References
E.S. Husebye, B. Allolio, W. Arlt et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 275(2), 104–115 (2014). https://doi.org/10.1111/joim.12162
G. Johannsson, A. Falorni, S. Skrtic et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin. Endocrinol. (Oxf) 82(1), 2–11 (2015). https://doi.org/10.1111/cen.12603
V. Guarnotta, C. Di Stefano, C. Giordano, Long-term outcomes of conventional and novel steroid replacement therapy on bone health in primary adrenal insufficiency. Sci. Rep. 12(1), 13280 (2022). https://doi.org/10.1038/s41598-022-13506-5
K. Løvås, C.G. Gjesdal, M. Christensen et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur. J. Endocrinol. 160(6), 993–1002 (2009). https://doi.org/10.1530/EJE-08-0880
M. Yazidi, C. Danguir, D. Maamer et al. Impact of hydrocortisone replacement on bone mineral density and bone turnover markers in patients with primary adrenal insufficiency. Endocr. Regul. 56(3), 209–215 (2022). https://doi.org/10.2478/enr-2022-0022
L. Li, S. Bensing, H. Falhammar, Rate of fracture in patients with glucocorticoid replacement therapy: a systematic review and meta-analysis. Endocrine 74, 29–37 (2021). https://doi.org/10.1007/s12020-021-02723-z
J. Compston, Glucocorticoid-induced osteoporosis: an update. Endocrine 61(1), 7–16 (2018). https://doi.org/10.1007/s12020-018-1588-2
Z.E. Belaya, T.A. Grebennikova, G.A. Melnichenko et al. Effects of endogenous hypercortisolism on bone mRNA and microRNA expression in humans. Osteoporos. Int. 29(1), 211–221 (2018). https://doi.org/10.1007/s00198-017-4241-7
T. van Staa, H.G. Leufkens, C. Cooper, The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos. Int. 13(10), 777–787 (2002). https://doi.org/10.1007/s001980200108
K.R. Koetz, M. Ventz, S. Diederich, M. Quinkler, Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97(1), 85–92 (2012). https://doi.org/10.1210/jc.2011-2036
D.D. Chandy, E. Bhatia, Bone mineral density in patients with Addison’s disease on replacement therapy with prednisolone. Endocr. Pract. 22(4), 434–439 (2016). https://doi.org/10.4158/EP151014.OR
K.R. Frey, T. Kienitz, J. Schulz et al. Prednisolone is associated with a worse bone mineral density in primary adrenal insufficiency. Endocr. Connect. 7(6), 811–818 (2018). https://doi.org/10.1530/EC-18-0160
N. Esteban, T. Loughlin, A.L. Yergey et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J. Clin. Endocrinol. Metab. 72(1), 39–45 (1991). https://doi.org/10.1210/jcem-72-1-39
W. Arlt, C. Rosenthal, S. Hahner, B. Allolio, Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin. Endocrinol. (Oxf) 64(4), 384–389 (2006). https://doi.org/10.1111/j.1365-2265.2006.02473.x
M. Fichna, M. Gryczyńska, A. Sowińska, J.Sowiński, Ocena metaboliczna terapii substytucyjnej hydrokortyzonem u pacjentów z pierwotna niedoczynnościa kory nadnerczy [Metabolic assessment of hydrocortisone replacement therapy in patients with primary adrenocortical insufficiency]. Przegl. Lek. 68(2), 96–102 (2011)
V. Camozzi, C. Betterle, A.C. Frigo et al. Vertebral fractures assessed with dual-energy X-ray absorptiometry in patients with Addison’s disease on glucocorticoid and mineralocorticoid replacement therapy. Endocrine 59(2), 319–329 (2018). https://doi.org/10.1007/s12020-017-1380-8
F. Heureux, D. Maiter, Y. Boutsen, J.P. Devogelaer, J. Jamart, J. Donckier, Evaluation de la substitution par corticostéroïdes et de ses répercussions osseuses dans la maladie d’Addison [Evaluation of corticosteroid replacement therapy and its effect on bones in Addison’s disease]. Ann. Endocrinol. (Paris) 61(3), 179–183 (2000)
S. Kaptoge, L.I. Benevolenskaya, A.K. Bhalla et al. Low BMD is less predictive than reported falls for future limb fractures in women across Europe: results from the European Prospective Osteoporosis Study. Bone 36(3), 387–398 (2005). https://doi.org/10.1016/j.bone.2004.11.012
P.D. Delmas, R. Eastell, P. Garnero, M.J. Seibel, J. Stepan; Committee of Scientific Advisors of the International Osteoporosis Foundation, The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos. Int. 6, S2–S17 (2000). https://doi.org/10.1007/s001980070002
S. Vasikaran, R. Eastell, O. Bruyère et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos. Int. 22(2), 391–420 (2011). https://doi.org/10.1007/s00198-010-1501-1
J. Delgado-Calle, A.Y. Sato, T. Bellido, Role and mechanism of action of sclerostin in bone. Bone 96, 29–37 (2017). https://doi.org/10.1016/j.bone.2016.10.007
P. Garnero, E. Sornay-Rendu, B. Claustrat, P.D. Delmas, Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone Miner. Res. 15(8), 1526–1536 (2000). https://doi.org/10.1359/jbmr.2000.15.8.1526
L. Gifre, S. Ruiz-Gaspà, A. Monegal et al. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 57(1), 272–276 (2013). https://doi.org/10.1016/j.bone.2013.08.016
A.L. Burshell, R. Möricke, R. Correa-Rotter et al. Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone 46(4), 935–939 (2010). https://doi.org/10.1016/j.bone.2009.12.032
M. Tóth, A. Grossman, A. Glucocorticoid-induced osteoporosis: lessons from Cushing’s syndrome. Clin. Endocrinol. (Oxf) 79(1), 1–11 (2013). https://doi.org/10.1111/cen.12189
M.A. Valero, M. Leon, M.P. Ruiz Valdepeñas et al. Bone density and turnover in Addison’s disease: effect of glucocorticoid treatment. Bone Miner 26(1), 9–17 (1994). https://doi.org/10.1016/s0169-6009(08)80158-4
S.R. Peacey, C.Y. Guo, A.M. Robinson et al. Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin. Endocrinol. (Oxf) 46(3), 255–261 (1997). https://doi.org/10.1046/j.1365-2265.1997.780907.x
M. Wichers, W. Springer, F. Bidlingmaier, D. Klingmüller, The influence of hydrocortisone substitution on the quality of life and parameters of bone metabolism in patients with secondary hypocortisolism. Clin. Endocrinol. (Oxf) 50(6), 759–765 (1999). https://doi.org/10.1046/j.1365-2265.1999.00723.x
A. Zdrojowy-Wełna, J. Halupczok-Żyła, N. Słoka, J. Syrycka, Ł. Gojny, M. Bolanowski, Bolanowski Trabecular bone score and sclerostin concentrations in patients with primary adrenal insufficiency. Front. Endocrinol. (Lausanne) 13, 996157 (2022). https://doi.org/10.3389/fendo.2022.996157
J.A. Kanis, L.J. Melton 3rd, C. Christiansen, C.C. Johnston, N. Khaltaev, The diagnosis of osteoporosis. J. Bone. Miner. Res. 9(8), 1137–1141 (1994). https://doi.org/10.1002/jbmr.5650090802
P. Głuszko, E. Sewerynek, W. Misiorowski et al. Guidelines for the diagnosis and management of osteoporosis in Poland. Update 2022. Endokrynol. Pol. 74(1), 5–15 (2023). https://doi.org/10.5603/EP.a2023.0012
M. Penna-Martinez, G. Meyer, A.B. Wolff et al. Vitamin D status and pathway genes in five European autoimmune Addison’s disease cohorts. Eur. J. Endocrinol. 184(3), 373–381 (2021). https://doi.org/10.1530/EJE-20-0956
W. Guo, F. Li, C. Zhu et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J. Int. Med. Res. 46(1), 492–503 (2018). https://doi.org/10.1177/0300060517725660
F.N. Ton, S.C. Gunawardene, H. Lee, R.M. Neer, Effects of low-dose prednisone on bone metabolism. J. Bone Miner. Res. 20(3), 464–470 (2005). https://doi.org/10.1359/JBMR.041125
A.M. Suliman, R. Freaney, T.P. Smith, Y. McBrinn, B. Murray, T.J. McKenna, The impact of different glucocorticoid replacement schedules on bone turnover and insulin sensitivity in patients with adrenal insufficiency. Clin. Endocrinol. (Oxf) 59(3), 380–387 (2003). https://doi.org/10.1046/j.1365-2265.2003.01860.x
J.T. Schousboe, J.A. Shepherd, J.P. Bilezikian, S. Baim, Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J. Clin. Densitom. 16(4), 455–466 (2013). https://doi.org/10.1016/j.jocd.2013.08.004
J.P. Devogelaer, J. Crabbé, C. Nagant de Deuxchaisnes, Bone mineral density in Addison’s disease: evidence for an effect of adrenal androgens on bone mass. Br. Med. J. (Clin Res Ed 294(6575), 798–800 (1987). https://doi.org/10.1136/bmj.294.6575.798
G.D. Braatvedt, M. Joyce, M. Evans, J. Clearwater, I.R. Reid, Bone mineral density in patients with treated Addison’s disease. Osteoporos. Int. 10(6), 435–440 (1999). https://doi.org/10.1007/s001980050251
E.M. Gurnell, P.J. Hunt, S.E. Curran et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 93(2), 400–409 (2008). https://doi.org/10.1210/jc.2007-1134
K. Mukaiyama, M. Kamimura, S. Uchiyama, S. Ikegami, Y. Nakamura, H. Kato, Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin. Exp. Res. 27(4), 413–418 (2015). https://doi.org/10.1007/s40520-014-0296-x
A.H. van Lierop, A.W. van der Eerden, N.A. Hamdy, A.R. Hermus, M. den Heijer, S.E. Papapoulos, Circulating sclerostin levels are decreased in patients with endogenous hypercortisolism and increase after treatment. J. Clin. Endocrinol. Metab. 97(10), E1953–E1957 (2012). https://doi.org/10.1210/jc.2012-2218
C. Fernández-Roldán, F. Genre, R. López-Mejías et al. Sclerostin serum levels in patients with systemic autoimmune diseases. Bonekey Rep. 5, 775 (2016). https://doi.org/10.1038/bonekey.2016.2
E. Shevroja, J.Y. Reginster, O. Lamy et al. Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos. Int. 34(9), 1501–1529 (2023). https://doi.org/10.1007/s00198-023-06817-4
F. Bioletto, M. Barale, M. Parasiliti-Caprino et al. Bone safety of dual-release hydrocortisone in patients with autoimmune primary adrenal insufficiency. Front. Endocrinol (Lausanne). 11(14), 1234237 (2023). https://doi.org/10.3389/fendo.2023.1234237
Acknowledgements
We express our gratitude to all our patients with Addison’s disease for their participation and comprehension.
Author contributions
All authors contributed to the study conception. K.F., P.G. and M.F. were involved in study design. Material preparation was performed by P.G., M.R. and M.F. Data collection and analysis was done by K.F., A.S. and M.F. The first draft of the manuscript was written by K.F. and all authors commented and provided feedback to the final version. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the principles of the Declaration of Helsinki. Its protocol was approved by the local Ethics Committee of Poznan University of Medical Sciences (decision 68/19). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furman, K., Gut, P., Sowińska, A. et al. Predictors of bone mineral density in patients receiving glucocorticoid replacement for Addison’s disease. Endocrine 84, 711–719 (2024). https://doi.org/10.1007/s12020-024-03709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03709-3