Abstract
Purpose
The association between glucocorticoid replacement therapy for adrenal insufficiency (AI) and osteoporosis is unclear. Fracture is a major cause of morbidity in patients with osteoporosis. This study aims to determine if patients on glucocorticoid replacement therapy for AI have an increased rate of fractures compared to the general population.
Methods
We included all studies with adult patients receiving glucocorticoid replacement therapy for either congenital adrenal hyperplasia (CAH), primary adrenal insufficiency (PAI), or secondary adrenal insufficiency (SAI). Studies without fracture data were excluded, as well as meeting abstracts. Studies with fractures but without a control group were eligible to be included in the systematic review but not in the meta-analysis. The primary outcome was the number of fractures, which was further differentiated into osteoporotic fractures. In addition, the glucocorticoid dose equivalents used were noted whenever possible.
Results
Seventeen studies were included in the systematic review. Seven were used in the meta-analysis of any fracture and six were used for osteoporotic fracture. The reported fracture rate ranged between no fracture to 60.8% in the patient group and no fracture to 43.8% in the control group. The odds ratio (OR) for any fracture was 2.71 (95%CI: 1.36–5.43, P = 0.005) and for osteoporotic fracture 2.76 (95%CI: 2.39–3.19 P < 0.00001), favoring the control group.
Conclusions
Patients with AI on glucocorticoid replacement therapy have a higher rate of fractures compared to the control population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal insufficiency (AI) is associated with increased morbidity and mortality compared to healthy subjects regardless of etiology [1,2,3,4,5,6,7,8]. Patients with AI require lifelong glucocorticoid replacement therapy and dose monitoring to avoid complications from either over- or under-supplementation. There is a clear association between glucocorticoid use and osteoporosis in subjects who receive immunosuppressive doses [9]. The evidence for increased risk of osteoporosis in those who receive glucocorticoid replacement therapy is less clear.
Bone mineral density (BMD) is often the main outcome measured in studies evaluating the risk of osteoporosis in patients with AI. In general, reduction in BMD has been shown to be associated with the cumulative glucocorticoid dose and disease duration [10], but there are many inconsistencies. Some studies have reported reduced BMD in patients with primary adrenal insufficiency (PAI) [11], while others reported no significant difference in BMD compared to matched controls [12]. Several studies reported reduced BMD in only postmenopausal women [13] or only in men [14]. A recent meta-analysis of adults with congenital adrenal hyperplasia (CAH) found a slight decrease in BMD compared to matched controls [15].
The main morbidity from osteoporosis comes from fragility fractures. However, there is a poor association between BMD and fracture risk, especially in glucocorticoid-induced osteoporosis [16, 17]. To date, there has been no comprehensive summary of data about glucocorticoid replacement and fracture rate. Despite this, several society practice guidelines have made recommendations on osteoporosis screening in patients based on very limited evidence. For instance, screening of BMD in patients with CAH is recommended if subjected to a prolonged period of higher-than-average glucocorticoid dosing or prior history of fragility fracture [18], a recommendation based on only two studies, neither of which looked for the correlation between glucocorticoid doses and fracture rates [19, 20]. The endocrine society clinical practice guideline for patients with PAI acknowledged the paucity of data on BMD in these patients and does not comment on fracture rate [21]. While the guideline for patients with secondary adrenal insufficiency (SAI) related to hypopituitarism referred to only one study for their statement of increased vertebral fracture risk related to glucocorticoid overreplacement [22], despite there was no control group in their cited reference [10]. Thus, the aim of the current study is to provide a comprehensive review of fracture incidence in patients on glucocorticoid replacement therapy.
Methods
This systematic review and meta-analysis were based on a prespecified protocol and is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [23].
Search strategy and selection criteria
The Independent literature search was performed by the first and last authors (LL and HF) using PubMed and Web of Science from inception to 11 January 2021. We used a combination of keyword/MeSH terms: “adrenal hyperplasia, congenital/complications”, “steroid 21-hydroxylase/genetic*”, “Addison disease/complications”, “Addison disease/drug therapy”, “primary adrenal insufficiency”, “hypopituitarism/complications”, “secondary adrenal insufficiency”, “glucocorticoid/therapeutic use*”, “glucocorticoid/adverse effects”, “fractures, bone/epidemiology”, “fractures, bone/etiology*”, “bone density*”, “bone density/drug effect*” and “bone density/physiology”. We imposed no restrictions based on language, publication status, or type of studies. Inclusion or exclusion of articles was agreed on by consensus. We sought additional studies from relevant references of included records.
We included all studies with adult patients receiving glucocorticoid replacement therapy for either PAI, CAH, or SAI. Addison’s disease was the main etiology for PAI in most studies. Only one study did not specify the etiology of PAI and included both PAI (n = 36) and CAH (n = 8) patients, which were not possible to be analyzed separately [24]. Hypopituitarism was the main cause of SAI in all of the studies included [10, 25]. Studies without fracture data were excluded, as well as reviews and meeting abstracts. Studies with fracture data but without a control group (either matched or reference control) were included in the systematic review but not in the meta-analysis.
Data extraction
Initially, records were screened at the title and abstract level, and potentially eligible studies were assessed in full text. We extracted data for study and participant baseline characteristics, interventions, comparators, and clinical outcomes. The quality of the studies that were included in the meta-analysis was assessed using the Newcastle- Ottawa quality assessment scale for case-control studies (www.ohri.ca/programs/clinical_epidemiology/oxford.asp) (Table 1).
Outcome
The primary outcome was the rate (%) of fractures in patients receiving glucocorticoid replacement therapy. Where possible, the details of fracture type (any fracture or osteoporotic fracture), location, and definition (self-report, X-ray, and international classification of diseases code (ICD)) were included (Table 2). In the meta-analysis, any fractures and osteoporotic fractures were analyzed separately. Fractures of the vertebrae, hip, or wrist without significant trauma were considered osteoporotic fractures. Hydrocortisone dose equivalents were expressed either as total daily milligram (mg) or mg per body surface area (mg/m²) (Table 2). All daily doses were converted to mg using an average body surface area of 1.79 m2 for the purpose of demonstrating a dose fracture correlation.
Statistical analysis
A cumulative odds ratio (OR) was calculated by pooling the reported fractures from the included studies using the Mantel-Haenszel method for dichotomous outcomes. Statistical heterogeneity among the OR was quantified by the I2 statistic (low heterogeneity I2 < 50% and high heterogeneity I2 > 50%). When high statistical heterogeneity was identified, a random-effect model was preferentially used over a fixed-effect model. 95% Confidence Interval (CI) not surpassing 1 and a P-value <0.05 were considered statistically significant. We performed a sensitivity analysis to assess the contribution of each study to the pooled estimation by excluding one study at a time and recalculating the pooled OR estimation for the remaining studies. Publication bias was not performed due to the small number of included studies. All analysis was performed and figures generated using Review Manager (RevMan) Software (Version 5.2. Copenhagen: the Nordic Cochrane Center, the Cochrane Collaboration, 2014). The effect of hydrocortisone dose equivalent on fracture rate (R2) was calculated using the linear regression function in Microsoft Excel.
Results
Through PubMed and Web of Science searches 289 records were identified and screened at the title and abstract level once duplicates were removed (Fig. 1). Finally, 17 studies were included in the systematic review, and seven studies were ultimately included in the meta-analysis.
Systematic review
Table 2 summarizes the important characteristics of the 17 studies included in the systematic review. Ten studies included fracture data in patients with CAH [12, 19, 20, 26,27,28,29,30,31,32]. Four out of the ten studies had age- and sex-matched controls [19, 20, 26, 29]. One out of the ten studies used historical controls only [27]. Six studies included fracture data in patients with PAI [11, 12, 24, 33,34,35]. Two of these had matched control subjects [34, 36] and one with historical control [11]. The study by Koetz et al. included both CAH and PAI patients which were presented separately in Table 2. Two studies had fracture data in patients with SAI [10, 25], of which one used reference controls [25].
The reported fracture rate ranged between 0–60.8% in the patient group and 0–43.8% in the control group (Table 2). In patients with CAH, fracture rate was 16.6% (range 0–53.3%) and 4.3% (range 0–20%) for any fracture and osteoporotic fracture, respectively [12, 19, 20, 26,27,28,29,30,31,32]. In patients with PAI, fracture rate was 16.7% (range 5.8–34.3%) and 7.5% (range 5.8–21.8%) for any fracture and osteoporotic fracture, respectively [11, 12, 24, 33,34,35]. In patients with SAI osteoporotic fracture rate was 37.3% (range 26.2–60.8%)[10, 25].
Three studies did not differentiate between osteoporotic versus non-osteoporotic fractures [27, 30, 33]. Vertebral fractures were the primary outcome reported by three studies [10, 11, 35]. Hip fracture was reported as the primary outcome of one study [34]. Björnsdottir et al. also kindly provided unpublished data of the rate of any fracture, which was included in the meta-analysis. Five studies reported a combination of any and osteoporotic fractures [19, 24, 26, 31, 32], while one study reported only osteoporotic fractures which included traditional sites such as wrist, vertebra, pelvis, and femur, but also non-traditional sites such as upper arm, tibia, ankle, foot, ribs, and clavicles [25]. One study reported a combination of any fractures and vertebral fractures [12]. The study by Ceccato et al. looked for evidence of any fractures as well as osteoporotic fractures, but only one traumatic fracture was found [20]. Two studies did not find any fracture [28, 29].
Various methods were used to identify fractures in different studies. These included self-reporting surveys [12, 24, 25, 27, 30, 32], medical records [19, 33, 34], history and examination [29], as well as radiographs [11, 19, 26, 35] or a combination of several of the previously mentioned methods [20]. Two studies did not state their fracture definition [28, 31]. In some instances, it was not possible to discern if the fractures included were symptomatic. It may be assumed that self-reported fractures were symptomatic fractures, but those diagnosed on X-ray or found through ICD codes could include asymptomatic fractures also.
Daily hydrocortisone equivalent doses were reported by 13 out of the 17 studies (Table 2). Most of these are within or at the higher end of the range typical for replacement regime (21.1–35 mg or 9–17.9 mg/m²) and are similar across the studies.
Meta-analysis
Seven studies were included in the meta-analysis for any fractures (Fig. 2) [19, 20, 25, 26, 29, 34, 35]. A total of 3282 patients and 29,980 controls were included, of which total events were 527 (16.1%) in the patient group and 3571 (11.9%) in the control group giving an OR of 2.71 (95%CI 1.36–5.43). The OR for any fracture was not able to be estimated in one study as it showed no fracture outcome in either patient or control groups [29].
Six studies were included in the meta-analysis for osteoporotic fractures (Fig. 2) [19, 20, 25, 26, 34, 35]. A total of 3542 patients and 32,092 controls were included, of which total events were 283 (8.0%) in the patient group and 887 (2.8%) in the control group giving an OR of 2.76 (95%CI 2.39–3.19). One study found no osteoporotic fractures in either patient or control group and therefore an OR could not be generated from this study [20].
Sensitivity analysis was done by excluding each study in turn. Any fracture OR ranged from 2.18 (95%CI 1.15–4.13) to 3.58 (95%CI 2.36–5.43). Osteoporotic fractures OR ranged from 2.70 (95%CI 2.32–3.13) to 3.35 (95%CI 2.14–5.23).
In a separate analysis with CAH patients, although the OR of osteoporotic fractures was 2.5 (95%CI 0.95–6.55, P = 0.06), it just failed to reach statistical significance [19, 20, 26, 29] (Fig. 3). In contrast, patients with either PAI or SAI showed statistically significantly higher rates of osteoporotic fractures compared to controls (OR 2.77, 95%CI 2.39–3.20) [25, 34, 35] (Fig. 3). The OR for osteoporotic fracture in patients with PAI and SAI ranged from 2.68 (95%CI 2.30–3.12) to 3.73 (95%CI 2.09–6.67) [25, 34, 35].
The rate of any fracture did not appear to have any correlation with hydrocortisone equivalent doses (R² = 0.059, P = 0.47), while the rate of osteoporotic fracture had only a weak correlation that did not reach statistical significance (R² = 0.250, P = 0.08). Separate correlation analysis of osteoporotic fractures in CAH patients and PAI/SAI patients also did not reach statistical significance (R² = 0.205, P = 0.44 and R² = 0.416, P = 0.17, respectively).
Discussion
This is the first systematic review and meta-analysis on fracture rate in patients receiving glucocorticoid replacement for either CAH, PAI, or SAI and it demonstrated an overall higher rate in patients compared to controls. This was true for both any and osteoporotic fractures. Fracture incidence reported by different studies varied greatly owing to differences in patient age, sex, and comorbidities, the etiology of AI as well as the type of fracture and method of screening.
Studies that reported low osteoporotic fracture rates were more likely to include younger patients. For instance, the studies with zero reported osteoporotic fracture had an age range of 24–32 years [20, 28, 29] compared to the median age of 55 years in the study population with the highest fracture rate [10]. Patients with CAH had a lower osteoporotic fracture rate compared to patients with PAI or SAI and were younger (30–40 years CAH patients versus 43–61 years PAI patients and 53–55 years SAI patients).
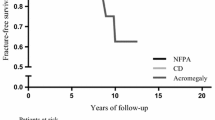
Studies with patients with SAI had the highest osteoporotic fracture rate, followed by patients with PAI. As well as older age, those with SAI were more likely to have other risk factors for osteoporosis such as hypogonadism and growth hormone deficiency [10, 25, 37]. For example, Mazziotti et al. reported the highest rate of osteoporotic fracture (60.8%) in their cohort of patients with hypopituitarism, all of whom with GH deficiency, and a proportion of these patients did not receive recombinant GH (rGH) treatment [10]. Similarly, 101 out of their 107 patients in Rosen et al.’s study had concurrent glucocorticoid and growth hormone deficiency. None of the patients were on rGH replacement, and a proportion of these patients also had untreated hypogonadism [25]. In addition, patients with Addison’s disease can have other autoimmune manifestations such as premature ovarian insufficiency, atrophic gastritis and celiac disease that may negatively impact bone health [34].
Androgen excess may be responsible for lower fracture rate in CAH patients due to its protective role. However, the true effect of androgens on fracture is difficult to tease out due to the interplay of many additional confounding factors mentioned earlier. It should also be considered that poor glucocorticoid adherence increases androgen concentrations, especially in women with CAH which may increase BMD, while good adherence may lead to low androgens [38, 39] and decreased BMD. In contrast, in males with CAH, increased adrenal androgen concentrations may decrease testosterone concentration due to inhibition of gonadotropins [38].
Glucocorticoid doses and regimens differ depending on the etiology of AI [40]. For instance, relatively higher doses of glucocorticoids are required for the suppression of adrenal androgen production in CAH compared to replacement doses for PAI or SAI [18, 41]. Our study showed glucocorticoid replacement doses had minimal effect on the overall fracture rate. Although there is a weak to moderate correlation of glucocorticoid equivalent dose with osteoporotic fracture rate, particularly in patients with PAI and SAI, these did not reach statistical significance. We acknowledged that the major limitations included the heterogeneity of fracture definition and the actual dose used was not too different between the studies. Moreover, we only used the current glucocorticoid dose and we could not calculate the lifetime glucocorticoid exposure due to lack of data. Thus, more studies are needed for analysis of the effect of glucocorticoid doses on fracture rate.
Osteoporotic fracture rates in patients with CAH, although 2.5 times higher than that of controls, just failed to reach statistical significance [19, 20, 26, 29]. The two studies in patients with CAH that contributed to the calculation of the final OR had vastly different fracture incidence with significantly increased fracture rate in females with CAH compared to controls (OR = 5.11, 1.05–24.71) but no increase in fracture rate in males with CAH (OR = 1.35, 0.36–4.99) [19, 26]. The latter also reported a higher than usual fracture rate in their male control subjects, which may be related to a high prevalence of sports injuries in young males [26]. A later study by the same authors on a female cohort reported that rough sport and outdoor activities were more common in women with CAH than their matched controls, probably due to high fetal androgen exposure and therefore more masculinized behavior [42], which may explain the relatively high fracture rate in these females.
The study by Björnsdottir et al. reported a relatively low rate of osteoporotic hip fractures at 6.9% [34], despite that the median age of their patient cohort was 61 years, compared to the rate of vertebral fractures which ranged between 5.8% [12] in patient with PAI, to up to 60.8% in a group of patients with SAI [10]. It should be noted that hip fractures occur at an older age compared to vertebral fractures which may explain the difference. Björnsdottir’s study accounts for the majority of combined subjects, which may underestimate the difference between fracture rate in patients and control subjects. Excluding Björnsdottir et al.’s study increased OR for both any and osteoporotic fractures.
Fracture definition is also an important determinant for fracture rate. For instance, one study reported relatively low rates of vertebral fractures (around 2.4% for CAH patients and 5.8% for PAI patients) when the method of data collection was done via self-reporting using surveys [12]. Other studies have looked for fractures extensively through history and examination as well as utilizing radiography if symptoms or signs suggest vertebral fractures but did not find any fractures except for one case of self-reported traumatic fracture of the wrist [20]. Three studies using radiological diagnosis reported much higher vertebral fracture rates at 16.7% [11], 21.8% [36], and 60.8% [10], further highlighting that spinal fractures are often underdiagnosed as they are asymptomatic. In two of three cases that reported zero any/osteoporotic fractures, fracture definition was not defined [28, 31].
This systematic review and meta-analysis have several limitations, which we must acknowledge when considering the internal and external validity of our conclusion. E.g., the studies included were highly heterogeneous with many confounders as discussed previously. There were no randomized controlled trials, most studies were small and the study design varied between prospective to retrospective cohort/case-control studies. Out of the seven studies included in the meta-analysis, three studies were deemed “poor quality” due to a lack of independent validation (blinded interview/examination or X-ray) in their outcome measures in both patients and controls and the use of reference or poorly matched controls [20, 25, 29]. It is worth noting that two of the three “poor quality” studies found no osteoporotic fractures in either patient or control groups and one found only one traumatic fracture in the patient group [20, 29]. Thus, the inclusion of these studies may underrepresent fracture incidence overall. Furthermore, fracture rate was not the primary outcome for some of the studies included, so it was difficult to discern the type/location of the fracture, fracture definition, or whether the fracture was symptomatic. Subgroup analysis of these would have helped further with understanding the diagnosis and management of fractures in this group of patients. Finally, many of the studies were Swedish or Scandinavian and almost all were from Europe/US which may make the data less generalizable.
Conclusion
AI is a chronic condition with many long-term complications. In this first systematic review and meta-analysis analyzing fracture rates, we could demonstrate that patients with AI on glucocorticoid replacement therapy had an increased incidence of fractures compared to the control population. Supraphysiological glucocorticoid doses probably play a role. Despite the study’s limitations, it is important to make clinicians aware of the association between glucocorticoid replacement and increased fracture rate while managing the bone health of these patients.
References
L. Leelarathna, L. Breen, J.K. Powrie, S.M. Thomas, R. Guzder, B. McGowan, P.V. Carroll, Co-morbidities, management and clinical outcome of auto-immune Addison’s disease. Endocrine 38, 113–117 (2010)
H. Falhammar, L. Frisén, C. Norrby, A.L. Hirschberg, C. Almqvist, A. Nordenskjöld, A. Nordenström, Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 99, E2715–E2721 (2014)
S. Bensing, L. Brandt, F. Tabaroj, O. Sjöberg, B. Nilsson, A. Ekbom, P. Blomqvist, O. Kämpe, Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clin. Endocrinol. 69, 697–704 (2008)
P. Burman, A.F. Mattsson, G. Johannsson, C. Höybye, H. Holmer, P. Dahlqvist, K. Berinder, B.E. Engström, B. Ekman, E.M. Erfurth, J. Svensson, J. Wahlberg, F.A. Karlsson, Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J. Clin. Endocrinol. Metab. 98, 1466–1475 (2013)
M.M. Erichsen, K. Løvås, K.J. Fougner, J. Svartberg, E.R. Hauge, J. Bollerslev, J.P. Berg, B. Mella, E.S. Husebye, Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur. J. Endocrinol. 160, 233–237 (2009)
H. Falhammar, L. Frisén, A.L. Hirschberg, C. Norrby, C. Almqvist, A. Nordenskjöld, A. Nordenström, Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J. Clin. Endocrinol. Metab. 100, 3520–3528 (2015)
R.L. Rushworth, D.J. Torpy, H. Falhammar, Adrenal crisis. N. Engl. J. Med 381, 852–861 (2019)
R. Bergthorsdottir, O. Ragnarsson, S. Skrtic, C.A.M. Glad, S. Nilsson, I.L. Ross, M. Leonsson-Zachrisson, G. Johannsson, Visceral fat and novel biomarkers of cardiovascular disease in patients with Addison’s disease: a case-control study. J. Clin. Endocrinol. Metab. 102, 4264–4272 (2017)
J.L. Shaker, B.P. Lukert, Osteoporosis associated with excess glucocorticoids. Endocrinol. Metab. Clin. North Am. 34, 341–356 (2005). viii–ix
G. Mazziotti, T. Porcelli, A. Bianchi, V. Cimino, I. Patelli, C. Mejia, A. Fusco, A. Giampietro, L. De Marinis, A. Giustina, Glucocorticoid replacement therapy and vertebral fractures in hypopituitary adult males with GH deficiency. Eur. J. Endocrinol. 163, 15–20 (2010)
K. Løvås, C.G. Gjesdal, M. Christensen, A.B. Wolff, B. Almås, J. Svartberg, K.J. Fougner, U. Syversen, J. Bollerslev, J.A. Falch, P.J. Hunt, V.K.K. Chatterjee, E.S. Husebye, Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur. J. Endocrinol. 160, 993–1002 (2009)
K.R. Koetz, M. Ventz, S. Diederich, M. Quinkler, Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97, 85–92 (2012)
M.A. Valero, M. Leon, M.P. Ruiz Valdepeñas, L. Larrodera, M.B. Lopez, K. Papapietro, A. Jara, F. Hawkins, Bone density and turnover in Addison’s disease: effect of glucocorticoid treatment. Bone Min. 26, 9–17 (1994)
P.M. Zelissen, R.J. Croughs, P.P. van Rijk, J.A. Raymakers, Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann. Intern. Med. 120, 207–210 (1994)
S. Rangaswamaiah, V. Gangathimmaiah, A. Nordenstrom, H. Falhammar, Bone mineral density in adults with congenital adrenal hyperplasia: a systematic review and meta-analysis. Front. Endocrinol. 11, 493 (2020)
N.F. Peel, D.J. Moore, N.A. Barrington, D.E. Bax, R. Eastell, Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann. Rheum. Dis. 54, 801–806 (1995)
T.P. van Staa, H.G.M. Leufkens, C. Cooper, The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos. Int. 13, 777–787 (2002)
P.W. Speiser, W. Arlt, R.J. Auchus, L.S. Baskin, G.S. Conway, D.P. Merke, H.F.L. Meyer-Bahlburg, W.L. Miller, M.H. Murad, S.E. Oberfield, P.C. White, Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 103, 4043–4088 (2018)
H. Falhammar, H. Filipsson, G. Holmdahl, P.-O. Janson, A. Nordenskjöld, K. Hagenfeldt, M. Thorén, Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92, 4643–4649 (2007)
F. Ceccato, M. Barbot, N. Albiger, M. Zilio, P. De Toni, G. Luisetto, M. Zaninotto, N.A. Greggio, M. Boscaro, C. Scaroni, V. Camozzi, Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 175, 101–106 (2016)
S.R. Bornstein, B. Allolio, W. Arlt, A. Barthel, A. Don-Wauchope, G.D. Hammer, E.S. Husebye, D.P. Merke, M.H. Murad, C.A. Stratakis, D.J. Torpy, Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 364–389 (2016)
M. Fleseriu, I.A. Hashim, N. Karavitaki, S. Melmed, M.H. Murad, R. Salvatori, M.H. Samuels, Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 3888–3921 (2016)
A. Liberati, D.G. Altman, J. Tetzlaff, C. Mulrow, P.C. Gøtzsche, J.P.A. Ioannidis, M. Clarke, P.J. Devereaux, J. Kleijnen, D. Moher, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6, e1000100 (2009)
K.R. Frey, T. Kienitz, J. Schulz, M. Ventz, K. Zopf, M. Quinkler, Prednisolone is associated with a worse bone mineral density in primary adrenal insufficiency. Endocr. Connect. 7, 811–818 (2018)
T. Rosén, L. Wilhelmsen, K. Landin-Wilhelmsen, G. Lappas, B.A. Bengtsson, Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur. J. Endocrinol. 137, 240–245 (1997)
H. Falhammar, H. Filipsson Nyström, A. Wedell, K. Brismar, M. Thorén, Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 168, 331–341 (2013)
H. Falhammar, H. Claahsen-van der Grinten, N. Reisch, J. Slowikowska-Hilczer, A. Nordenström, R. Roehle, C. Bouvattier, B.P.C. Kreukels, B. Köhler; dsd-LIFE group, Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr. Connect 7, 466–478 (2018)
Z. Chakhtoura, A. Bachelot, D. Samara-Boustani, J.-C. Ruiz, B. Donadille, J. Dulon, S. Christin-Maître, C. Bouvattier, M.-C. Raux-Demay, P. Bouchard, J.-C. Carel, J. Leger, F. Kuttenn, M. Polak, P. Touraine, Centre des maladies endocriniennes rares de la croissance and association surrénales, impact of total cumulative glucocorticoid dose on bone mineral density in patients with 21-hydroxylase deficiency. Eur. J. Endocrinol. 158, 879–887 (2008)
N. Raizada, V.P. Jyotsna, A.D. Upadhyay, N. Gupta, Bone mineral density in young adult women with congenital adrenal hyperplasia, Indian. J. Endocrinol. Metab. 20, 62–66 (2016)
D. El-Maouche, S. Collier, M. Prasad, J.C. Reynolds, D.P. Merke, Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin. Endocrinol. 82, 330–337 (2015)
M.K. Auer, L. Paizoni, L.C. Hofbauer, M. Rauner, Y. Chen, H. Schmidt, A. Huebner, M. Bidlingmaier, N. Reisch, Effects of androgen excess and glucocorticoid exposure on bone health in adult patients with 21-hydroxylase deficiency. J. Steroid Biochem. Mol. Biol. 204, 105734 (2020). https://doi.org/10.1016/j.jsbmb.2020.105734
G. Riehl, N. Reisch, R. Roehle, H. Claahsen van der Grinten, H. Falhammar, M. Quinkler, Bone mineral density and fractures in congenital adrenal hyperplasia: findings from the dsd-LIFE study. Clin. Endocrinol. 92, 284–294 (2020)
P. Vestergaard, L. Rejnmark, L. Mosekilde, Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif. Tissue Int. 82, 249–257 (2008)
S. Björnsdottir, M. Sääf, S. Bensing, O. Kämpe, K. Michaëlsson, J.F. Ludvigsson, Risk of hip fracture in Addison’s disease: a population-based cohort study. J. Intern. Med. 270, 187–195 (2011)
V. Camozzi, C. Betterle, A.C. Frigo, V. Zaccariotto, M. Zaninotto, E. De Caneva, P. Lucato, W. Gomiero, S. Garelli, C. Sabbadin, M. Salvà, M.D. Costa, M. Boscaro, G. Luisetto, Vertebral fractures assessed with dual-energy X-ray absorptiometry in patients with Addison’s disease on glucocorticoid and mineralocorticoid replacement therapy. Endocrine 59, 319–329 (2018)
V. Camozzi, V. Carraro, M. Zangari, F. Fallo, F. Mantero, G. Luisetto, Use of quantitative ultrasound of the hand phalanges in the diagnosis of two different osteoporotic syndromes: Cushing’s syndrome and postmenopausal osteoporosis. J. Endocrinol. Investig. 27, 510–515 (2004)
G. Mazziotti, M. Doga, S. Frara, F. Maffezzoni, T. Porcelli, L. Cerri, R. Maroldi, A. Giustina, Incidence of morphometric vertebral fractures in adult patients with growth hormone deficiency. Endocrine 52, 103–110 (2016)
H. Falhammar, H. Filipsson, G. Holmdahl, P.-O. Janson, A. Nordenskjöld, K. Hagenfeldt, M. Thorén, Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92, 110–116 (2007)
A. Nordenström, H. Falhammar, Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency, Eur. J. Endocrinol. R127–R145 (2019). https://doi.org/10.1530/eje-18-0712.
E. Whittle, H. Falhammar, Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J. Endocr. Soc. 3, 1227–1245 (2019)
H. Falhammar, M. Thorén, Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine 41, 355–373 (2012)
L. Frisen, A. Nordenstrom, H. Falhammar, H. Filipsson, G. Holmdahl, P.O. Janson, M. Thoren, K. Hagenfeldt, A. Moller, A. Nordenskjold, Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439 (2009)
Funding
This project was supported by grants from the Magnus Bergvall Foundation (grant number 2017–02138, 2018–02566, and 2019–03149 Henrik Falhammar).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.L. declares that she has no conflict of interest. S.B. declares that she has no conflict of interest. H.F. declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Bensing, S. & Falhammar, H. Rate of fracture in patients with glucocorticoid replacement therapy: a systematic review and meta-analysis. Endocrine 74, 29–37 (2021). https://doi.org/10.1007/s12020-021-02723-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02723-z