Abstract
In randomized controlled trials (RCTs), more intensive glucose control in patients with type 2 diabetes leads to a modest (9%) reduction in major cardiovascular events (MACE), associated with a 20% reduction of kidney events and 13% reduction of eye events. The FDA issued guidance in 2008 led to the conduct of numerous cardiovascular outcomes (CVOT) trials to assess cardiovascular safety of new antihyperglycemic therapies in patients with type 2 diabetes. The results of these trials show that insulin glargine, three different dipeptidyl peptidase-4 (DPP-4) inhibitors (saxagliptin, alogliptin, and sitagliptin) and lixisenatide (a glucagon like peptide-1 receptor agonist) produce no significant difference in CVOT when compared with usual care or placebo. Other trials with newer diabetes drugs, including empagliflozin and canagliflozin (two sodium-glucose co-transporter-2 inhibitors), liraglutide and semaglutide (two GLP-1 receptor agonists) succeeded in demonstrating CV benefit in people with type 2 diabetes. In the last two decades, the equation “diabetes equals myocardial infarction” have contributed to the development of preventive therapy for risk factors in diabetes. In both primary and secondary prevention, the diabetic patients with high rates of statin and aspirin treatment have improved CV outcome, as compared with non-users. The drugs used to reduce glucose levels in patients with type 2 diabetes seem important for the ultimate cardiovascular outcome: the combination of intensive glycemic control, when safely attainable, with newer diabetes drugs (empagliflozin, canagliflozin, liraglutide, and semaglutide) may decrease the incidence of MACE, nephropathy and retinopathy. Moreover, depending on the drug used, CV mortality and heart failure may also be reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
At least in westernized countries, patients with type 2 diabetes mellitus seem to live longer lives with fewer complications [1]. However, cardiovascular (CV) complications remain more common among patients with type 2 diabetes than among persons without diabetes. In the past two decades, some dogmas have influenced clinicians’ mind about cardiovascular prevention in type 2 diabetes.
First dogma: reducing HbA1c is enough
In the 1990s, the US FDA (Food and Drug Administration) started approving drugs for the treatment of type 2 diabetes based on their capacity to reduce hemoglobin A1c (HbA1c) levels. The thought was that, being specific to diabetes, microvascular complications were more likely to be positively influenced by improved glycemic control; therefore, any diabetes drug could reasonably be expected to reduce microvascular complications as a consequence of its HbA1c-lowering effect. This concept has still inspired ongoing trials [2] comparing antihyperglycemic drugs on their ability to reduce HbA1c, rather than to reduce the risk of diabetes complications.
In randomized controlled trials (RCTs), intensive glycemic control in patients with type 2 diabetes is associated with a HbA1c value between 6.4 and 7.0%, as compared with conventional control (HbA1c between 7.9 and 8.4%). In the first meta-analysis [3] by the Collaborators on Trials of Glucose Lowering (CONTROL) from more than 27,000 patients with type 2 diabetes included in 4 RCTs (UKPDS, ACCORD, ADVANCE, and VADT) [4–7], more intensive glucose control led to a significant but modest (9%) reduction in major cardiovascular events (MACE, i.e., time to the first event of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke), with no reduction in cardiovascular (CV) mortality and an increase in severe hypoglycemia. Since then, uncertainty has surrounded the clinical benefit of intensive glycemic control on the risk of CV disease in type 2 diabetes. The latest CONTROL meta-analysis [8] of individual participant data shows that more intensive glucose control over 5 years reduced both kidney and eye events. The composite primary kidney outcome, including development of overt nephropathy (macroalbuminuria), end-stage kidney disease, and renal death, was reduced by 20%, and the composite primary eye outcome, including development and progression of retinopathy, was reduced by 13%. By contrast, intensive glucose control did not reduce the frequency of primary nerve events, probably related to the subjective and variable methodology (monofilament and reflex testing) used to assess diabetic neuropathy. Glucose lowering remains important for the prevention of vascular complications in adults with type 2 diabetes; however, residual microvascular and macrovascular risk remains high despite intensive glycemic control (Fig. 1).
Residual risk of major cardiovascular and microvascular events in patients with type 2 diabetes following intensive glycemic control. MACE, major cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stoke); MI myocardial infarction, HF heart failure, NEPHRO nephropathy, RETINO retinopathy, NEURO neuropathy. For MACE, for example, CV risk reduction following intensive glycemic control is 9% and, therefore, residual vascular risk is 91%. For nephropathy, the reduction of kidney events following intensive glycemic control is 20%, and therefore residual microvascular risk is 80%
Second dogma: all diabetes drugs reduce HbA1c, “ergo” all drugs are equal
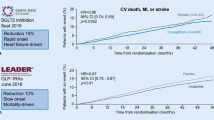
In contrast to the leitmotiv derived from dogma 1, the results of recent studies with newer diabetes drugs support the importance of the drug used in order to abate CV outcomes (CVOT) in type 2 diabetes. The FDA issued guidance in 2008 [9] intensified the need to establish the cardiovascular safety of new antihyperglycemic therapies and led to the conduct of numerous CVOT trials involving patients with type 2 diabetes. The ORIGIN trial [10], which was specifically sought to evaluate the cardiovascular safety of glargine, showed no significant difference in CVOT with glargine as compared with standard care, although glargine attenuated the risk of microvascular outcomes (kidney and eye) in participants with a baseline HbA1c level ≥6.4% [11]. Subsequent trials with three different dipeptidyl peptidase-4 (DPP-4) inhibitors (saxagliptin, alogliptin, and sitagliptin) also showed no significant difference in CVOT when compared with placebo [12–14], although treatment with saxagliptin was associated with a significant increased risk for hospital admission for heart failure [12], and a significant improvement in albumin/creatinine ratio, even in the normoalbuminuric range [15]. It remains unclear why none of the DPP-4 inhibitors was superior to placebo in terms of CVOT, contrasting early signals of CV benefit based on short-term preliminary studies [16]. In the same year, the results of the ELIXA trial [17] were released: in patients with type 2 diabetes and a recent acute coronary syndrome, the addition of lixisenatide, a glucagon-like peptide 1 (GLP-1) receptor agonist, to usual care did not significantly alter the rate of CVOT (Table 1).
Although the CV neutrality of these trials [12–14, 17] satisfied the primary objective, as they excluded significant CV harms, the enrollment of more than 40,000 patients with type 2 diabetes for demonstrating the CV safety of these drugs left something missing. Luckily, other trials with newer diabetes drugs, including empagliflozin, canagliflozin, liraglutide, and semaglutide, succeeded in demonstrating CV benefit in people with type 2 diabetes. The all-cause mortality reduction observed with empagliflozin (a sodium-glucose co-transporter-2 inhibitor) and liraglutide (a GLP-1 receptor agonist), primarily driven by a reduction in CV mortality [18, 19], seems not mediated through HbA1c reduction, as in both trial it was modest, and less than the half of that seen in trials of intensive glycemic control, none of which showed reductions in mortality (Table 1). Moreover, there was a disproportion between the modest reduction in CVOT (myocardial infarction and stroke) and the large reduction of CV mortality, suggesting that both drugs may be working through mechanisms other than atherosclerosis. Furthermore, the improvement in CV mortality was evident earlier than expected (within the first 6 months) in relation to the long natural history of the progression of atherosclerosis attributable to hyperglycemia [20]. Finally, there may be differences across drugs belonging to the same class, too. Both semaglutide (a weekly GLP-1 receptor agonist) and canagliflozin (another SGLT-2 inhibitor) failed to affect mortality, while they significantly reduced MACE (semaglutide reduced stroke only, canagliflozin reduced no single outcome among CV death, non-fatal myocardial infarction, or stroke) [21, 22]. Thus, the reduction in CV mortality observed with empagliflozin and liraglutide seems better attributable to drug-specific effects on other mechanistic pathways. Interestingly, all four drugs can reduce kidney events [23], although the increase of eye events with semaglutide [21] and the occurrence of more distal amputations with canagliflozin [22] deserve attention.
Paradoxically, newer diabetes drugs that have demonstrated CV benefit when compared with placebo, lack head-to-head comparison with other active drugs; and other drugs, like insulin, that has not demonstrated CV benefit when compared with placebo, now has a head-to-head trial. In the devote trial [24], conducted among patients with type 2 diabetes at high risk for cardiovascular events, degludec was non-inferior to glargine with respect to the incidence of major cardiovascular events, despite higher rates of severe hypoglycemia in the glargine group.
Third dogma: the diabetic patient behaves as a non-diabetic patient with heart attack
About 20 years ago, it was shown that diabetic patients without previous myocardial infarction had as high a risk of myocardial infarction as nondiabetic patients with previous myocardial infarction (MI) [25]. Although subsequent studies suggested that the impact of diabetes on the risk of future coronary artery disease could be overestimated, with hindsight the equation “diabetes equals myocardial infarction” have contributed to the development of preventive therapy for risk factors in diabetes. In the primary prevention setting [26], a real-world population of 93,866 Danish patients (12,544 with diabetes), followed for a median of 4.1 years, shows that, in the absence of angiographically significant coronary disease, the diabetic patients with high rates of statin and aspirin treatment had the same risk of cardiovascular events (death, cardiac death, and MI) as patients without diabetes. Thus, a high level of preventive therapy in patients with diabetes without CAD may remove the diabetes-associated increased risk of MI and cardiac death for at least a 7-year period. In the secondary prevention setting [27], a trial population of 13,616 patients with type 2 diabetes and known CV disease enrolled in trial evaluating cardiovascular outcomes with sitagliptin (TECOS) shows that statin and aspirin users, as compared to non-users, had improved CV outcome (25 and 21% MACE reduction, respectively); moreover, those diabetic patients achieving all five preventive measures (use of aspirin, statin, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, blood pressure <140/90 mmHg, non-smoking status) had 40% reduction of MACE vs. those achieving ≤2 measures.
Combination strategies: the next dogma?
The drugs used to reduce glucose levels in patients with type 2 diabetes seem important for the ultimate cardiovascular outcome (Table 2). Some strategies to improve the poor cardiovascular outlook of the diabetic patient may be considered: the combination of intensive glycemic control, when safely attainable, with some newer diabetes drugs may decrease the incidence of MACE, nephropathy, and retinopathy. Moreover, depending on the drug used, CV mortality and heart failure may also be reduced. Tight glycemic control, a newer diabetes drug (empagliflozin, canagliflozin, liraglutide, and semaglutide), statin and aspirin might well be the four musketeers for preventing and fighting cardiovascular complications in type 2 diabetes.
References
E.W. Gregg, X. Zhuo, Y.J. Cheng, A.L. Albright, K.M. Narayan, T.J. Thompson, Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modeling study. Lancet Diabetes Endocrinol. 2, 867–874 (2014)
D.M. Nathan, J.B. Buse, S.E. Kahn et al., GRADE Study Research Group, Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 36, 2254–2261 (2013)
F.M. Turnbull, C. Abraira, R.J. Anderson et al., Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298 (2009)
UK Prospective Diabetes Study (UKPDS) Group, Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998)
The Action to Control Cardiovascular Risk in Diabetes Study Group, Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008)
The ADVANCE Collaborative Group, Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008)
W. Duckworth, C. Abraira, T. Moritz et al., Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009)
S. Zoungas, H. Arima, H.C. Gerstein et al., Collaborators on Trials of Lowering Glucose (CONTROL) group, Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomized controlled trials. Lancet Diabetes Endocrinol. 5, 431–377 (2017)
FDA, Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes: guidance for industry. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed December 2008
ORIGIN trial Investigators, Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328 (2012)
ORIGIN Trial Investigators, Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the outcome reduction with an initial glargine intervention (ORIGIN) trial. Diabetologia 57, 1325–1331 (2014)
B.M. Scirica, D.L. Bhatt, E. Braunwald et al., SAVOR-TIMI 53 steering committee and investigators, saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013)
W.B. White, C.P. Cannon, S.R. Heller et al., EXAMINE Investigators, Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013)
J.B. Green, M.A. Bethel, P.W. Armstrong et al., TECOS Study Group, Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015)
O. Mosenzon, G. Leibowitz, D.L. Bhatt et al., Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care 40, 69–76 (2017)
M. Monami, I. Dicembrini, E. Mannucci, Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 24, 689–697 (2014)
M.A. Pfeffer, B. Claggett, R. Diaz et al., ELIXA investigators, Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 373, 2247–2257 (2015)
B. Zinman, C. Wanner, J.M. Lachin et al., EMPA-REG OUTCOME Investigators, Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015)
S.P. Marso, G.H. Daniels, K. Brown-Frandsen et al., LEADER Trial Investigators, Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016)
C.C. Low Wang, C.N. Hess, W.R. Hiatt, A.B. Goldfine, Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation 133, 2459–2502 (2016)
S.P. Marso, S.C. Bain, A. Consoli et al., SUSTAIN-6 Investigators, Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016)
B. Neal, V. Perkovic, K.W. Mahaffey, et al., CANVAS Program Collaborative Group, Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017)
C. Wanner, S.E. Inzucchi, J.M. Lachin et al., EMPA-REG OUTCOME Investigators, Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 323–334 (2016)
S.P. Marso, D.K. McGuire, B. Zinman, et al., DEVOTE Study Group, Efficacy and safety of degludec versus glargine in type 2 diabetes. N. Engl. J. Med. 377, 723–732 (2017)
S.M. Haffner, S. Lehto, T. Rönnemaa, K. Pyörälä, M. Laaks, Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 (1998)
K.K.W. Olesen, M. Madsen, G. Egholm et al., Patients with and without diabetes without significant angiographic coronary artery disease have the same risk of myocardial infarction in a real-world population receiving appropriate prophylactic treatment. Diabetes Care 40, 1103–1110 (2017)
N.J. Pagidipati, A.M. Navar, K.S. Pieper, et al., On behalf of the TECOS Study Group, Secondary prevention of cardiovascular disease in patients with type 2 diabetes: international insights from the TECOS trial. Circulation. (2017). https://doi.org/10.1161/CIRCULATIONAHA.117.027252
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.G received speaker fee from Lilly, NOVO, Sanofi, Roche and Novartis; K.E. received speaker fee from NOVO, Sanofi, Roche, Novartis, and Merck. The remaining authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Dario Giugliano, Maria Ida Maiorino, Giuseppe Bellastella and katherine Esposito contributed equally to this work.
Rights and permissions
About this article
Cite this article
Giugliano, D., Maiorino, M.I., Bellastella, G. et al. Type 2 diabetes and cardiovascular prevention: the dogmas disputed. Endocrine 60, 224–228 (2018). https://doi.org/10.1007/s12020-017-1418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1418-y