Abstract
Purpose of Review
Seven trials of new agents to treat type 2 diabetes (T2DM) have been performed to assess cardiovascular (CV) safety. A significant amount of information regarding the effects of drugs in three classes is available, with new data from multiple other trials expected shortly. This article provides a summary of recently completed trials.
Recent Findings
The dipeptidyl peptidase-4 inhibitors studied thus far do not alter the risk of major adverse CV events (MACE). Glucagon like peptide-1 receptor agonists liraglutide and semaglutide, and the sodium glucose cotransporter-2 inhibitor empagliflozin, significantly reduced the risk of MACE. Empagliflozin also decreased the risk of hospitalization for heart failure. Agents demonstrating a CV outcome benefit also improved parameters of renal function.
Summary
Several newer antihyperglycemic agents have been found to reduce the risk of important CV complications in high-risk patients with T2DM. Future trials are needed to assess the effects of additional drugs and the impact of therapy in lower risk patients and provide additional information regarding non-CV safety outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management of type 2 diabetes (T2DM) is evolving at a rapid pace as several new classes of medications have been introduced over the past decade. The recent FDA requirements for a thorough assessment of cardiovascular (CV) safety through specific CV outcome trials (CVOTs) have shifted the focus of care from glycemic targets to the impact of therapies upon important clinical complications. The completed trials designed to meet these regulatory requirements have provided an immense amount of CV and other safety data, and further CVOTs will report outcome data in the near future. As seven trials of antihyperglycemic agents from different classes have now been concluded, this provides an opportunity to compare and contrast drug effects both within and between classes. In order to enhance translation of the trial findings into clinical care practices, this article will summarize, compare, and contrast the key CV and non-CV findings from the trials through May, 2017.
Relationship Between Glycemia and Cardiovascular Risk
For patients with diabetes, CV disease (CVD) is the leading cause of morbidity and mortality. Individuals with diabetes are two to four times more likely to die from CVD than people without diabetes [1]. New diabetes therapies have traditionally been approved based upon efficacy in hemoglobin A1c (HbA1c) lowering, which has served as a reliable surrogate for the risk of microvascular complications. The UK Prospective Diabetes Study (UKPDS) has demonstrated that better glycemic control initiated around the time of diagnosis of T2DM is associated with a reduction in the risk of microvascular complications, as well as a reduction in rates of CV complications and death over the long term [2, 3]. The Diabetes Complications and Control Trial (DCCT) and its long-term follow-up study found similar benefits to intensive glycemic control in patients with type 1 diabetes [4,5,6]. However, diabetes management strategies targeting very tight glycemic control in higher risk patients have not been found to consistently reduce the risk of macrovascular complications. Compared to a standard approach to glycemic control, medical management designed to achieve near-normoglycemia in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the Action in Diabetes and Vascular Disease–Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, and the Veterans Affairs Diabetes Trial (VADT) provided no reduction in rates of CV complications [7,8,9]. In addition, randomization to the intensive glycemic control strategy in ACCORD was associated with an increased risk of death [7]. These unexpected results suggested that the implementation of very intensive glycemic control in patients with longstanding, complicated T2DM does not convey a CV benefit and may in fact be harmful. The findings have significantly impacted diabetes care guidelines by emphasizing individualized HbA1c targets, with recommendations for more relaxed glycemic goals in people with long disease duration or in those with established complications [10]. Although the increased risk of death in ACCORD could not be directly attributed to hypoglycemia, rates of hypoglycemia were increased in the intensive versus standard treatment groups [11]. Given this, concerns regarding potential adverse side effects of the drugs available at the time to achieve intensive glycemic control in ACCORD have persisted.
Rationale for Cardiovascular Outcome Trials of Diabetes Medications
In addition to the issues outlined above, concerns related to the CV effects of specific antihyperglycemic medications have been described. The example of rosiglitazone, a drug in the thiazolidinedione class, is most notable. Rosiglitazone was initially approved by the United States Food and Drug Administration (FDA) in 1999 based upon relatively limited prior data (five trials of relatively short duration, with 2902 patients total), not unlike other medications of the time [12]. Concerns regarding the potential for drugs in the class to increase the risk of heart failure emerged shortly afterwards [13]. In addition, in 2007, a meta-analysis of 42 trials concluded that rosiglitazone treatment was associated with a significantly increased risk of myocardial infarction (MI) and death from CVD [14]. Although the subsequently completed Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial did not find an increased risk of MI, stroke, or CV death associated with rosiglitazone use, when published the Nissen meta-analysis elicited significant concern on the part of patients, physicians, and regulatory agencies [14, 15].
The inability to adequately assess the CV impact of antihyperglycemic therapies under the traditionally required phases of drug development has led the United States FDA and European Medicines Agency (EMA) to issue similar new drug development guidelines [16••, 17]. The FDA guidelines specify that clinical trials must be conducted to specifically assess CV outcomes in trials conducted during the development of new glucose-lowering medications, and that new drugs must not increase the risk of CV events to an unacceptable degree. These trials must prospectively collect and adjudicate major adverse CV events (MACE) including CV mortality, MI, and stroke. The mandate also specifies that such trials should include patients with high CV risk (such as patients with established CVD, renal disease, and of older age) and should be of a duration long enough to reliably assess the CV impact of administered medications. In addition to lowering glucose, new medications must be found to have an estimated premarket risk ratio for MACE of <1.8 and postmarket risk ratio <1.3. That is, the upper bound of the two-sided 95% confidence interval for the estimated hazard ratio (HR) must be demonstrated as <1.8 in order for the drug to receive initial approval, and the upper bound of the two-sided 95% confidence interval must be shown to be <1.3 in order for a drug to remain on the market. Demonstration of the latter is generally expected to require conduct of a large, long-term designated CVOT. New antihyperglycemic drugs are not required to demonstrate a decrease in CV risk associated with use, but must clearly demonstrate noninferiority to comparator therapy [16••].
Completed Cardiovascular Outcome Trials of Diabetes Medications
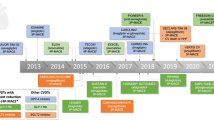
Following issuance of the 2008 FDA guidance, seven large international, randomized controlled trials designed to assess the CV impact of treatment with a specific new antihyperglycemic agent have been completed and provided published results at the time of submission of this manuscript. Three trials have been completed of agents in the dipeptidyl peptidase-4 inhibitor (DPP-4) class, including the The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial; the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial; and the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) [18••, 19••, 20••]. Similarly, three trials of agents in the glucagon like peptide-1 receptor agonist (GLP-1 RA) class have been completed. These include the Evaluation of LIXisenatide in Acute Coronary Syndrome (ELIXA); the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial; and the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) [21••, 22••, 23••]. The EMPA-REG OUTCOME trial of empagliflozin represents the first completed CVOT of a drug in the sodium glucose cotransporter-2 inhibitor (SGLT-2) class [24••]. As outlined visually in Fig. 1, the seven trials have varied with respect to the types of patients which have been enrolled, the duration of trial follow-up, and the primary composite CV endpoints which were selected for analysis. However, all of the trials have compared the effects of the studied antihyperglycemic medication on MACE outcomes compared to placebo, superimposed on a background of usual diabetes and CV care. All of the trials have enrolled older patient populations with fairly longstanding T2DM, who are at elevated risk for CV events due to either a prior diagnosis of atherosclerotic CVD or by having multiple CV risk factors [18••, 19••, 20••, 21••, 22••, 23••, 24••]. The EXAMINE and ELIXA trials have enrolled the highest risk populations, as the inclusion criteria for those trials required patients to have had a recent hospitalization for acute coronary syndrome (ACS) [19••, 21••].
Cardiovascular outcome trials of newer antihyperglycemic agents (through May 2017). a DPP-4 Inhibitor Trials (adapted from: Coch R, Green JB. Nutrition, Metabolism and Cardiovascular Diseases 2016: 26(9); 767–772) [25]. b GLP-1 RA Trials. c SGLT2 Inhibitor Trial. DPP-4 = dipeptidyl peptidase-4; GLP-1 RA = glucagon-like peptide-1 receptor agonist; SGLT-2 = sodium-glucose co-transporter-2; ACS = acute coronary syndrome; CV = cardiovascular; MI = myocardial infarction; CVD = cardiovascular disease; RFs = risk factors; A1c = hemoglobin A1c; UA = unstable angina; CRF = chronic renal failure; HF = heart failure. 1 White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369(14):1327–35. 2 Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369(14):1317–26. 3 Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373(3):232–42. 4 Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–57. 5 Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375(4):311–322. 6 Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. 7 Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128
Major CV outcome findings from these completed antihyperglycemic agent trials are summarized in Table 1. The trials of the DPP-4 inhibitors saxagliptin, alogliptin, and sitagliptin have all concluded that those antihyperglycemic agents are noninferior, but not superior, to placebo with respect to MACE outcomes. However, SAVOR-TIMI 53 reported an unanticipated significant increase in the risk of hospitalization for heart failure (hHF) associated with saxagliptin treatment compared to placebo (HR [95% CI]: 1.27 [1.07–1.51]; P = 0.007) [18••]. Subgroup analyses suggest that estimated glomerular filtration rate (eGFR) ≤60 mL/min, prior history of heart failure, or a baseline NT-proBNP level in the highest quartile were associated with an increased risk of hHF with saxagliptin treatment [26•]. In EXAMINE, the relative risk of hHF was increased by 19% with alogliptin therapy compared to placebo; however, this difference was not found statistically significant [27]. No difference in hHF risk was seen with sitagliptin therapy compared to placebo in TECOS [20••]. Thus far, there is no clear physiologic explanation for the possible increase in hHF risk described in SAVOR-TIMI or for possible disparities in effects noted between the different drugs in the DPP-4 inhibitor class.
The results of the CVOTs of GLP-1 RA medications have been somewhat heterogenous so far, perhaps a reflection of differences in trial design or the diversity of metabolic effects elicited by the agents in that class. Patients assigned to GLP-1 RA therapy in the CVOTs have generally had modest reductions in HbA1c, body weight, and blood pressure; slight reductions in LDL cholesterol; and slight increases in heart rate when compared to placebo [21••, 22••, 23••]. Despite these differences, the ELIXA trial reported that lixisenatide treatment of patients with T2DM and recent ACS neither increased nor decreased the risk of MACE outcomes when compared to placebo [21••]. On the other hand, liraglutide treatment in the LEADER trial significantly reduced the risk of the primary composite MACE outcome compared to placebo (HR [95% CI]: 0.87 [0.78–0.97]; P = <0.001 for noninferiority and P = 0.01 for superiority) [22••]. In addition, liraglutide therapy was associated with a statistically significant 22% reduction in the risk of CV death. Estimated risks of non-fatal MI and non-fatal stroke were also lower with liraglutide treatment compared to placebo, but the differences were not statistically significant [22••]. Similarly, the risk of the primary composite outcome of CV death, non-fatal MI, or non-fatal stroke was significantly reduced with semaglutide treatment compared to placebo in the SUSTAIN-6 trial (HR [95% CI]: 0.74 [0.58–0.95]; P = <0.001 for noninferiority and P = 0.02 for superiority) [23••]. Although semaglutide treatment did not significantly reduce the risk of the individual endpoints of CV death or non-fatal MI, treatment with the GLP-1 RA was associated with a significant reduction in the risk of non-fatal stroke [23••]. No significant increase or decrease in the risk of hHF was found with GLP-1 RA therapy in any of the three trials [21••, 22••, 23••]. These fascinating differences in the CV effects of the studied GLP-1 RA agents also remain inadequately understood. Although physiologic differences elicited by lixisenatide, liraglutide, and semaglutide therapy may explain the varying impact of drug treatment on the CV outcomes studied, these drugs have only been directly compared to placebo; thus, our ability to make between-drug comparisons remains limited. Major differences in the types of patients enrolled in the GLP-1 RA trials may have significantly affected the outcomes; for example, the very high-risk post-ACS patients enrolled in ELIXA may have been unlikely to derive a CV benefit from any type of antihyperglycemic therapy [21••].
The first completed CVOT of an agent in the SGLT-2 inhibitor class, EMPA-REG OUTCOME, assessed the effect of empagliflozin therapy upon important CV complications in T2DM [24••]. In EMPA-REG, treatment with either 10 or 25 mg of empagliflozin daily was associated with modest reductions in HbA1c, body weight and blood pressure, and small increases in both LDL and HDL cholesterol, when compared to placebo. Treatment with empagliflozin, as assessed in both the individual dosing arms and pooled analyses, was found superior to placebo for the primary composite outcome of death from CV causes, non-fatal MI, or non-fatal stroke (HR [95% CI]: 0.86 [0.74–0.99]; P = <0.001 for noninferiority and P = 0.04 for superiority). The outcome difference between the empagliflozin and placebo group outcomes was driven by a significant reduction in the risk of CV death: a non-significant reduction in the risk of non-fatal MI and a non-significant increase in the risk of stroke was noted with empagliflozin therapy compared to placebo. As opposed to the results of CVOTs of drugs in the other studied classes, empagliflozin treatment also significantly reduced the risk of hHF (HR [95% CI]: 0.65 [0.50–0.85]; P = 0.002) [24••]. Results from other ongoing trials of medications in the SGLT-2 inhibitor class are eagerly anticipated.
Non-cardiovascular Safety Outcomes
Although these large randomized controlled trials were specifically designed to investigate CV outcomes, the immense data that they have collected also provide insights into the non-CV effects of these newer drug classes. However, conclusions regarding the impact of these newer medications on microvascular and other safety outcomes should be cautiously interpreted as there are several limitations to these findings. Most of the CV outcome trials are not powered to identify differences in rates of rare events, and in general the duration of follow-up insufficient to detect lasting effects of therapies upon microvascular complications or malignancy risk. Furthermore, the lack of inter-study standardization of non-CV event reporting and definitions compromises the ability to make between-trial safety comparisons. Table 2 outlines the findings related to the pancreatic, microvascular, and hypoglycemia outcomes described in the trials of DPP-4 and GLP-1 RA medications completed thus far [18••, 19••, 20••, 21••, 22••, 23••].
It has previously been demonstrated that patients with T2DM have an increased risk of pancreatic disease [28]. The pathophysiology is likely multi-factorial, but concerns regarding potential adverse effects of incretin-based antihyperglycemic medications upon pancreatic structure and function exist [29]. In the completed DPP-4 inhibitor CVOTs, no statistically significant increase in the risk of pancreatic cancer has been noted with use of active drug compared to placebo [18••, 19••, 20••, 30, 31]. Numerically, more events of acute pancreatitis were reported with DPP-4 inhibitor therapy compared to placebo in SAVOR TIMI-53, EXAMINE, and TECOS, but the between-group differences did not reach statistical significance in any of the individual trials [18••, 19••, 20••, 30, 31]. A recently published meta-analysis of data from the three trials suggests a significant increase in the risk of acute pancreatitis with the use of DPP-4 inhibitor therapy compared to placebo, although the absolute increase in risk is small [32]. In the trials of the GLP-1 RA medications, numerically, fewer events of both pancreatitis and pancreatic cancers were reported as occurring in the active treatment groups compared to placebo. However, the numbers of such events were small and the differences were non-significant when analyzed [21••, 22••, 23••]. Again, the overall incidence rate of each of these outcomes is low and the duration of drug exposure variable, which limits interpretation of the findings. These data do, however, provide reassurance that the rates of pancreatic complications are low and not markedly increased with use of incretin-based therapies to manage T2DM during the time periods studied.
Renal outcomes reported in the CV outcome trials of DPP-4 inhibitors have varied, both with respect to chosen endpoints and the findings of the effects of therapies compared to placebo. In SAVOR-TIMI, patients treated with saxagliptin had reduced progression of albuminuria compared to placebo, but no significant difference was found for a pre-specified composite renal outcome including doubling of creatinine, initiation of renal replacement therapy, or creatinine >6.0 mg/dl [18••, 33]. Alogliptin therapy in EXAMINE resulted in changes in estimated glomerular filtration rate (eGFR) and an incidence of dialysis initiation comparable to that of the placebo group [19••]. The sitagliptin-treated group in TECOS had a slight but significantly greater mean reduction in eGFR during the study period compared to placebo, but no between-group difference in renal failure was reported [20••]. Although modest differences in some parameters of renal function have been reported for this class, no difference in clinically important renal outcomes was noted with use of DPP-4 inhibitor therapy in the CV outcome trials.
Promisingly, outcomes from two of the CV outcome trials of GLP-1 RA therapy suggest a renal benefit to the use of these agents. Although ELIXA demonstrated only a smaller percent increase in urinary albumin to creatinine ratio (UACR) with lixisenatide therapy compared to placebo, more meaningful changes in renal outcomes have been seen in the trials of liraglutide and semaglutide therapy [21••, 22••, 23••]. In both the LEADER and SUSTAIN-6 trials, GLP-1 RA-treated patients had a significantly lower risk of new or worsening nephropathy compared to placebo, and semaglutide also significantly reduced the risk of new macroalbuminuria [22••, 23••]. On the other hand, an increase in retinopathy complications was reported in semaglutide-treated patients in SUSTAIN-6, perhaps attributable to the significant and rapid decline in HbA1c experienced by that treatment group [23••].
Other safety outcomes collected from the CV outcome trials of agents in the DPP-4 inhibitor and GLP-1RA classes suggest no major differences in the risk of severe hypoglycemia with the active therapies compared to placebo, although the definitions of such events have varied between trials [18••, 19••, 20••, 21••, 22••, 23••]. Although preclinical findings suggested a possible increase in the risk of medullary thyroid cancer with GLP-1 RA therapy, a clinical increase in this risk has not been noted in the CV outcome trials of that class. In fact, only one such malignancy has been reported as occurring in the placebo-treated group in LEADER [22••]. In addition, no increase in the risk of fracture has been associated with the use of saxagliptin or sitagliptin in SAVOR-TIMI 53 or TECOS [34, 35].
Data from the EMPA-REG trial of the SGLT-2 inhibitor empagliflozin has provided meaningful information regarding the non-CV effects of that medication. Unsurprisingly, use of empagliflozin increased the risk of genital infections compared to placebo, but no clinically important increase in rates of urinary tract infections, diabetic ketoacidosis, acute kidney injury, volume depletion, severe hypoglycemia, or bone fractures were found [24••]. Furthermore, compared to placebo, the empagliflozin-treated group had significant reductions in the risk of important renal outcomes including new or worsening nephropathy; new-onset macroalbuminuria; and the need for renal replacement therapy [24••, 36].
Conclusions
The recent FDA requirement to comprehensively assess the CV safety of new drugs for T2DM has had a dramatic impact upon our understanding of the effects of antihyperglycemic drug therapy. In addition, these trials have shifted the focus of diabetes care away from primarily glycemic goals to our ability to impact more important health-related outcomes. The effects of care upon rates of serious CV complications and death are likely to be of significant interest to patients, providers, and insurers alike. The trial results so far suggest that although there appear to be some similarities of drug effects within classes, important differences may also be present. In particular, the unexpected increase in the risk of heart failure hospitalization seen with saxagliptin therapy in SAVOR-TIMI 53 has both highlighted potential within-class differences in CV outcomes and emphasized the clinical importance of this relatively under-recognized complication of T2DM [18••]. Several of the agents studied so far have demonstrated CV benefits which are complementary to those achieved via traditional CV risk factor modification; furthermore, a tantalizing link between CV and renal benefits has been identified which clearly warrants further investigation. Enrollment of large, diverse patient populations into these trials offers opportunities to better understand the effects of therapies in the many different types of people affected by T2DM. In addition to providing robust information regarding CV outcomes, these trials have provided sorely needed insight into the effects of therapies upon pancreatic, bone, and other microvascular events.
On the other hand, CV outcome trials are costly to perform and the drugs studied may not be readily accessible to all patients. In addition, some have argued that findings from trials which enroll primarily high CV risk populations may yield results not applicable to lower risk patients. Restriction of the enrolled population to extremely high-risk patients, as was the case in EXAMINE and ELIXA, may in fact compromise the ability to detect potential benefits to therapy [19••, 21••]. Within-trial comparisons thus far have also been restricted to the effects of active therapies versus placebo. Although such a strategy does enhance the ability to determine the effects of a specific intervention, this feature of trial design limits the ability to make comparisons between antihyperglycemic agents and has also resulted in differences in glycemic control between treatment groups. These concerns may have contributed to delays in the meaningful incorporation of trial findings into current diabetes care guidelines.
Rather than serving as a barrier, these concerns should provide a framework to enhance the conduct of further trials. Creative strategies in trial design and data collection will be needed to incorporate lower CV risk patients into trials of diabetes drug safety, to permit comparisons between active therapies, and to minimize differences in glycemia between treatment groups. Longer follow-up of enrolled subjects, either during or subsequent to an active study period, will enhance our understanding of drug effects upon outcomes which are either rare or unlikely to occur during short courses of drug exposure. Furthermore, enhanced and more fully standardized collection of heart failure measures, as well as non-CV outcomes such as hypoglycemia and pancreatitis, are clearly needed. Finally, studies are needed to determine whether combination therapy with agents shown independently to provide a CV benefit (such as empagliflozin plus semaglutide) would provide an additive reduction in risk.
The diabetes care community will need to be poised to absorb and incorporate the rapidly evolving findings from CV outcome trials into clinical practice. Although only some of the agents studied thus far have demonstrated a CV benefit, fortunately the rest do not appear to significantly increase the risk of major adverse cardiovascular events. In high-risk patients, preferential use of antihyperglycemic agents shown to significantly reduce CV risk is appropriate. However, it is likely that continued advocacy for evidence-based approaches to care will be needed to ensure accessibility of beneficial drugs to those who need them.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose and risk of cause specific death. N Engl J Med. 2011;364(9):829–41. doi:10.1056/NEJMoa1008862.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53. doi:10.1016/S0140-6736(98)07019-6.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in typediabetes. N Engl J Med. 2008;359(15):1577–89. doi:10.1056/NEJMoa0806470.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi:10.1056/NEJM199309303291401.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9. doi:10.1056/NEJM200002103420603.
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi:10.1056/NEJMoa052187.
Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi:10.1056/NEJMoa0802743.
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi:10.1056/NEJMoa0802987.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi:10.1056/NEJMoa0808431.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. doi:10.2337/dc12-0413.
Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC Jr, et al. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012;35(2):409–14. doi:10.2337/dc11-0996.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21071_Avandia_Approv.pdf. Accessed 30 Apr 2017.
https://www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm143413.pdf. Accessed 30 Apr 2017.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi:10.1056/NEJMoa072761.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35. doi:10.1016/S0140-6736(09)60953-3.
•• Guidance for Industry. Diabetes Mellitus — Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). December 2008. https://www.fda.gov/downloads/Drugs/.../Guidances/ucm071627.pdf. Accessed 30 Apr 2017. This document outlines the requirements and specifications for cardiovascular outcomes trials of new medications for diabetes.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus.14 May 2012 CPMP/EWP/1080/00 Rev. 1. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 30 Apr2017.
•• Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi:10.1056/NEJMoa1307684. Primary results for saxagliptin CVOT
•• White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. doi:10.1056/NEJMoa1305889. Primary results for alogliptin CVOT
•• Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. doi:10.1056/NEJMoa1501352. Primary results for sitagliptin CVOT
•• Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi:10.1056/NEJMoa1509225. Primary results for lixisenatide CVOT
•• Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. doi:10.1056/NEJMoa1603827. Primary results for liraglutide CVOT
•• Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi:10.1056/NEJMoa1607141. Primary results for semaglutide CVOT
•• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi:10.1056/NEJMoa1504720. Primary results for empagliflozin CVOT
Coch RW, Green JB. Current cardiovascular outcomes trials in type 2 diabetes: perspectives and insight. Nutr Metab Cardiovasc Dis. 2016;26(9):767–72.
• Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–88. doi:10.1161/CIRCULATIONAHA.114.010389. In-depth analysis of heart failure findings from SAVOR-TIMI 53 CVOT
Zannad Z, Cannon CP, Cushman WC, Bakris GL, Menon V, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385(9982):2067–76. doi:10.1016/S0140-6736(14)62225-X.
Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32(5):834–8. doi:10.2337/dc08-1755.
Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–6. doi:10.1053/j.gastro.2011.02.018.
Raz I, Bhatt DL, Hirshberg B, Mosenzon O, Scirica BM, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor Saxagliptin. Diabetes Care. 2014;37(9):2435–41. doi:10.2337/dc13-2546.
Buse JB, Bethel MA, Green JB, Stevens SR, Lokhnygina Y, et al. Pancreatic safety of sitagliptin in the TECOS study. Diabetes Care. 2017;40(2):164–70. doi:10.2337/dc15-2780.
Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284–6. doi:10.2337/dc15-1707.
Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(4):696–705. doi:10.2337/dc14-1850.
Mosenzon O, Wei C, Davidson J, Scirica BM. Yanuv, et al. incidence of fractures in patients with type 2 diabetes in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38(11):2142–50. doi:10.2337/dc15-1068.
Josse RG, Majumdar SR, Zheng Y, Adler A, Bethel MA, et al. Sitagliptin and risk of fractures in type 2 diabetes: results from the TECOS trial. Diabetes Obes Metab. 2017;19(1):78–86. doi:10.1111/dom.12786.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi:10.1056/NEJMoa1515920.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rebecca Herbst, Wilburn Bolton, and Afreen Shariff declare that they have no conflict of interest. Jennifer B. Green report grants and personal fees from Merck, grants from AstraZeneca, grants from GlaxoSmithKline, personal fees from Daiichi, and personal fees from Boehringer Ingelheim.
Human and Animal Rights and Informed Consent
This article does not contain any previously unpublished data from human or animal studies.
Additional information
This article is part of the Topical Collection on Macrovascular Complications in Diabetes
Rights and permissions
About this article
Cite this article
Herbst, R., Bolton, W., Shariff, A. et al. Cardiovascular Outcome Trial Update in Diabetes: New Evidence, Remaining Questions. Curr Diab Rep 17, 67 (2017). https://doi.org/10.1007/s11892-017-0898-8
Published:
DOI: https://doi.org/10.1007/s11892-017-0898-8