Abstract
Aims/hypothesis
As glycaemia and the incidence of microvascular diabetes complications follow a log-linear relationship, it becomes increasingly difficult to demonstrate a microvascular benefit of glucose-lowering when the HbA1c level is close to normal.
Methods
The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial randomised 12,537 people with diabetes, impaired glucose tolerance or impaired fasting glucose to receive standard glycaemic care or standard care with the addition of basal insulin glargine (A21Gly,B31Arg,B32Arg human insulin), targeting a fasting plasma glucose level ≤5.3 mmol/l. Microvascular outcomes during a median follow-up of 6.2 years were examined in participants whose baseline HbA1c was above or below the median of 6.4% (46.4 mmol/mol).
Results
Allocation to the insulin glargine group reduced the incidence of the primary microvascular composite outcome of kidney and eye disease in participants whose baseline HbA1c level was ≥6.4% (46.4 mmol/mol; HR 0.90 [95% CI 0.81, 0.99]) but not in participants with a lower baseline HbA1c (HR 1.07 [95% CI 0.95, 1.20]; p value for interaction 0.031). In people whose baseline HbA1c level was ≥6.4% (46.4 mmol/mol), the median post-randomisation change in HbA1c was −0.65% (interquartile range −0.16, −0.91%) after allocation to insulin glargine and −0.33% (−0.83, 0.13%) after allocation to standard care (median HbA1c difference 0.33%; p < 0.0001). A smaller median difference of 0.22% was noted in people whose baseline HbA1c was <6.4% (p < 0.0001).
Conclusions/interpretation
In patients with dysglycaemia, intervention targeting normal fasting glucose levels reduced HbA1c and attenuated the risk of microvascular outcomes in participants with a baseline HbA1c level ≥6.4% (46.4 mmol/mol). A neutral effect was seen in those with a lower baseline HbA1c level.
Trial registration:
ClinicalTrials.gov NCT00069784
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma glucose concentrations are distributed over a wide range in any given population. Within this continuum, the threshold for the diagnosis of diabetes is based largely on epidemiological studies wherein a plasma glucose cut-off point largely separates individuals who are at substantial risk of diabetic retinopathy from those who are not [1]. For individuals with diabetes, numerous observational studies have indicated that the likelihood of developing both eye and kidney disease steeply increases as glucose levels rise above the diabetes threshold [2–5].
Several intervention studies have shown the effectiveness of glucose-lowering in reducing microvascular outcomes of type 2 diabetes using a range of anti-hyperglycaemic agents [6–8]. In light of these data, together with observational studies showing a log-linear relationship between HbA1c and the risk of diabetes complications [4], an intervention that lowers glucose levels may be expected to have a greater effect in people with higher vs those with lower HbA1c levels. We tested this possibility using data from the recently completed Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial [9], wherein 12,537 people (after exclusion of 75 individuals by the health authorities; see electronic supplementary material [ESM] Fig. 1) with a median baseline HbA1c level of 6.4% (46.4 mmol/mol; interquartile range [IQR] 5.8–7.2% [39.9–55.2 mmol/mol]) were allocated to receive standard glycaemic care or standard care with the addition of basal insulin glargine (A21Gly,B31Arg,B32Arg human insulin), and followed for a median of 6.2 years (IQR 5.8–6.7 years) for the development of cardiovascular, microvascular and other clinical outcomes. Specifically, we compared the effect of the intervention on microvascular outcomes in participants within two pre-specified subgroups: those with baseline HbA1c levels above and those with levels below the median.
Methods
Study design
The design and main results of the ORIGIN trial have been previously reported [9–11]. Briefly, men and women aged 50 years or older with either type 2 diabetes on not more than one oral glucose-lowering drug, impaired glucose tolerance or impaired fasting glucose, and with either a prior cardiovascular event or at high risk of vascular disease were recruited. In addition to the insulin glargine vs standard care intervention, participants were randomly allocated to receive a n-3 fatty acid supplement or placebo according to a 2 × 2 factorial design. Screening, randomisation and follow-up of ORIGIN trial patients are described in ESM Fig. 1 and in further detail in the appendix to the main ORIGIN results paper [9]. Study participants provided written informed consent, with the study approved by the ethics committee at each study site.
ORIGIN intervention with insulin glargine
As described in detail previously [11], participants assigned to the insulin glargine group added an evening injection to their pre-existing anti-hyperglycaemic regimen, increasing its dose to attain a self-measured fasting plasma glucose level of ≤5.3 mmol/l (95 mg/dl). Participants assigned to standard care were treated according to the investigator’s discretion in alignment with local guidelines. The ORIGIN trial was designed to assess the effect of basal insulin on health outcomes, and was neither designed nor powered to assess the effect of more vs less intensive glucose-lowering. Nevertheless, the difference in insulin use led to a modest difference in fasting plasma glucose level and HbA1c whereby at 2 years, the median fasting plasma glucose concentrations and HbA1c were 1.6 mmol/l and 0.3% lower, respectively, in the insulin glargine group compared with the standard care group [9].
Microvascular outcomes
The pre-defined composite secondary outcome of the ORIGIN trial was the first occurrence of a microvascular event defined as a doubling of serum creatinine from the baseline screening value, worsening of albuminuria category (i.e. from normoalbuminuria to either microalbuminuria or clinical proteinuria, or from microalbuminuria to clinical proteinuria), renal replacement therapy, death due to renal failure or diabetic retinopathy requiring retinal photocoagulation or vitrectomy. All clinical outcomes were adjudicated by a committee that was blinded to treatment allocation. Participants were asked about laser eye therapy, kidney failure, dialysis, vitrectomy, photocoagulation and other serious health outcomes every 4 months, and serum creatinine was measured at baseline, 2 years and study end in local laboratories. First-voided urine samples were collected at the research sites according to the same schedule and forwarded to the Clinical Trials Laboratory at the Hamilton General Hospital (Toronto, ON, Canada) for the measurement of urine albumin and urine creatinine concentrations. Urine albumin was measured using a turbidimetric method (with the Beckman MA reagent) and urine creatinine was measured using a modified Jaffé reaction (with the Beckman CR-S reagent) on a Beckman UniCel DxC 600 Instrument (Beckman Coulter, Fullerton, CA, USA). Coefficients of variation for both albumin and creatinine at different concentrations varied from 2.4 to 4.4%.
Statistical analysis
Categorical data were summarised as counts and percentages, and continuous data were summarised as either means and SDs or medians and IQRs. Medians were compared using Wilcoxon rank-sum tests, means were compared using Student’s t tests and counts and proportions were compared using Χ 2 tests. The effect of allocation to the insulin glargine intervention on microvascular outcomes was assessed by constructing time-to-event curves using product-limit estimation that were compared using log-rank tests. HRs were estimated using Cox regression models that were stratified according to the factorial allocation, diabetes status at baseline and pre-randomisation cardiovascular event. Moreover, as reported in the main ORIGIN paper [9], Cox regression models with adjustment for these factors as covariates were used when there were fewer than five participants with events within any stratum. The differential effect of the intervention within the two subgroups of individuals identified according to whether the baseline HbA1c level was below or at or above the median baseline value of 6.4% (46.4 mmol/mol) was assessed by including this subgroup as an independent variable as well as an interaction variable comprising the subgroup and intervention. When there was evidence of an interaction, separate HRs were estimated within each subgroup using the above approach. The subgroups were defined in this way to be consistent with the ORIGIN statistical analysis plan, which pre-specified that the effect of the intervention on the primary outcome would be assessed within subgroups defined by the median baseline HbA1c level.
The epidemiological relationship between baseline HbA1c levels and the subsequent occurrence of microvascular outcomes (i.e. independent of treatment allocation) was assessed using a Cox proportional hazards model with adjustment for age. In a supplemental exploratory analysis, the HbA1c–microvascular relationship was also assessed by dividing baseline levels into fifths using quintiles and estimating the outcome incidence during follow-up within each fifth. Two-sided Cochran–Armitage trend tests were used to test for a progressive increase in outcomes across fifths. All statistical analyses were done using SAS software (version 9.1 for Solaris, Cary, NC, USA). A nominal p value of <0.05 was pre-specified as indicating significance for tests for interactions and epidemiological relationships.
Results
Baseline characteristics and microvascular outcome
Among the 12,537 participants enrolled, 2,686 (21%) experienced a microvascular outcome during the study (Table 1). These participants were older and had higher baseline BP, serum creatinine, urine albumin/creatinine ratio, fasting plasma glucose and HbA1c levels than participants who did not experience a microvascular outcome. They were also more likely to have had a history of prior diabetes, laser photocoagulation or vitrectomy, or be using either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), although they were less likely to have had a prior cardiovascular event.
Effects of insulin glargine intervention on microvascular outcomes
As reported previously, the incidence of the composite microvascular outcome did not differ between the glargine intervention and standard care groups overall (HR 0.97, 95% CI 0.90, 1.05; p = 0.43) [9]. However, when participants were analysed within the two subgroups defined by the median HbA1c of 6.4% (46.4 mmol/mol; ESM Table 1), an interaction (p = 0.031) between the insulin glargine allocation and HbA1c subgroup was evident for the composite microvascular outcome (Fig. 1). Specifically, allocation to insulin glargine reduced the incidence of the primary microvascular composite outcome in participants whose baseline HbA1c was ≥6.4% (46.4 mmol/mol; HR 0.90 [95% CI 0.81, 0.99]) but had no effect in participants with a lower baseline HbA1c (<6.4% [46.4 mmol/mol]; HR 1.07 [95% CI 0.95, 1.20]). This finding, which did not change when death was added to the composite outcome to account for the possibility of competing risk, was also apparent in the time-to-event curves for microvascular outcomes (Fig. 2). As noted in Fig. 1, no effect of allocation to insulin glargine intervention on the composite microvascular outcome was evident in other subgroups (defined by the presence or absence of hypertension, treatment with an ACE inhibitor or ARB, diabetes, baseline albuminuria or obesity).
Effect of allocation to insulin glargine vs standard care on microvascular outcomes overall and within subgroups; to convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929 or use the conversion calculator at www.hba1c.nu/eng/. ACEI, ACE inhibitor; ACR, albumin/creatinine ratio
Time-to-event curves for the composite microvascular outcome (doubling of serum creatinine, worsening of albuminuria, renal replacement therapy or death due to renal failure, or diabetic retinopathy requiring retinal photocoagulation or vitrectomy) in patients assigned to receive insulin glargine (blue line) or standard care (red line) in (a) the entire study population, (b) patients with baseline HbA1c ≥6.4% (46.4 mmol/mol) and (c) those with baseline HbA1c <6.4% (46.4 mmol/mol). The numbers below the graphs show the numbers of patients at each time point
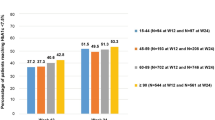
Within the subgroup whose baseline HbA1c was ≥6.4% (46.4 mmol/mol), the median (IQR) post-randomisation change in HbA1c levels was −0.65% (−0.16, −0.91%) in participants allocated to the glargine group and −0.33% (−0.83, 0.13%) in participants allocated to the standard group (median HbA1c difference 0.33%; p < 0.0001). Among those whose baseline HbA1c was <6.4% (46.4 mmol/mol), changes in median (IQR) HbA1c levels were 0.06% (−0.21, 0.40%) and 0.27% (−0.02, 0.64%) for the glargine and standard care group, respectively (median HbA1c difference 0.22%; p < 0.0001). Changes in HbA1c over time by subgroup are noted in the ESM Fig. 2, and differences in the use of glucose-lowering drugs within these two subgroups are shown in ESM Table 2.
Epidemiological relationship between categories of HbA1c and microvascular outcomes
When the effect of fifths of HbA1c (defined by quintiles) was analysed in all participants, a progressive relationship was evident between the baseline HbA1c and composite microvascular outcome in the entire study population (Fig. 3a, ESM Table 3). Similar trends were noted for the insulin glargine and standard care groups separately (ESM Table 3), and exploratory analyses suggested a marginally blunted rise in outcome incidence across categories in the insulin glargine vs standard care group (p value for interaction 0.076). In analyses of HbA1c as both a categorical and continuous variable adjusted for age (Fig. 3b), the HR of the microvascular composite outcome was 1.20 (95% CI 1.16, 1.25) per 1% higher baseline HbA1c (p < 0.001). Additional adjustment for sex, diabetes status, serum creatinine, history of retinopathy and log albumin/creatinine attenuated the relationship (HR 1.12 [95% CI 1.07, 1.16]), which nevertheless remained significant (p < 0.001). Similar relationships were noted for the individual outcomes of albuminuria progression, renal failure, retinal therapy and doubling of serum creatinine, as well as for a clinical microvascular composite outcome that excluded albuminuria progression from the pre-defined microvascular composite (ESM Table 4).
Discussion
Glycaemia and the incidence of microvascular complications follow a log-linear relationship in both type 1 and type 2 diabetes [4, 5]. As such, it may be difficult to demonstrate a microvascular benefit of further glucose-lowering when glycaemic levels are already close to normal. Accordingly, the glycaemic threshold at which intervention remains beneficial is uncertain. Similar difficulty is encountered in providing support for improved glucose control when glycaemic separation between control and intervention groups is comparatively modest. The current analysis of the ORIGIN trial addresses both issues. Here we show, first, that an intervention targeting normal fasting glucose levels reduced microvascular outcomes in participants whose baseline HbA1c was at or above 6.4% (46.4 mmol/mol; i.e. the baseline median HbA1c that was pre-specified as defining a subgroup of interest prior to analysis of the ORIGIN trial results). Second, we show that this effect was evident in the setting of a modest relative reduction in median HbA1c of 0.33% (i.e. a HbA1c reduction of 0.65% vs 0.33% in the glargine and standard arms, respectively) over a 7-year period.
Previous studies have examined the impact of lowering blood glucose on microvascular outcomes in type 2 diabetes. In the UK Prospective Diabetes Study (UKPDS), published 20 years ago, participants assigned to the intensive therapy group experienced a 25% reduction in microvascular complications [8]. While the baseline HbA1c in that study was 6.2% (44.3 mmol/mol), it rose substantially during follow-up, so that by 10 years HbA1c levels were 8.7% (71.6 mmol/mol) and 8.1% (65 mmol/mol) in the standard and intensive therapy arms, respectively [8]. Thus, glycaemic control in both arms was above currently recommended targets for a large part of the UKPDS. In ORIGIN, on the other hand, the median HbA1c in all participants was 6.4% (46.4 mmol/mol) at baseline, rising minimally during the 7 years of follow-up to 6.5% (47.5 mmol/mol) in those randomised to receive standard therapy and falling to 6.2% (44.3 mmol/mol) in the glargine intervention group [9].
At first glance it might seem somewhat surprising that a modest 0.33% lowering of HbA1c in the ORIGIN subgroup whose baseline HbA1c was ≥6.4% (46.4 mmol/mol) was associated with a 10% reduction in microvascular events. However, the findings are consistent with both the UKPDS and the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study [6]. In the UKPDS, a 0.9% lowering of HbA1c was associated with a 25% reduction in microvascular events and in ADVANCE a 0.6% HbA1c lowering led to a 14% reduction in microvascular events [6].
Observational analyses from the Diabetes Control and Complications Trial (DCCT) and UKPDS indicate a log-linear relationship between glycaemia and the incidence of microvascular complications in both type 1 and type 2 diabetes [3–5]. These studies did not identify a glycaemic threshold below which glycaemic control was ineffective, while a threshold of 6.5% (47.5 mmol/mol) was reported in the ADVANCE trial [12]. Taking advantage of the comparatively low baseline HbA1c of 6.4% (46.4 mmol/mol) in the ORIGIN trial, in conjunction with the long follow-up time and large patient numbers, we explored the relationship between fifths of glycaemia and microvascular events in ORIGIN. No HbA1c threshold was evident in our analysis, with the incidence of microvascular outcomes rising progressively from the lowest fifth of <5.7% (38.8 mmol/mol) to the highest of ≥7.4% (57.4 mmol/mol), although an effect of intervention was only evident among individuals with HbA1c levels >6.4%. While the number of events in the composite was largely driven by changes in albuminuria, the step-wise progression between baseline HbA1c and outcome was still evident even after albuminuria-based events were excluded.
The current analysis of the ORIGIN trial has several strengths. These relate primarily to the study itself, with a duration exceeding 6 years, high rates of follow-up and treatment adherence along with the large numbers of microvascular endpoints. These factors, in addition to the prospective collection and adjudication of outcomes, provide sufficient power to detect clinically important effects. Moreover, the prospective ascertainment of data pertaining to hypoglycaemia and weight gain assists in assessing risks and benefits. Finally, although conducted after analysing the primary cardiovascular outcome, it is notable that the statistical analysis plan used in the original trial pre-specified both that the effect of the intervention on outcomes would be examined in subgroups defined by the median HbA1c levels at baseline and the composite microvascular outcome as the main secondary outcome.
This study has a number of limitations. Although it is possible that the reduction in microvascular events was due to the use of insulin vs no insulin rather than to glucose-lowering, the fact that the effect was only observed in the subgroup with the higher HbA1c, and that the difference in insulin use was smaller in this group than in the subgroup with a lower baseline HbA1c, makes that unlikely. Thus the absence of an effect on microvascular outcomes among individuals whose HbA1c was below the 6.4% (46.4 mmol/mol) median is likely to be due to the smaller glycaemic separation between the intervention and standard care groups. This lack of microvascular outcome may also have been due to the possibility that the lower absolute rate of events in people with a lower baseline HbA1c reduced the power to detect a difference in this group compared with those with higher baseline HbA1c. Furthermore, only clinical retinal outcomes were recorded and fundus photographs were not done, the latter potentially providing additional useful information regarding the progression of retinopathy.
In summary, the present analysis demonstrates that an insulin glargine-based intervention targeting fasting normoglycaemia in people with good glycaemic control reduces HbA1c and the risk of microvascular complications in those with HbA1c levels >6.4% (46.4 mmol/mol). Whether or not achieving such control with other anti-hyperglycaemic agents would also reduce microvascular complications at such low baseline HbA1c levels remains unknown.
Abbreviations
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation
- ACE:
-
Angiotensin-converting enzyme
- ARB:
-
Angiotensin receptor blocker
- IQR:
-
Interquartile range
- ORIGIN:
-
Outcome Reduction with an Initial Glargine Intervention
- UKPDS:
-
UK Prospective Diabetes Study
References
(1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197
Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH (1995) Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332:1251–1255
Raskin P, Rosenstock J (1986) Blood glucose control and diabetic complications. Ann Intern Med 105:254–263
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Patel A, MacMahon S, Chalmers J et al (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Ismail-Beigi F, Craven T, Banerji MA et al (2010) Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376:419–430
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
ORIGIN Trial Investigators, Gerstein HC, Bosch J et al (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 367:319–328
ORIGIN Trial Investigators, Bosch J, Gerstein HC et al (2012) n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 367:309–318
Origin Trial Investigators, Gerstein H, Yusuf S et al (2008) Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 155:26–32
Zoungas S, Chalmers J, Ninomiya T et al (2012) Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 55:636–643
Funding
The ORIGIN trial was funded by Sanofi, who also provided regulatory support, site monitoring and insulin glargine. Pronova Bio Pharma Norge supplied the n-3 fatty acids supplements and placebo.
Duality of interest
REG received consulting and lecture fees from Sanofi, Merck, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Boehringer Ingelheim and other funds through his institution from Merck, AstraZeneca and Bristol-Myers Squibb. JFEM received consulting and lecture fees from Abbott, NovoNordisk, Novartis, Amgen, Roche, and Boehringer Ingelheim and other funds through his institution from Roche, Celgene and the European Union through framework-7 grant SysKid 241544. GS received consulting and lecture fees from Eli Lilly, Merck, AstraZeneca, Novo Nordisk, Novartis, Sanofi-Aventis and Servier, and research support from Novo Nordisk. MH received speaker honoraria from Takeda, Glaxo SmithKline, Roche, Bayer, Eli Lilly and Sanofi and advisory board honoraria from Sanofi, Takeda, Bristol-Myers Squibb and Glaxo SmithKline. SY received consulting and lecture fees and grant support from Sanofi. HCG reports consulting and lecture fees from Sanofi, Bayer, Merck, Glaxo SmithKline, Roche, Novartis, Janssen, Abbott and AstraZeneca and other funds through his institution from Sanofi, Merck, Novo Nordisk, Eli Lilly and Boehringer Ingelheim. JB reports no duality of interest associated with her contribution to this manuscript.
Contribution statement
The work presented here was undertaken in collaboration between all authors. REG and HCG defined the research idea, designed the study’s methodology and wrote the report. REG, HCG, JFEM, MH, GS, JB and SY contributed to the data collection, study design and discussion, review and editing of the report. All authors have seen and approved the current version of the report. HCG is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 192 kb)
ESM Fig. 2
(PDF 47 kb)
ESM Table 1
(PDF 58 kb)
ESM Table 2
(PDF 59 kb)
ESM Table 3
(PDF 58 kb)
ESM Table 4
(PDF 47 kb)
Rights and permissions
About this article
Cite this article
The ORIGIN trial investigators., Gilbert, R.E., Mann, J.F.E. et al. Basal insulin glargine and microvascular outcomes in dysglycaemic individuals: results of the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial. Diabetologia 57, 1325–1331 (2014). https://doi.org/10.1007/s00125-014-3238-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3238-4