Abstract
In this study, we investigated the combined effect of pioglitazone (PIO) with alpha lipoic acid (ALA) on the peripheral nerves of diabetic rats. Animals were divided into 8 groups (N = 6–8) and designated according to ALA (100 mg/kg/day) and PIO (10 mg/kg/day) treatment: Normal, Normal + ALA, Normal + PIO, Normal + ALA + PIO, DM, DM + ALA, DM + PIO, and DM + ALA + PIO. After 24 weeks, current perception threshold, mechanical allodynia, oxidative stresses, intraepidermal nerve fiber density (IENFD), and axonal morphology in the sciatic nerve were compared among groups. IENFD in the DM + ALA + PIO group was significantly less reduced than in other DM groups (7.61 ± 0.52 vs. 5.62 ± 0.96, 5.56 ± 0.60, and 7.10 ± 0.70 for DM, DM + ALA, and DM + PIO, respectively P < 0.05). The mean myelinated axonal area in the sciatic nerves was significantly higher in the DM + ALA + PIO group compared with non-treated DM group (70.2 ± 3.46 vs. 61.1 ± 2.91, P < 0.05) although significant differences were not present between combination therapy and monotherapy independent of ALA or PIO. Our results demonstrated that combination therapy using PIO based on ALA can give an additional benefit in peripheral nerve preservation in diabetes. Moreover, PIO can be preferentially considered when additional glucose-lowering agent is required in DPN patients treated with ALA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are many limitations in treating diabetic peripheral neuropathy (DPN) using current therapeutic strategies, and so new management tools based on DPN pathogenesis are necessary. Alpha-lipoic acid is a well-known pathogenically effective agent that reverses or prevents diabetic peripheral neuropathy via diverse anti-oxidant properties [1–3]. In addition to alpha lipoic acid, new agents targeting the detailed pathogenic mechanism of DPN are warranted, and many drugs have been attempted [4, 5]. Among these, evidence suggests that thiazolidinedione (TZD) reduces diabetic complications independent of glycemic control [6]. In the field of neuropathy, troglitazone, rosiglitazone, and pioglitazone (PIO) were reported to have neuroprotective effects in an animal model by showing diverse neurologic parameters [7–10]. However, there are little data about the combination of therapeutic approaches using pathogenically effective agents such as alpha lipoic acid, gamma linoleic acid, neurotrophics, and TZD for DPN.
Actually, all kind of oral anti-diabetic drug (OAD) can be added as 2nd line agent for the glucose control next to metformin based on the individualization. However, at this time, combined complications from diabetes also need to be considered in the OAD selection. In this concept, OAD which has additional effect beyond glycemic control will be better for diabetes management. Therefore, it is worthy investigating whether PIO, which is one of OAD and has been reported to have neuronal effect previously, can give an additional benefit on the DPN management especially, based on the ALA treatment because PIO is not a neuropathic agent as well as the combination of well-known two pathogenic agents of DPN.
In the present study, we investigated the combined effect of PIO and ALA on peripheral nerve preservation in diabetic rats.
Materials and methods
Animals and experimental design
All experiments were performed after approval from the Institutional Rat Care and Use Committee of Chonbuk National University Medical School (CBU 2011-0027). Six to eight-week-old male Sprague–Dawley (SD) rats weighing 160–180 g were purchased from Damool Science (Daejeon, Chungnam, Korea) and housed under optimal conditions. Rats were kept in a pathogen-free rat-rearing facility with a 12 h light and dark cycle. The room’s temperature (23 ± 1 °C) and humidity (53 ± 2 %) were maintained, and rats were provided with food and water ad libitum. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) (60 mg/kg body weight) (Sigma Chemical, St. Louis, MO, USA) dissolved in 0.1 mol/L citrate buffer (pH 4.5). Forty-eight hours after the STZ injection, diabetes was verified in rats showing blood glucose levels higher than 350 mg/dL, as measured by Precision Xtra Plus® (Abbot Laboratories, MediSence Products, Bedford, MA, USA) following overnight fasting. Age-matched control rats received an equal volume of vehicle (sodium citrate buffer) in the same manner. During the experimental period, body weight and blood glucose were measured every week after 8 h of fasting. Fasting glucose levels were assessed using blood samples drawn from a tail vein. HbA1c levels were compared using a commercially available kit (NycoCard, Oslo, Norway).
Normal and diabetic rats were randomly assigned to 8 groups (n = 7–9 per group): normal, normal with alpha lipoic acid (Normal + ALA), normal with pioglitazone (Normal + PIO), normal with alpha lipoic acid and pioglitazone (Normal + ALA + PIO), diabetes (DM), DM with alpha lipoic acid (DM + ALA), DM with pioglitazone (DM + PIO), and DM with alpha lipoic acid and pioglitazone (DM + ALA + PIO). ALA (supplied by Bukwang Pharm. South Korea) was in powdered form. ALA which was used in this experiment was racemic mixture, named of (R,S)-5-(1,2-dithiotane-3-yl) pentanoic acid. Each day, 0.5 % ALA (100 mg/kg/day) was mixed with food for the ALA-treated group. Pioglitazone (Cadila Pharmaceuticals Limited, Ankleshwar, India) was dissolved in water and administered orally at a dose of 10 mg/kg/day. Normal and diabetic rats without ALA or PIO were treated with compound or placebo once a day for 24 weeks. After 24 weeks, biochemical measurement, allodynia assessment, and morphometric comparison were performed. Blood was collected from a cardiac puncture under deep anesthesia. Cutaneous nerves and sciatic nerves were isolated and stored at −80 °C until further processing for diverse parameters comparison.
Biochemical estimation
At week 25, blood was obtained by a cardiac puncture after killing all rats under deep anesthesia. Serum was collected by centrifugation for 15 min at 2,000×g at 4 °C. Total serum cholesterol and triglyceride were measured by means of an enzymatic method using a commercial kit (San Pharmaceutical Co., Seoul, Korea). The high-density lipoprotein cholesterol concentration was determined by the dextran sulfate-Mg++ method and the enzymatic method using the same commercial kit used to measure total serum cholesterol and triglyceride. Low-density lipoprotein cholesterol was calculated for each sample according to the following formula: LDL − C = total cholesterol − HDL − (TG/5). The serum insulin concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Linco Research, St. Charles, MO, USA). Left sciatic nerve segments were immediately dissected after sacrifice and homogenized in 20 mM HEPES buffer, pH 7.2, containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose. The supernatant was obtained after centrifugation at 1,500×g at 4 °C for 15 min. Total superoxide dismutase activity in the sciatic nerve supernatant and serum were assayed by means of a colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. One unit of superoxide dismutase activity was defined as the amount of enzyme needed to exhibit 50 % dismutation of superoxide radical. The supernatant protein concentration was quantified by means of the known Bradford method [11].
Sensory assessment
To measure mechanical allodynia and hypoalgesia, the rats were placed individually on mesh with 1 cm perforations in a plastic cage after adapting to the testing conditions for at least 10 min. Von Frey filaments (Stoelting Co., IL, USA) with calibrated bending forces (in g) were used to deliver mechanical stimuli of varying intensity. The procedure was performed according to the method of Chaplan et al. and some modification from Chaplan, old Von frey, and Weinstein method as our previous researches [12–14]. Each stimulation was performed five times with 5 s intervals, and immediate withdrawal was determined to be a positive response at least one time in the five applications. To quantify nerve dysfunction, current perception thresholds (CPTs) were also examined during the 24th week by the same method as in our previous study [15]. The minimum intensity value required to elicit a hind paw withdrawal reflex or the appearance of vocalization or agitation was defined as CPT. When the response occurred, the stimulus was immediately stopped, and the next stimulus began after an interval of at least 10 min. Every threshold of 2,000, 250, and 5 Hz was measured 3 or 4 times, and the mean value of intensities was expressed as the CPT.
Skin blood flow measurement
Skin blood flow under resting conditions was measured at 24 weeks on the dorsal aspect of the hind leg using a Laser Doppler Blood Perfusion Monitor (Periflux System 5000®; Perimed, Stockholm, Sweden). Arbitrary units were recorded according to the baseline skin blood flow, and these scores were compared among experimental groups without repeated measurements of the ischaemic block, cold constrictor, local warming stimuli, limb-raising, and limb-lowering tests because stimuli change was impossible in the rats.
Morphometric assessment
At the 0th and 12th week, 3 X 3 mm tissues were taken from the hind left index, left middle toe by a skin biopsy for the immunohistochemical analysis of intraepidermal nerve fiber (IENF). After sacrifice during the 25th week, cutaneous tissue samples from right middle toe and segments of the right sciatic nerve were obtained from each rat for morphometric analyses of IENF and myelinated fiber, respectively. Sciatic nerve tissues were post-fixed overnight in 4 % paraformaldehyde before embedding in JB-4 (Polysciences, Inc, Germany), and then 1.5 μm transverse sections were stained with toluidine blue. The procedures used for immunohistochemical analysis were the same as those described previously [15]. Skin tissue specimens were fixed with periodate–lysine–paraformaldehyde (PLP) (2 % paraformaldehyde, 0.075 M lysine, 0.05 M phosphate buffer pH 7.4, 0.01 M sodium m-periodate) solution for 24 h. Tissue specimens were cryoprotected with Tissue-Tec® (OCT compound) (Miles, Elkhart, IN, USA) after thorough rinsing for 48 h in phosphate-buffered saline (PBS) containing 20 % glycerol–0.1 M phosphate buffer at 4 °C. Sections perpendicular to the dermis and 40 μm in thickness were prepared with a sliding cryostat (Leica CM 1510®, Leica Microsystems AG, Wetzlar, Germany) and immersed in PBS for 15 min at room temperature. Samples were then transferred into microtubes containing Dako Protein Block Serum-Free® (Dako, Carpinteria, CA, USA) as a blocking buffer supplemented with 3 % goat serum. After 30 min of blocking on a shaker table kept at room temperature, the sectioned specimens were washed with PBS twice for 10 min. They were then incubated overnight with the primary antibody, rabbit anti-protein-gene-product 9.5 (PGP 9.5) (Biogenesis, Poole, UK) at a dilution of 1:100 at 4 °C. The antibodies were diluted in antibody diluent (Dako, Carpinteria, CA, USA) supplemented with 1 % goat serum. The relevant secondary antibody, goat anti-rabbit IgG-FITC (1:200, Vector, UK), was loaded for 1 h after complete washing at room temperature in a dark room. After washing with PBS as described above, the sections were placed on slides and mounted with fluorescent mounting media (Dako, Carpinteria, CA, USA).
Photomicrographs of the myelinated fiber and IENF were captured using a digital camera (Axiocam HRC®, Carl Zeiss, Goettingen, Germany) with a final magnification of 400 and 100 times. In the sciatic nerve, the myelinated fiber and axonal area represented by the outer or inner border of the myelin sheath were measured with analySIS® image software (Soft Imaging Systems GmbH, Munster, Germany). The mean fiber area and axon/fiber area ratio were determined.
PGP 9.5-immunoreactive nerve fibers in the epidermis of each section were counted as explained previously [16]. In cutaneous nerves, each individual nerve fiber with branching points inside the epidermis was counted as one fiber. The number of IENFs per length (fibers/mm) was used to quantify innervation. For epidermal nerve fibers with branching points in the dermis, each individual nerve fiber was counted as a separate fiber. To avoid any possible bias during preparation and calculation, two independent investigators were blinded to the experimental groups, and the slides were mixed with a set of normal slides before examination.
Statistical analysis
All data were expressed as mean ± SD. One-way ANOVA with Duncan’s post hoc test was used to compare experimental groups. The power to detect differences was 95 %, and data were considered statistically significant if the P value was less than 0.05. Statistical analyses were performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effects on body weight, insulin, lipid profile, and blood glucose
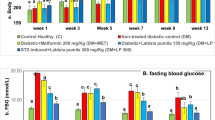
Diabetic animals exhibited significantly decreased body weight and increased blood glucose during the experimental period. There was a significant difference in body weight between normal groups and diabetic groups, however, significant differences were not observed according to ALA or PIO or both treatment in diabetic rats (Table 1). Fasting blood glucose and HbA1c levels in the diabetic animals were not affected by ALA, or PIO, or both combination treatment. The insulin levels in DM groups were lower than those of normal control rats, and the levels were similar irrespective of treatment strategies in diabetic rats (Table 1). The effect of ALA, or PIO, or combination treatment on lipid concentrations in blood is shown in Table 1. Triglyceride is especially high in the diabetic condition although total cholesterol and HDL cholesterol levels were similar between non-diabetic and diabetic group. However, there was a significant difference in the triglyceride and total cholesterol levels of diabetic rats according to ALA, or PIO, or combination treatment (P < 0.05). HDL cholesterol in ALA, or PIO, or combination treated diabetic group was significantly higher than that of non-treated DM group (P < 0.05) (Table 1).
Effect on mechanical allodynia, skin blood flow, and CPT
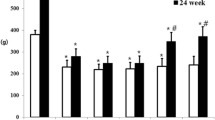
In weeks 12 and 24, tactile withdrawal threshold was assessed by response to a light touch with von Frey filaments. In week 12, the threshold was reduced about 50 % in the non-treated diabetic group (DM). In addition, the diabetic group with ALA and PIO combination treatment showed an enhanced threshold compared with DM group (P < 0.05), however, significant differences were not observed between combination treatment and ALA or PIO monotherapy (Fig. 1a). However, the threshold to light touch tended to be higher in non-treated diabetic groups according to the time passage, and so ALA or PIO or both treated diabetic group showed a significantly reduced threshold (P < 0.05) in week 24 compared with DM group (Fig. 1b) although significant differences between by sole ALA or PIO treatment and non-treated diabetic group at 12 week, and between both ALA and PIO combination and sole ALA or PIO treatment in diabetic rats at 24 week were not observed. In week 24, skin blood flow decreased in the diabetic condition compared with non-diabetic groups. However, ALA and PIO combination treatment was beneficial for maintaining skin blood flow at resting state (27.4. ± 4.2 and 43.1 ± 3.2 TPU for DM and DM + ALA + PIO, respectively, P < 0.05), as shown in Fig. 2. ALA or PIO monotherapy also showed the increased skin blood flow compared with DM group although significant differences were not present, and the degree did not reach to the level of normal group or both combination group. The CPTs of the non-treated diabetic group at all three frequencies were higher than those of the normal groups. However, ALA or PIO or both treatment reduced the threshold of diabetic rats significantly compared with non-treated diabetic group at three frequencies except sole ALA-treated diabetic group at 250 Hz (P < 0.05). Moreover, the diabetic group treated with both ALA and PIO combination showed more decreased trend in thresholds at all three frequencies compared with sole ALA- or PIO-treated diabetic group although significant differences were not present (Fig. 3).
a Threshold of von Frey filament at 12 weeks. Both ALA and PIO combination treatment enhanced the threshold in diabetic groups compared with the non-treated diabetic group. b Threshold of von Frey filament at 24 weeks. The trend was reversed in diabetic rats, and lower threshold was observed in the ALA or PIO or both treatment diabetic group than non-treated diabetic group. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO pioglitazone
Skin blood flow of experimental groups at resting state according to ALA or PIO administration at 24 weeks. Skin blood flow diminished significantly in the diabetic groups compared with the normal glucose groups. However, the PIO + ALA-treated diabetic group showed a significant increase in blood flow compared with non-treated DM group. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO pioglitazone
Current perception threshold comparison according to PIO and ALA treatment in each group. The thresholds for different stimuli were higher in the non-treated diabetic group than in the normal glucose group, and the value decreased significantly in the PIO or ALA or both treated diabetic groups compared with the non-treated diabetic group. The degree of reduction was most potent in ALA + PIO combination treatment among diabetic groups. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM. **P < 0.05 versus DM + ALA. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO pioglitazone
Effect on SOD activity
The SOD activities measured from blood and sciatic nerve samples were significantly lower in the non-treated diabetic group than in the normal group (P < 0.05). The ALA or PIO or both treated diabetic groups showed higher SOD activity than the non-treated diabetic group (P < 0.05). In diabetic groups, more improved trend was observed in the ALA and PIO combination treatment compared with ALA or PIO monotherapy; however, significant differences were not present according to the treatment strategies (Fig. 4).
Super oxide dismutase (SOD) levels in blood (a) and sciatic nerve (b). SOD level in non-treated diabetic group was lower than in the normal glucose group. This decreased level recovered after ALA, PIO, or combination treatment although there was no significant difference among treatment strategies. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO pioglitazone
Effect on IENF quantity
PGP 9.5-positive small nerve fibers in the epidermis obtained from both feet were analyzed for IENFD comparison among experimental groups. The IENFD of the foot dorsum was similar in all four diabetic groups at week 0. However, density decreased gradually with the passage of time. At the 24th week, the diabetes groups treated with PIO or ALA and PIO combination exhibited significant nerve fiber preservation compared with the non-treated diabetes group. There were no significant differences among the normal groups (Fig. 5). ALA monotherapy did not show the protective effect of peripheral nerve quantity although an additive benefit was observed when combined with PIO as shown in Fig. 5. Figure 6 shows the morphological pattern of PGP 9.5-positive small nerve fibers extending into the epidermis in each group. Less shortened and more preserved patterned nerve fibers were observed in the DM + ALA + PIO group than in other DM groups.
Comparison of the IENF density of experimental groups according to experimental time. The quantity of IENF reduced markedly in the non-treated diabetic group compared with the normal glucose group. However, this trend was blunted in the ALA and PIO combination treated diabetic group. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM, **P < 0.05 versus DM + ALA. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO Pioglitazone
Immunohistochemical labeling of PGP 9.5-positive small nerve fibers in normal and diabetic rat skin at week 24. PGP 9.5-positive nerve fiber (arrows) numbers of epidermis and dermis were maintained in the normal glucose group. A less decreased pattern in the ALA with PIO-treated diabetic group was shown compared with the non-treated diabetic group. White bar indicates 100 μm. ALA Alpha lipoic acid, DM diabetes, PIO pioglitazone
Effect on sciatic nerve
The estimated mean fiber area of the sciatic nerve was significantly larger in the ALA + PIO-treated diabetic group than in non-treated diabetic groups (70.23 ± 3.46 vs. 61.06 ± 2.91 μm2, P < 0.05) although a mild increasing trend was observed in the ALA or PIO single-treated diabetic groups. The axon/fiber ratio was significantly higher in the ALA + PIO-treated diabetic group than in the non-treated diabetic group (29.8 ± 1.02 vs. 25.1 ± 2.07 %, P < 0.05) (Fig. 7). Transverse sections of sciatic nerve samples showed the larger endoneurial area and fewer nerve fibers with a degenerated myelin sheath in the DM + ALA + PIO group compared with the non-treated diabetic group (Fig. 8).
Mean fiber area (a) and axon/fiber ratio of the sciatic nerve (b) in each group. This parameters were lower in the non-treated diabetic group than in the normal glucose group. PIO, and PIO with ALA combination prevented sciatic nerve fiber degeneration in diabetic groups although there was no significant difference between monotherapy and combination therapy. Data are given as mean ± SD. † P < 0.05 versus Normal group. ‡ P < 0.05 versus Normal group of ALA, PIO, or ALA + PIO. *P < 0.05 versus DM. N = 6–8 in each group. ALA Alpha lipoic acid, DM diabetes, PIO Pioglitazone
Sciatic nerve samples stained with toluidine blue. The endoneurial area appears larger and nerve fibers with degenerated myelin sheaths decreased in pattern in PIO and PIO with ALA combination treated DM groups compared with the non-treated diabetic group. ALA Alpha lipoic acid, DM diabetes, PIO Pioglitazone
Discussion
The multifactorial pathogenesis of DPN is currently poorly clarified, which limits the success of pathogenic treatment for DPN. A number of mechanisms have been suggested as causes of DPN, and therapeutic trials have investigated the polyol pathway, the advanced glycation end product, protein kinase C, poly ADP-ribose polymerase, and aldose reductase [17, 18]. The main problems are increases in oxidative stresses and the attenuation of anti-oxidative defense mechanisms in DPN. Therefore, alpha lipoic acid, which is a powerful free radical scavenger of the peripheral nerve, seems to play a basic role in the treatment of DPN. Until now, DPN has generally been accepted to result from hyperglycemia-induced oxidative stress [19], and ALA is well known as a pathogenically therapeutic agent. With this pathogenically targeting therapy, drugs which can alleviate DPN symptoms are also included in the DPN treatment. Therefore, when additional efforts to relieve the DPN symptoms are required, most of the current therapeutic strategies are limited to symptomatic management with pregabalin, tricyclic compounds (TCAs), selective serotonin noradrenaline reuptake inhibitors (SNRIs), anticonvulsants, opiates, membrane stabilizers, and capsaicin [20]. However, pathogenically oriented management may play an important role in delaying or reversing the progression of diabetic neuropathy, and DPN should be prevented or treated pathogenically, rather than merely relieving neuropathic symptoms. Therefore, the ideal therapeutic approach for DPN is combining a pathogenic oriented agent with a symptomatic relieving drug. Agents inhibiting or modulating different steps from ALA can be considered for obtaining synergic or additive effects when combined with ALA. In this concept, TZDs are agents that can be combined with ALA in diabetes management. Basically, TZDs are insulin sensitizers used in treating type 2 diabetes. These drugs have an anti-diabetic effect by peroxisome proliferator-activated receptor (PPAR) γ activation, which is important in lipid metabolism, insulin sensitization, and glucose-lowering process. This PPAR-γ is also expressed in neuronal cell such as Schwann cells in addition to adipose and liver tissue [8, 21]. Furthermore, TZDs can reduce oxidative stresses and inflammatory responses, which are important mechanisms in microvascular complications of diabetes [22]. Based on these effects, the neuroprotective potential of TZD treatment was investigated in an animal model [7, 9, 10] although this agent is not included in neuropathy treatment drug. These reports explain the neuroprotective effect of TZD by diverse effects of PPAR γ agonist in addition to insulin sensitization. In this study, we investigated the combined effect of two commonly used agents, ALA and PIO, expecting that their synergic or additive effect. Furthermore, glucose-lowering drugs that prevent diabetic complications additionally are more attractive in drug selection than those that just have a glucose-lowering effect.
Generally, a combination of drugs that act on different pathogenic pathways can give an additive or synergic effect and overcome adverse effects or insufficient efficacy that results from the maximal dose of monotherapy. This therapeutic trend is rare in neuropathy management, and most therapies use nonpathogenic drugs focused on symptom relief [23–25]. Combination of drugs known to be useful for DPN clinically or experimentally is worth of investigating as well as the development of novel drugs in DPN treatment.
As shown in the results, we reconfirmed that each ALA or PIO treatment significantly improved the threshold of current perception and von frey filament stimulation. They also protected IENFD decreases and reduced the degeneration of sciatic nerve fiber in STZ-induced diabetes. We found that this neuroprotective effect increased when these agents were combined although the exact mechanism is not explained in this study.
Treatment with ALA, or PIO, or both combination did not affect body weight or blood glucose levels in diabetic rats. The type 1 diabetic or late stage type 2 diabetic models induced in this experiment are thought to explain this result. To compare neuropathic pain of hyperalgesia and allodynia, the nociceptive threshold is generally used with von frey filaments [26]. A significant decrease in the threshold of diabetic rats was reported in previous studies [27–29]. However, our results showed a higher threshold in the force of von frey filaments in diabetic rats than in normal rats. The pattern of diabetes-related nociception, such as hyperalgesia, allodynia, and hypoalgesia, can be changed according to the various factors including disease severity and animal condition. Therefore, sensory parameters assessing these symptoms need to be interpreted by considering the morphological findings of involved nerves. Mechanical hyperalgesia was dominant in our experiment, and the threshold of nociception was reduced in diabetic rats in the early part of the experiment. This difference might be due to the timing of the experiment. An altered pattern of nociception is possible depending on the duration of diabetes. Hyperalgesia or allodynia may be dominant in early period of DPN, while negative symptoms including hypoalgesia are more prevalent as neuropathy progresses. We compared nociception at 24 weeks, and rats were exposed to hyperglycemia for a long duration, and so mechanical hypoalgesia was dominant. The diabetic group treated with ALA combined with PIO showed a significantly reduced threshold in hypoalgesia assessment compared with other diabetic groups. CPT showed a similar trend in the threshold level as the von frey filament test. However, the response pattern of CPT according to the PIO or ALA treatment were different at 250 Hz. This difference basically was due to the dissimilarity in the nerve fibers which were assessed by each frequency. In general, 2,000 Hz assesses pressure in the A-beta fiber (5–12 μm in diameter), 250 Hz assesses vibration in the A-delta fibers (2–5 μm in diameter), and 5 Hz assesses the temperature, or pain threshold in C-fiber (0.1–1.5 μm in diameter) although the identification of the nerve type from PIO and ALA treatment was not performed. We just observed the different threshold of treated groups compared to diabetes, and so the exact reason of the more sensitive response at 250 Hz in PIO treatment was not clear. It is postulated that nerve fibers responding at 250 Hz may be more sensitive to PIO or PIO + ALA combination; however, it is difficult to confirm that. Therefore, the more detailed classification of affected nerve fibers needs to be documented in the future. However, nerve conduction velocity experiment in addition to CPT is more supportable to compare the neuroprotective effect among experimental groups. In the data of skin blood flow, PIO and ALA combination therapy showed the increased trend compared with PIO or ALA monotherapy and non-treated diabetic groups; however, it was not documented whether additive or synergic effect was present or not in this study. Furthermore, the role of skin blood flow in the neuronal protection is not clear until now. It will be more valuable to evaluate vessel density in the endoneurium of each group to determine whether the improvement of vessel function is implicated in the change of vessel pathology.
Diverse oxidative stresses and anti-oxidants are involved in the development and pain transmission of neuropathy in diabetes. SOD is one of the major anti-oxidant enzymes involved in DPN [30]. Results of the present study are in accordance with those of previous studies [30, 31], and a decrease in SOD activity was observed in the plasma and sciatic nerve in diabetes. However, ALA and PIO administration recovered the SOD level similar to the non-diabetic group. As expected, lipid profiles were significantly improved in the ALA with PIO-treated diabetic group and mildly improved in the ALA or PIO monotherapy groups. Dyslipidemia management using fenofibrate was reported to be effective in DPN [32]. For this reason, improvements in the lipid profile could be suggested to play a role in the neuroprotection in this study partly. However, other possibilities including anti-inflammatory mediators such as TNF-alpha and IL-6 also need to be considered in the explanation for our results of mechanical allodynia and skin blood flow as previous studies [10, 33]. Besides, the addition of anti-inflammation from PIO and anti-oxidant enhancement from ALA may improve mechanical allodynia and skin blood flow although resultant-improved mediators from the combination of PIO and ALA need to be identified.
For more objective evidences in neuroprotection, we compared morphological features among experimental groups in sciatic nerves and small peripheral nerves within the epidermis. PIO treatment was more potent than ALA in the morphologic comparison in this study, although ALA is a favorable agent for DPN. PIO may be more effective than ALA due to the experimental type of diabetes, and the delay in morphological recovery could partly explain the discrepancy between functional parameters and morphologic quantification of peripheral nerves. ALA is considered less effective in preserving the peripheral nerve in the STZ-induced diabetic model than in typical type 2 diabetes such as an OLEFT animal model. There is a limitation of ALA effect on the protection from nerve degeneration caused by toxic damage due to STZ or excessive hyperglycemia. The difference between R(+) enantiomer and racemic mixture in the therapeutic potential for DPN also needs to be investigated in the future. However, the general trend of neuronal quantification increased in the both PIO and ALA-treated diabetic group. This result supports the concept that two agents with pathogenic benefits for neuronal protection can be combined for additional effects in DPN. Besides, PIO can be preferentially selected for glucose control in DPN patients although PIO is not an agent for DPN. To enhance practical use in clinical situations, epidemiologic comparisons need to be performed with ALA, or PIO, or both combination.
Of course, there are several limitations in this study. First, it is important to interpret the functional metrics in the neuropathy experiment, and careful explaining is necessary. As we mentioned in the previous part, our test results can be interpreted with complexity. The neuronal damage assessed by von frey filament can be delayed in the treated diabetic group and more diverse factors can influence on the animal condition such as time passage or cachexic state. Furthermore, diverse neuronal symptoms including hyperalgeia, hypoalgesia, and allodynia can occur or mixed in the later period of experiment as shown in diabetic patients. These components were mixed, so there is a difficulty in the clear interpretation of neuronal parameters in the comparison between ALA and PIO effect. Therefore, more frequent and short-term assessment using functional parameters can show more consistent results than our data. Second, the neuroprotective potential of ALA needs to be clarified according to diabetes type, diabetes stage, and ALA dose in the future. Third, this experiment is just observational experiment of neuronal parameters according to the PIO addition in the course of diabetes management rather than mechanism demonstration of two drug combination. To know how much PIO signaling is implicated in the combined therapy, evaluation of signaling related to PIO therapy such as PKC activity, inflammatory cell infiltration, and 8-hydroxy deoxyguanogine expression on peripheral nerve will be necessary in the future. Our study was just descriptive of DPN parameters. Therefore, more detailed explanation is necessary including exact additive or synergic mechanisms of involved factors. Moreover, the combination of other agents which have neuroprotective effect in pathogenesis of DPN such as ARI needs to be performed instead of PIO in the future.
In summary, the present study revealed that PIO addition based on the ALA can give a more increased neuroprotective effect in DPN. Therefore, two agents including PIO or ALA that are pathogenically beneficial in DPN can be combined in the DPN management.
Abbreviations
- ALA:
-
Alpha lipoic acid
- CPT:
-
Current perception threshold
- DM:
-
Diabetes
- DPN:
-
Diabetic peripheral neuropathy
- IENF:
-
Intraepidermal nerve fiber density
- PPAR:
-
Peroxisome proliferator-activated receptor
- PIO:
-
Pioglitazone
- SOD:
-
Super oxide dismutase
- TZD:
-
Thiazolidinedione
References
F. Becic, E. Kapic, M. Rakanovic-Todic, Pharmacological significance of alpha lipoic acid in up to date treatment of diabetic neuropathy. Med. Arh. 62, 45–48 (2008)
J.L. Evans, I.D. Goldfine, Alpha-lipoic acid: a multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Ther. 2, 401–413 (2000)
D. Ziegler, M. Reljanovic, H. Mehnert, F.A. Gries, Alpha-lipoic acid in the treatment of diabetic polyneuropathy in Germany: current evidence from clinical trials. Exp. Clin. Endocrinol. Diabetes 107, 421–430 (1999)
J. Shakher, M.J. Stevens, Update on the management of diabetic polyneuropathies. Diabetes Metab. Syndr. Obes. 4, 289–305 (2011)
I.G. Obrosova, Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics 6, 638–647 (2009)
R. Dumasia, K.A. Eagle, E. Kline-Rogers, N. May, L. Cho et al., Role of PPAR- gamma agonist thiazolidinediones in treatment of pre-diabetic and diabetic individuals: a cardiovascular perspective. Curr. Drug Targets Cardiovasc. Haematol. Disord. 5, 377–386 (2005)
X. Qiang, J. Satoh, M. Sagara, M. Fukuzawa, T. Masuda et al., Inhibitory effect of troglitazone on diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetologia 41, 1321–1326 (1998)
S. Yamagishi, S. Ogasawara, H. Mizukami, N. Yajima, R. Wada et al., Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J. Neurochem. 104, 491–499 (2008)
T.D. Wiggin, M. Kretzler, S. Pennathur, K.A. Sullivan, F.C. Brosius et al., Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2 J mice. Endocrinology 149, 4928–4937 (2008)
T. Maeda, N. Kiguchi, Y. Kobayashi, M. Ozaki, S. Kishioka, Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J. Pharmacol. Sci. 108, 341–347 (2008)
T. Zor, Z. Selinger, Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal. Biochem. 236, 302–308 (1996)
S. Weinstein, Tactile sensitivity of the phalanges. Percept. Mot. Skills 14, 351–354 (1962)
H.Y. Jin, S.H. Kim, H.M. Yu, H.S. Baek, T.S. Park, Therapeutic potential of dioscorea extract (DA-9801) in comparison with alpha lipoic acid on the peripheral nerves in experimental diabetes. J. Diabetes Res. 2013, 631218 (2013)
S.R. Chaplan, F.W. Bach, J.W. Pogrel, J.M. Chung, T.L. Yaksh, Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994)
H.Y. Jin, W.J. Liu, J.H. Park, H.S. Baek, T.S. Park, Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch. Med. Res. 40, 536–544 (2009)
E. Asensio-Pinilla, E. Udina, J. Jaramillo, X. Navarro, Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 219, 258–265 (2009)
J.L. Evans, I.D. Goldfine, B.A. Maddux, G.M. Grodsky, Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23, 599–622 (2002)
Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, et al. (2005) Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes 54: 3435-3441
A.M. Vincent, J.W. Russell, P. Low, E.L. Feldman, Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 25, 612–628 (2004)
S. Tesfaye, Advances in the management of diabetic peripheral neuropathy. Curr. Opin. Support Palliat. Care 3, 136–143 (2009)
B. Desvergne, W. Wahli, Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20, 649–688 (1999)
S. Giannini, M. Serio, A. Galli, Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J. Endocrinol. Invest. 27, 982–991 (2004)
Y.H. Gong, X.R. Yu, H.L. Liu, N. Yang, P.P. Zuo et al., Antinociceptive effects of combination of Tramadol and Acetaminophen on painful diabetic neuropathy in streptozotocin-induced diabetic rats. Acta Anaesthesiol. Taiwan 49, 16–20 (2011)
M.J. Walker Jr, L.M. Morris, D. Cheng, Improvement of cutaneous sensitivity in diabetic peripheral neuropathy with combination l-methylfolate, methylcobalamin, and pyridoxal 5’-phosphate. Rev. Neurol. Dis. 7, 132–139 (2010)
Y. Liu, N. Li, X.W. Ran, Clinical effect of ligustrazine combined with citicoline for treatment of diabetic peripheral neuropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi 28, 606–609 (2008)
H. Gul, O. Yildiz, A. Dogrul, O. Yesilyurt, A. Isimer, The interaction between IL-1beta and morphine: possible mechanism of the deficiency of morphine-induced analgesia in diabetic mice. Pain 89, 39–45 (2000)
V. Tiwari, A. Kuhad, K. Chopra, Emblica officinalis corrects functional, biochemical and molecular deficits in experimental diabetic neuropathy by targeting the oxido-nitrosative stress mediated inflammatory cascade. Phytother. Res. 25, 1527–1536 (2011)
A. Kuhad, K. Chopra, Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 57, 456–462 (2009)
V. Tiwari, A. Kuhad, K. Chopra, Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain 145, 129–135 (2009)
K. Arai, S. Maguchi, S. Fujii, H. Ishibashi, K. Oikawa et al., Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J. Biol. Chem. 262, 16969–16972 (1987)
X.P. Cui, B.Y. Li, H.Q. Gao, N. Wei, W.L. Wang et al., Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. (Tokyo) 54, 321–328 (2008)
J.C. Ansquer, C. Foucher, P. Aubonnet, K. Le Malicot, Fibrates and microvascular complications in diabetes–insight from the FIELD study. Curr. Pharm. Des. 15, 537–552 (2009)
G. Chinetti, J.C. Fruchart, B. Staels, Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 49, 497–505 (2000)
Acknowledgments
The authors thank to the Research Institute of Clinical Medicine of Chonbuk National University—Biomedical Research Institute of Chonbuk National University Hospital for supporting our researches in a grant partly and experimental system.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, H.Y., Lee, K.A., Wu, J.Z. et al. The neuroprotective benefit from pioglitazone (PIO) addition on the alpha lipoic acid (ALA)-based treatment in experimental diabetic rats. Endocrine 47, 772–782 (2014). https://doi.org/10.1007/s12020-014-0198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0198-x