Abstract
Glucocorticoid-induced osteoporosis (GIOP) continues to be the most common cause of secondary osteoporosis because at any time about 1% of the adult population has been prescribed oral glucocorticoids. Surprisingly, there are relatively few treatment studies of GIOP, particularly of younger individuals including women of child bearing potential and children. Thus, recommendations for management of patients at risk for fracture or who have already suffered an osteoporotic fracture are often based more on clinical experience than randomized controlled trials. Nonetheless, organizations such as the American College of Rheumatology have provided guidance on management of GIOP. In this review, the treatment of GIOP is discussed in light of the specific pathophysiology of this disorder. What separates GIOP from other types of osteoporosis is the profound decrease in osteoblastic function and fact that 3 months (or less) of glucocorticoid therapy leads to a demonstrable increase in fracture incidence. The new ACR Guideline uses FRAX and bone mineral density to categorize fracture risks in GIOP and advocates use of oral bisphosphonates for most patients. Other new findings and alternative management approaches are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
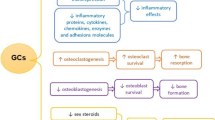
While endogenous Cushing’s syndrome, glucocorticoid excess, leads to osteoporosis and fracture, it is relatively rare. Much more common is osteoporosis and fracture due to exogenous glucocorticoids such as prednisone. Despite recognition of this important side effect of chronic glucocorticoid treatment, osteoporosis and fracture remain under appreciated and not commonly evaluated and treated. Importantly, glucocorticoid-induced osteoporosis (GIOP) is different from post-menopausal osteoporosis in women and age-related osteoporosis in men in very specific ways. First, the mechanism of the osteoporosis is different [1]. In most other types of osteoporosis, bone resorption increases and bone formation is unable to accelerate adequately, leading to gradual loss of bone mass and microarchitecture. In GIOP, while there may be increased bone resorption early on, the major difference is a profound loss of osteoblast and osteocyte function, with increased apoptosis of osteoblasts. This means that bone formation is markedly decreased very soon after starting glucocorticoid therapy. Decreases in serum bone formation markers can be detected after a single dose of prednisone [2]. The consequences of this different mechanism include rapid loss of bone mass and rapid increase in fragility fracture risk. In an oft-cited study [3] from the UK General Practice Research Database (UKGPRD), 3 months of approximately 5 mg of prednisone equivalent therapy was associated with a demonstrable increase in fracture risk. Even the low dose of 2.5 mg of prednisolone (probably slightly more than 2.5 mg of prednisone and approximately equal to 2.5 mg of methylprednisolone, 10 mg of hydrocortisone, and 0.375 mg of dexamethasone) raised fracture risk. In contrast, while fracture risk rises after the menopause, the increase occurs over years or decades in most women. In age-related osteoporosis in men, the hip fracture rate only accelerates after age 80. Thus, the time to identify fracture risk in patients with GIOP is much shorter than in other types of osteoporosis. Finally, management of GIOP is complicated by the plethora of reasons for glucocorticoid therapy. Prednisone and similar drugs are used for a variety of inflammatory conditions, some of which also put patients at risk for fracture. Therefore, many different types of clinicians will prescribe glucocorticoids. Interventions to improve management of GIOP have to be aimed at a variety of clinicians and settings. In an attempt to improve care of GIOP patients who may be at high risk for fracture soon after starting glucocorticoid therapy, medical and scientific groups such as the American College of Rheumatology (ACR) have devised guidelines. Indeed, in 2017, an updated version [4] of the ACR Guideline has been published. I will use this guideline to illustrate the challenges of GIOP that remain today.
The Updated GIOP Guideline from the ACR
It is impossible to simplify the new ACR Guideline [4], which provides guidance for patients on at least 2.5 mg of prednisone equivalent daily. The authors define a high daily dose as ≥30 mg/day of prednisone equivalent and cumulative dose of >5 g in the previous year. A recent observational study [5] in patients with new onset rheumatoid arthritis confirmed increased fracture risk with cumulative doses of >5 g but found a much greater fracture risk with daily doses of ≥15 mg. In the study from the UKGPRD [3], the rate of fracture increased markedly at a dose of 20 mg of prednisolone daily. After assessment of dosing, the ACR Guideline categorizes GIOP patients as low, medium, or high risk by fracture history, bone mineral density by dual energy X-ray absorptiometry (DXA), and 10-year fracture risk calculated by FRAX. Hence, a high-risk patient 40 years or older would be one who had already suffered an osteoporotic fracture or (if postmenopausal or over age 50) had a spine or hip T-score <−2.5 or by FRAX had a 10-year risk of major osteoporotic fracture (MOF) of ≥20% or hip fracture risk of ≥3%. For the patient younger than 40 years old, high risk was defined as having or had an osteoporotic fracture, and moderate risk was a DXA Z-score of <−3 or ≥10% bone density loss in 1 year and a prednisone dose of ≥7.5 mg prednisone equivalent for >6 months. For adults >40 years old, moderate risk was a FRAX MOF risk of 10–19% or hip fracture risk between 1 and 3%. Low risk for patients 40 or older was FRAX MOF risk of ≤10% and hip fracture risk ≤1%. Low risk in the younger group was defined simply as no osteoporotic fractures. The complexity of the guideline is apparent from this paragraph. It is further complicated by the adjustment of FRAX for prednisone dose. If the patient is taking ≥7.5 mg of prednisone equivalent daily, the FRAX risk should be multiplied by 1.15 for MOF and 1.2 for hip fracture risk. All patients need a full assessment at baseline, clinical assessment annually, and fuller assessment every 2 years. For the busy clinician prescribing prednisone as an anti-inflammatory agent, this stratification scheme is daunting, but management depends upon it to a great extent.
According to the ACR Guideline [4], treatment for all patients at risk for GIOP should include adequate calcium intake (1000 to 1200 mg daily of elemental calcium), vitamin D supplements of 600 to 800 international units daily, smoking cessation, minimizing alcohol intake, and increasing weight bearing/resistance exercise. Many bone experts would add that attaining a serum 25-hydroxyvitamin D level of 30 ng/ml would be an important goal [6]. For patients >40 years old, the treatment of choice for moderate and high-risk patients was oral bisphosphonates, based on efficacy, ease of use, and cost [4]. The ACR Guideline voting group chose oral bisphosphonates over intravenous bisphosphonates because of fewer side effects, over teriparatide because of cost and injection burden, over denosumab because of lack of safety data in immunosuppressed patients, and over raloxifene because of lack of data in GIOP and potential harms from increased clotting risk. Here is where expert opinion was used in the guideline as opposed to evidence, not that there are many head-to-head trials in GIOP. In a 1-year study [7] of intravenous zoledronic acid versus oral risedronate, zoledronic acid increased lumbar spine and femoral neck bone mineral density by DXA more than risedronate. In a 1.5-year study [8] using quantitative computed tomography (QCT), teriparatide was shown to increase trabecular bone density more than risedronate, and while there were only five clinical fractures in the subjects receiving risedronate, there were none in the teriparatide group. In a 3-year study [9] of teriparatide versus alendronate, not only did the patients receiving teriparatide have greater bone density response (in the spine and hip) than those receiving alendronate, there were fewer morphometric and clinical vertebral fractures in the subjects who received teriparatide. By network meta-analysis [10], only teriparatide, risedronate, and etidronate were associated with lower risk for vertebral fracture. Some experts would thus have difficulty reconciling these findings with the general recommendation of the ACR to use oral bisphosphonates for most patients at risk for fracture because of GIOP. In addition, although the teriparatide versus alendronate study was a 3-year study, the FDA approves use of anabolic bone drugs (teriparatide and abaloparatide) for only 2 years because of concerns raised by the findings of osteosarcoma in a certain strain of rats prone to this cancer [11].
The ACR Guideline attempts to provide some guidance for the younger patient on systemic glucocorticoids. No pharmacologic treatment is advocated unless the person under age 40 has had a low trauma fracture while on glucocorticoids (defined as high risk) or has a DXA Z-score of −3 or a 10% loss of bone density in a year and is on7.5 mg of prednisone equivalent daily (moderate risk). For younger patients, even those with childbearing potential, the ACR recommends oral bisphosphonates, if treatment is to be given.
While some of the concerns [12] about the previous iteration of the ACR Guideline have been addressed, the guideline is still complex and not all that different from the 2010 version [13]. Part of this is due to lack of new large scale studies of GIOP treatment, including in younger patients. Thus, while the method used for creating the guideline was different from the previous version, the outcome was similar.
Other New Findings in GIOP
In the past few years, there have been relatively few additions to our knowledge base of GIOP, but some are worthy of mention. The new ACR Guideline recommended evaluation of fracture risk within 6 months of starting prednisone or its equivalent. This was based on the UKPCRD data [3] demonstrating increased fracture risk with as little as 3 months of oral glucocorticoid. Using a large private insurance database, Waljee et al. [14] reported that some clinically important side effects were found in patients on short courses of glucocorticoid therapy. Specifically, increased fracture risk was noted in patients within the first month of glucocorticoid use. A conclusion from this study would be that evaluation of fracture risk should take place when the patient commences glucocorticoid treatment. In a small open-label study [15], Sawamura et al. reported that denosumab increased spine and femoral neck bone density in patients taking an average of 7.5 mg of prednisone daily. In another small, open-label 1.5-year study [16], Seno et al. noted that once weekly teriparatide increased lumbar spine bone density but not the hip in patients with GIOP, in contrast to the 3-year study [9] mentioned above. Interestingly, Ishiguro et al. [17] reported that denosumab did not increase hip bone density at 12 months but did at 28 months. Saag et al. updated that randomized trial [9] of teriparatide versus alendronate by reporting that trabecular bone score [18] improved in teriparatide treated subjects but not those on alendronate, perhaps a partial explanation for teriparatide’s better vertebral fracture risk reduction in this trial. While all of these studies enrolled subjects on oral glucocorticoids, there has been concern that other forms of glucocorticoid therapy might also lead to increased fracture risk. A recent review [19] of inhaled glucocorticoids concluded that in patients with asthma, those on higher doses of inhaled glucocorticoids were at higher risk for fracture than those on lower doses. In chronic obstructive pulmonary disease (COPD), the dose response was less clear. In patients with asthma or COPD, short courses of oral glucocorticoids may be used in addition to the inhaled steroids. The study [14] of Waljee noted above stating that short courses of oral glucocorticoids lead to increased fracture risk makes attention to patients with inflammatory lung diseases more pressing.
Mazziotti et al. [20] provided highlights from the ninth international glucocorticoid-induced osteoporosis meeting held in Rome in 2016. Among the findings, the potential pathogenic roles of the growth hormone/IGF-I axis and parathyroid hormone/vitamin D axis were discussed. It was speculated that growth hormone might have a therapeutic role for some patients with GIOP. Current treatment was also reviewed concluding that some studies show potential advantages to begin therapy with teriparatide rather than those with anti-resorptive drugs. Not in the meeting but of importance, two cases of calciphylaxis have been reported in patients without renal failure but being treated with teriparatide for osteoporosis while on glucocorticoids [21, 22].
At the GIOP meeting [20], the previous ACR and other GIOP guidelines were described, but multiple methods to improve adherence to the various guidelines were reported as mostly unsuccessful. In contrast, GIOP management has been improved in closed systems [23] using methods similar to a fracture liaison service for patients on chronic doses of glucocorticoids. The meeting report concluded that while progress has been made in understanding and managing GIOP, much more work is needed to implement current evidence-based management while at the same time increasing the knowledge base.
Finally, in a very recent, large observational Swedish cohort study [24], patients older than 65 years (average age about 80) who were given alendronate after least 3 months of prednisolone (dose ≥5 mg daily) had fewer hip fractures compared to those not treated with bisphosphonate. The hazard ratio was 0.35 versus propensity-matched patients in a short study (median follow-up of 15 months). This adds to the evidence that prompt attention to fracture risk in patients on glucocorticoids leads to better outcomes.
Long-Term Management
Unfortunately, clinical trials of GIOP treatment have lasted up to 3 years only, so evidence for long-term management is based mostly on clinical experience and pathophysiology. Glucocorticoids suppress osteoblasts, and long-term management is likely to be with drugs that decrease bone turnover, especially osteoclasts. Attempts have been made to provide guidance on long-term management [25] in light of potential additive effects on side effect risk of glucocorticoids and bisphosphonates. While there is evidence that such potentiation exists, it cannot be quantitated at this time. Nonetheless, the clinician must be cognizant of the possible increase in side effects in the patient taking both long-term glucocorticoids and long-term bisphosphonates.
Conclusion
GlOP remains an important but underestimated cause of fracture. Such fractures surely complicate management of the underlying disorders for which glucocorticoids are prescribed, plus all osteoporotic fractures lead to increased morbidity and sometimes mortality. The new ACR Guideline is based on contemporary methods, but the research database is limited, preventing more definitive recommendations, particularly for younger patients. The recent observational study [24] provides evidence that following the guidelines should lower fracture risk. While oral bisphosphonates were advocated because of efficacy, safety, and costs, there are studies that demonstrate advantages to anabolic agents in some cases. In GIOP, there is severe loss of osteoblastic function, and drugs that stimulate osteoblasts theoretically and to some extent empirically are superior to drugs that decrease osteoclastic function. Nonetheless, the huge price differential between these drug classes and the need for daily subcutaneous injection of anabolics led the ACR to recommend oral bisphosphonates for the majority of GIOP patients. The clinician must therefore determine which patients have such high fracture risk that anabolic therapy’s advantages clearly outweigh the disadvantages and convince both the patients and third party payers that such treatment is right for the particular individual. Long-term management studies are needed but will be very difficult to accomplish.
References
Adler RA, Weinstein RS, Saag KG. Glucocorticoid-induced osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. 4th ed. Waltham: Academic Press; 2013. p. 1191–223.
Kauh E, Mixson L, Malice MP, Mesens S, Ramael S, Burke J, et al. Prednisone affects inflammation, glucose tolerance, and bone turnover within hours of treatment in healthy individuals. Eur J Endocrinol. 2012;166:459–67.
van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39:1383–9.
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017; doi:10.1002/art.40137.
Balasubramanian A, Wade SW, Adler RA, Lin CJF, Maricic M, O’Malley CD, et al. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos Int. 2016;27:3239–49.
El-Hajj Fuleihan G, Bouillon R, Clarke B, Chakhtoura M, Cooper C, McClung M, et al. Serum 25-hydroxyvitamin D levels: variability, knowledge gaps, and the concept of a desirable range. J Bone Miner Res. 2015;30:1119–33.
Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al., HORIZON Investigators. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicenter, double-dummy, randomized controlled trial. Lancet. 2009;373:1253–63.
Gluer C-C, Marin F, Ringe JD, Hawkins F, Moricke R, Papaioannu N, et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res. 2013;28:1355–68.
Saag KG, Zanchetta JR, Dveogelaer J-P, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2009;60:3346–55.
Amiche MA, Albaum JM, Tadrous M, Pechlivanoglou P, Levesque LE, Adachi JD, et al. Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporosis Int. 2016;27:1989–98.
Jolette J, Attalla B, Varela A, Long GG, Mellal N, Trim S, et al. Comparing the incidence of bone tumors in rats chronically exposed to the selective PTH type 1 receptor agonist abaloparatide or PTH (1-34). Regul Toxicol Pharmacol. 2017;86:356–65.
Hansen KE, Wilson HA, Zapalowski C, Fink HA, Minisola S, Adler RA. Uncertainties in the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Res. 2011;26:1–8.
Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010;62:1515–26.
Waljee AK, Rogers MAM, Lin P, Singal AG, Stein JD, Marks RM, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
Sawamura M, Komatsuda A, Togashi M, Wakui H, Takahashi N. Effects of denosumab on bone metabolic markers and bone mineral density in patients treated with glucocorticoids. Intern Med. 2017;56:631–6.
Seno T, Yamamoto A, Kukida Y, Hirano A, Kida T, Nakabayashi A, et al. Once-weekly teriparatide improves glucocorticoid-induced osteoporosis in patients with inadequate response to bisphosphonates. SpringerPlus. 2016;5:1056.
Ishiguro S, Ito K, Nakagawa S, Hataji O, Sudo A. The clinical benefits of denosumab for prophylaxis of steroid-induced osteoporosis in patients with pulmonary disease. Arch Osteoporos. 2017;12:44.
Saag KG, Agnusdei D, Hans D, Kohlmeier LA, Krohn KD, Leib ES, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68:2122–8.
Sutter SA, Stein EM. The skeletal effects of inhaled glucocorticoids. Curr Osteoporos Rep. 2016;14:106–13.
Mazziotti FA, Adler RA, Bilezikian JP, Grossman A, Shardella E, Minisola S, et al. Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/Vitamin D axes, treatment options, and guidelines. Endocrine. 2016;54:603–11.
Spanakis EK, Sellmeyer DE. Nonuremic calciphylaxis precipitated by teriparatide (rhPTH 1-34) therapy in the setting of chronic warfarin and glucocorticoid treatment. Osteoporos Int. 2014;25:1411–4.
Dominguez AR, Goldman SE. Nonuremic calciphylaxis in a patient with rheumatoid arthritis and osteoporosis treated with teriparatide. J Am Acad Dermatol. 2014;70:e41–2.
Newman ED, Matzko CK, Olenginski TP, Perruquet JL, Harrington TM, Maloney-Saxon G, et al. Glucocorticoid-induced osteoporosis program (GIOP): a novel, comprehensive, and highly successful care program with improved outcomes at 1 year. Osteoporosis Int. 2006;17:1428–34.
Axelsson KF, Nilsson AG, Wedel H, Lundh D, Lorentzon M. Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA. 2017;318:146–55.
Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authorship and Disclosure Form
Robert A. Adler, MD is the sole author of this manuscript.
There was no funding for this manuscript.
The manuscript was done as part of regular duties as an endocrinologist for the Department of Veterans Affairs. The opinions are those of the author and not necessarily those of the Department of Veterans Affairs.
The author has no relevant disclosures.
The manuscript is a review and thus does not contain primary data on any study in humans or animals performed by the author.
The submission has not been published before nor has it been submitted to any other publication.
Rights and permissions
About this article
Cite this article
Adler, R.A. Glucocorticoid-Induced Osteoporosis and the New ACR Guideline. Clinic Rev Bone Miner Metab 15, 123–127 (2017). https://doi.org/10.1007/s12018-017-9234-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-017-9234-8