Abstract
Summary
Retrospective claims analysis indicated that high levels of daily and cumulative doses of systemic glucocorticoids were associated with elevated fracture risk in a large cohort of new RA patients under age 65. Heightened risk began to decline within months of discontinuation. Findings were similar among patients age <50 years.
Introduction
We evaluated the impact of systemic glucocorticoid exposure on fracture risk among relatively young patients with new-onset rheumatoid arthritis (RA).
Methods
Using administrative data, we identified 42,127 RA patients diagnosed January 1, 2005–December 31, 2012, age 18–64 years, with benefits coverage for ≥12 months before RA diagnosis. Follow-up extended to clinical fracture, cancer diagnosis, or December 31, 2012. Glucocorticoid users were new to therapy. Fracture incidence rates (IR) were stratified by glucocorticoid exposure expressed as prednisone equivalent doses. Cox’s proportional hazards models estimated fracture risk adjusted for demographics and baseline clinical characteristics to assess dose-response relationships with current (daily) and prior (cumulative) dose, and by time since discontinuation.
Results
Most patients (85 %) had glucocorticoid exposure. Exposed and unexposed patients were demographically similar (74 % female; mean age 49.7 and 48.8 years); 1 % had prior fracture. Fracture IRs (95 % confidence intervals) were 5 to 9 per 1000 person-years at doses <15 mg/day, 16.0 (11.0, 22.6) at doses ≥15 mg/day, and 13.4 (10.7, 16.7) at cumulative doses ≥5400 mg. Adjusted fracture risk was approximately 2-fold higher at highest dose levels compared with 0 mg/day current daily dose and <675 mg cumulative dose, respectively. Fracture risk was 29 % lower at 60–182 days post-discontinuation compared with ongoing use and was similar to unexposed patients by 12 months. Findings were similar among patients age <50 years.
Conclusions
Among younger, new-onset RA patients, fracture risk was significantly elevated at high levels of daily and cumulative dose, and was similar to unexposed patients by 12 months post-discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic glucocorticoids are a mainstay in the treatment of rheumatoid arthritis (RA) and offer both short-term inflammation control and the longer-term potential to modify RA disease progression and reduce radiographic damage [1, 2]. In addition to these therapeutic benefits, use of systemic glucocorticoids is associated with a range of adverse effects, including increased risk of osteoporosis and fracture, beginning within the first 3 months of use [3–5].
Glucocorticoid use is associated with a rapid and transient increase in bone resorption followed by a decrease in bone formation that persists throughout the duration of use [3, 6]. Fractures related to glucocorticoid-induced osteoporosis (GIOP) have been reported in as many as 30–50 % of patients receiving chronic glucocorticoid therapy for various rheumatologic, pulmonary, and other diseases and followed for up to 1 year [7, 8]. Previous studies suggest that fracture risk increases soon after therapy initiation, is generally dose-dependent, and at least partially independent of bone mineral density (BMD) [4, 9–15]. There is insufficient or inconsistent evidence, however, regarding the steroid dose at which fracture risk increases and the relative effects of cumulative versus daily dose [4, 16–20]. Some studies suggest that a safe dose of glucocorticoid does not exist with respect to bone health [4, 21]. Though limited, published data also suggest that risk may increase only slightly with intermittent use and that risk may decrease within a year after the discontinuation of glucocorticoids [3, 10, 14, 22].
Assessment of the relationship between glucocorticoid use and fracture risk is challenging because exposures are dynamic and difficult to measure, and the association may be confounded by independent associations of fracture risk with underlying diseases, disease activity, patient age, and sex. This may partially explain why evidence on glucocorticoid-related fracture risk is sparse and GIOP treatment guidelines are limited or lacking for specific diseases and age groups, especially young and middle-aged adults. Additionally, many studies include patients with prevalent disease who are continuing glucocorticoid therapy, making the impact of cumulative glucocorticoid dose difficult to assess. Studying such patients may yield substantial misclassification of past glucocorticoid use, suggesting the need to study patients with new-onset disease who are initiating glucocorticoid therapy. Furthermore, patients initiating glucocorticoids appear to lose bone early, leading some guidelines to preferentially highlight management recommendations for patients newly initiating therapy [3, 13, 23]. This study examined glucocorticoid-related fracture risk in adults under age 65 who were newly diagnosed with RA. We followed patients from RA treatment onset and richly characterized systemic glucocorticoid exposure from the time of first use to assess the impact of dose and timing on fracture risk.
Materials and methods
Data source and study population
The MarketScan® Commercial Claims and Encounters Database used for this retrospective analysis contains information on health plan enrollment and enrollee demographics, in addition to administrative claims data for inpatient and outpatient medical services and outpatient prescription drug fills for approximately 39 million commercially insured individuals (2011) in the USA. These data are statistically de-identified per Section 164.514 (b) [1] (i–ii) of the Health Insurance Portability and Accountability Act Privacy Regulations, and Institutional Review Board approval was not required.
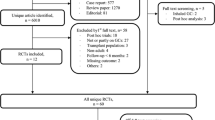
The study population includes individuals with at least one inpatient or two outpatient claims on different days with an RA diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 714.X; claims for diagnostic tests [e.g., X-rays, laboratory testing] were not considered); at least one claim was incurred between January 1, 2005, and December 31, 2012. Patients were required to have no RA claims during a 12-month period of medical and pharmacy benefits coverage prior to their first RA claim in this cohort selection period (Fig. 1). To improve the specificity of the RA disease classification, patients were also required to have received RA treatment (i.e., claim(s) for an RA-related medication [Supplemental Table 1]) within 12 months (±) of their RA diagnosis. For each patient, the index date marking the beginning of follow-up was the latter of the RA diagnosis date or first RA medication date. All patients were aged 18 to 64 years at index and had no pre-index cancer diagnoses (excluding nonmelanoma skin cancer).
In addition to the requirement for a 12-month baseline period prior to the first RA diagnosis, patients with claims for any glucocorticoid use had at least a 12-month glucocorticoid-free clean period prior to their first glucocorticoid claim in the study period. In addition, patients with pre-index glucocorticoid use were required to have continuous benefits coverage between their first glucocorticoid claim and index date. Together, these two requirements helped ensure complete capture of new glucocorticoid initiation, regardless of whether glucocorticoids were initiated prior to or following RA onset.
For each patient, follow-up for fracture outcomes began at the index date and extended through the earliest censoring event: incident fracture, cancer diagnosis (excluding nonmelanoma skin cancer), end of benefits coverage, or December 31, 2012. Figure 1 illustrates the study design and assessment windows.
Systemic glucocorticoid exposure
Systemic glucocorticoid exposure was assessed from the earliest pre-index claim through the end of follow-up for each patient. Exposure was defined by claims for oral, intravenous (IV), and injected glucocorticoids, excluding intra-articular injections. Inhaled steroids were not considered to provide a quantifiable level of systemic exposure and were excluded. Relevant claims were identified by National Drug Codes, Healthcare Common Procedure Coding System codes, and Current Procedural Terminology procedure codes. Data on strength and quantity for each glucocorticoid claim were used to determine the amount of glucocorticoid dispensed, which was converted to prednisone equivalents.
Exposure was assessed as a yes/no indicator of any use, current daily dose, cumulative dose, cumulative exposure days, peak dose, and days since most recent exposure. Exposure metrics were assessed and updated for each patient-day during the study period. Cumulative exposures were assessed from the date of the first use; for some patients, first use occurred in the pre-index period.
Fracture outcomes
We evaluated the incidence of fragility fractures, a composite outcome that included closed fractures of the hip, distal radius/ulna, other parts of radius/ulna, pelvis, humerus, shaft/unspecified parts of femur, and clinically diagnosed vertebral fractures. The only open fracture site included was the distal radius/ulna. We also separately examined the incidence of hip and vertebral fractures. An incident fracture was identified by a relevant ICD-9-CM diagnosis code which was either (1) preceded by at least 180 days with no diagnosis codes for the same fracture site or (2) which appeared in conjunction with a surgical repair procedure [24–26].
Statistical methods
Patient characteristics assessed during the 12-month pre-index period were summarized descriptively for patients with and without systemic glucocorticoid exposure at any point during the study period. To fully capture historical fragility fractures, we evaluated all available pre-index data, after December 31, 2000. Standardized mean differences were computed to compare characteristics of exposed and unexposed patients; differences ≤0.1 were considered negligible [27]. Exposure variables were summarized into predefined categories based on treatment guidelines and the expected glucocorticoid use per authors’ clinical judgment [28]. Recommendations for osteoporosis treatment among glucocorticoid users vary by dose and duration of exposure, with 5 mg/day, 7.5 mg/day, and 3 months of exposure representing key cut-points [28]. These thresholds, along with the expected dose of exposure in RA patients, guided categorization of exposure variables. For example, 7.5 mg/day of exposure for 3 months represents approximately 675 mg of cumulative exposure; thus, cumulative exposure categories are multiples of 675 mg.
Fracture incidence rates per 1000 person-years were computed for the post-index period, stratified by glucocorticoid exposure levels. Glucocorticoid exposure was updated on a daily basis, and patients could contribute both unexposed and exposed time during follow-up. Glucocorticoid users were likewise allowed to contribute person time to various dose categories (e.g., 5 mg/day, 7.5 mg day), and this dose could change over time. For the composite fracture outcome, incidence rates among glucocorticoid exposure groups were also separately estimated for patients aged <50 and ≥50 years.
Among exposed patients, Cox’s proportional hazards regression was used to estimate the effects of cumulative and current daily dose on fracture risk, after adjusting for patient age and sex, pre-index use of anticonvulsant medications, antidepressants, osteoporosis therapies, and baseline disease burden (number of medications, Charlson Comorbidity Index score [29]) and selected comorbidities (asthma/COPD, inflammatory bowel disease, multiple sclerosis). These models were first run with all of these variables included as adjusters; the models were also run using these variables as stratification variables. Since the results were similar, we present the results only from the first approach in this paper. These regression analyses were repeated among all patients, including those with no exposure. Sensitivity analysis in which patients with pre-index fractures were excluded was also performed.
A separate Cox’s proportional hazards model was run to examine fracture risk associated with time since last exposure. This model, which shows the impact of glucocorticoid discontinuation, included only patients with at least some glucocorticoid exposure and adjusted for and the patient demographic and baseline clinical characteristics noted above as well as past cumulative dose. We used the chi-square test to evaluate the presence of linear trends in fracture rates and risk associated with increasing glucocorticoid dose.
Analyses were conducted in SAS Software, version 9.2 (SAS Institute, Cary, NC). This study complied with STROBE observational research guidelines.
Results
Of the 691,145 patients with at least one RA diagnosis during the selection period, 42,127 met all study inclusion criteria (Supplemental Table 1). The majority (85 %) of patients used systemic glucocorticoids in the study period (Table 1). In both the exposed and unexposed groups, nearly three-quarters of patients were women, the average age was approximately 49–50 years, and approximately 1 % of patients had fracture(s) pre-index. In both groups, approximately 1 % had a formal osteoporosis diagnosis and 7 % received osteoporosis medications pre-index.
The average (standard deviation) health plan enrollment time was 1482 days (875) pre-index and 807 days (700) post-index. The median follow-up in the study population was 595 days (1.6 years), which is slightly longer than the median for all adults in the source database (486 days, 1.3 years). Among all exposed patients, approximately half were long-term glucocorticoid users defined by >90 days of use and 14 % were exposed for >1 year (Table 2). Approximately 54 % of patients with at least 12 months of follow-up and 40 % of patients with shorter follow-up had >90 days of cumulative glucocorticoid use. Approximately 60, 22, and 9 % of exposed patients had cumulative dose of ≥675, ≥2700, and ≥5400 mg, respectively (i.e., the equivalent of more than approximately 90 days, 1 year, and 2 years, respectively, of physiologic range prednisone of 7.5 mg per day) (Table 2) at the end of follow-up. The majority of patients (71 %) had some systemic glucocorticoid exposure pre-index (prior to RA diagnosis date), and 2 % of all patients had accumulated ≥5400 mg of exposure pre-index (data not shown).
Use of osteoporosis medications was relatively uncommon in the post-index period (following RA diagnosis), ranging from 7 % in patients who never received glucocorticoids and those with cumulative dose <675 mg to 8 % among patients with cumulative dose 675 to <1350 mg, 10 % among patients with cumulative dose 1350 to <2700 mg, 13 % among patients with cumulative dose 2700 to < 5400 mg, and then nearly doubling (25 %) in patients with cumulative dose ≥5400 mgs. The majority of patients used either alendronate (54 %) or risedronate (23 %), with oral ibandronate also commonly used (18 %). Similarly, post-index use of dual-energy X-ray absorptiometry (DXA) scans was low, ranging between 14 % among unexposed patients and 43 % among patients with cumulative dose ≥5400 mg.
We observed 519 incident post-index osteoporosis-related fractures, including 167 clinical vertebral fractures and 95 hip fractures. The unadjusted incidence rates of any osteoporosis-related fracture increased significantly in a dose-dependent manner for all exposure metrics evaluated (Table 3, Supplemental Table 2). The highest incidence rates (95 % CI) were 16.0 (11.0, 22.6) per 1000 person-years at daily doses of ≥15 mg/day, 13.4 (10.7, 16.7) per 1000 person-years at cumulative doses of ≥5400 mg, and 11.1 (9.1, 13.4) per 1000 person-years at cumulative exposures longer than 365 days. Similar trends existed when clinical vertebral and hip fractures were evaluated separately (Table 3, Supplemental Table 2), although small sample sizes resulted in less precise estimates that did not achieve statistical significance for daily and peak dose associations with hip fractures. In these unadjusted results, only the highest levels of daily dose and cumulative dose were associated with statistically higher fracture risks compared to unexposed. Age-stratified analyses of incidence rates of any osteoporosis-related fracture showed similar trends among patients younger vs. older than 50 years. These trends achieved statistical significance for measures of cumulative exposure in the younger age group and for all exposure metrics in the older age group (Supplemental Table 3).
We further evaluated the impact of daily and cumulative dose in regression analyses that adjusted for patient age and sex. In these analyses, fracture risk increased significantly with increasing steroid dose. Cumulative doses ≥5400 mgs were associated with an approximately 2-fold increased fracture risk compared to cumulative doses <675 mg, after adjusting for patient demographics, pre-index clinical characteristics, and daily glucocorticoid dose (Table 4). Compared to no current exposure (0 mg/day current daily dose), daily doses ≥15 mg/day were associated with an approximately 2.3-fold increased fracture risk, after adjusting for patient demographics, pre-index clinical characteristics, and cumulative dose. In sensitivity analysis excluding patients with fracture in prior to the index date from the model, the results were extremely similar for the highest levels of both cumulative dose and current daily dose (data not shown).
When considering time since discontinuation, the unadjusted fracture incidence rates were highest during current exposure and decreased significantly with longer time since discontinuation, returning to a level similar to that of unexposed patients by 12 months post-discontinuation (Table 3). In Cox’s models adjusted for patient sex and age and cumulative dose, fracture risk was 31 to 44 % lower beginning 60 days after discontinuation, compared with current exposure (Fig. 2, Supplemental Table 4).
Discussion
In this cohort of newly diagnosed RA patients with a mean age of only 49 years, many of whom initiated glucocorticoids during the study period, we found that fracture risk increased significantly with increasing steroid exposure. Osteoporosis-related fractures occurred at the rate of approximately 5 to 9 per 1000 person-years at low glucocorticoid doses, and this risk was elevated 2- to 2.5-fold at high doses (≥15 mg/day or ≥5400 mg cumulative dose). The increased fracture risk appeared to be reversible, decreasing by 31 to 44 % beginning at 60 days after glucocorticoid discontinuation and to the level observed among unexposed patients by 12 months post-discontinuation. Age-specific estimates were less precise, but trends were similar in patients under age 50 and those age 50 or older.
Our newer findings are consistent with the glucocorticoid-related fracture risk reported for general populations of steroid users. A 2002 meta-analysis estimated relative rates of any fracture in steroid users compared to nonusers of 1.33 (95 % CI 1.29–1.38) to 1.91 (95 % CI 1.68–2.15) and reported 61 to 101 % higher hip fracture risk and 160 to 185 % higher vertebral fracture risk among steroid users versus nonusers [30]. Decreased risk within 1 year following discontinuation of glucocorticoid therapy has also been reported for a large UK population [3]. In another study, recent prolonged (≥3 months) steroid exposure was associated with elevated fracture risk, while remote and short-term exposures were not [14]. In addition, individuals who were “on drug” have been shown to be at higher risk than unexposed individuals, and during times when exposed individuals were “off drug,” their risk was between the risk levels of the on drug and unexposed patients [12].
Evidence regarding the threshold and patterns of exposure that result in substantial increases in fracture risk are limited and inconsistent. In one large-scale study of a general population of steroid users, daily doses of 20 mg were associated with a 60 % higher rate of nonvertebral fracture compared with daily doses of less than 2.5 mg [4]. Moreover, cumulative dose was reported to be an important determinant of fracture risk among patients who were intermittently exposed: Intermittent exposure to high glucocorticoid doses (≥15 mg/day) was associated with significantly increased fracture risk at cumulative glucocorticoid doses >1 g but not when previous exposure was <1 g [10]. Studies in RA and lung disease populations have also reported associations between cumulative dose and fracture risk, and have found cumulative dose to be a stronger predictor of fracture compared to daily dose [15–17].
In our study, significant dose-response trends of increasing fracture risk with increasing levels of exposure were seen for both cumulative and daily dose. Rates of osteoporosis-related fracture increased from approximately 5 to 9 per 1000 person-years at the lower dose ranges to approximately 13 to 16 per 1000 person-years at the highest doses (≥15 mg/day and cumulative doses ≥5400 mg). Risk of osteoporosis-related fracture should warrant particular attention among younger RA patients exposed to glucocorticoids exceeding these high thresholds. We note, however, that incidence rates of vertebral and hip fracture observed among patients unexposed or exposed to low levels of steroids in our study are comparable to those reported for post-menopausal women aged 50–70 years in the general population [31, 32].
In contrast to previous studies of glucocorticoid users with diverse disease [15, 33–37], we may have better isolated the impact of glucocorticoid use on fracture risk by focusing on incident RA patients and, thus, decreasing the potential for disease-related confounding. This approach also reduced the heterogeneity in patterns of steroid use, which varies by disease. For each patient, we captured steroid use from first exposure and characterized exposure in multiple ways for each follow-up day. The result is a more precise and detailed assessment of exposures than reported in previous studies. In addition, our data source provided insights about younger patients which are generally not available elsewhere. Clinical guidelines do not specifically address the management of steroid-induced bone loss in patients under age 50 [23]. Although small sample sizes limited statistical power, our findings were similar in patients under age 50 and those age 50 or older.
We acknowledge limitations of this study. Patient identification can be challenging in administrative claims data studies, though we believe that our two-part selection process increased the likelihood that study patients had new onset RA at their index date. This was accomplished by selecting patients based on their first claim (excluding claims for diagnostic tests) with an RA diagnosis after at least 12 months with no RA claims, and requiring that each patient have also received medication indicative of RA therapy. Realizing the importance of accurately capturing exposures, we also required that all patients who used glucocorticoids before or after their index date were new users with a 12-month glucocorticoid-free interval prior to their first steroid claim in the study period. It is possible that left censoring of the data may have misclassified some patients with prior steroid exposure as being new glucocorticoid users, although the required 12-month period free of all glucocorticoid use likely minimized this misclassification. This approach also ensures capture of glucocorticoid exposure from first use forward through the end of follow-up (i.e., censoring event) for each patient.
Ascertainment of incident fractures is challenging in administrative claims data. We improved fracture identification by using algorithms that have been previously used in claims data studies, some of which have been validated against clinical records [24–26]. Even with this approach, we expect some underestimation of fracture events, particularly for clinical vertebral fractures which tend to be underdiagnosed. Thus, the absolute incidence rates reported for vertebral fractures are likely low, as only fractures that have come to clinical attention can be identified in the claims data. In addition, it is possible that some of the vertebral fractures considered incident in this study may have occurred earlier in the exposure period without being detected in the data at the time of occurrence. In any such instances, the fractures would have likely occurred at lower levels of cumulative glucocorticoid exposure suggesting that our results may be conservative. Although we restricted our study to RA patients in an effort to minimize confounding of the association between steroids and fracture by the underlying disease, residual confounding may remain since we could not adjust for disease activity and severity. Finally, the relatively small numbers of hip fractures reduced the precision of our estimates of hip fracture risk.
Despite the known deleterious impact of glucocorticoid use on bone, rates of diagnosis and treatment for steroid-induced bone loss tend to be low. In this study, although nearly one-quarter of patients had cumulative steroid exposure exceeding 1 year and most patients were exposed before their RA was diagnosed, the use of osteoporosis medications was low in both the pre- and post-index periods. Osteoporosis treatment was more common in the post-index period among patients with high dose steroid exposures, yet only one-quarter of patients with cumulative doses of ≥5400 mg used osteoporosis medication and less than half of patients at that dose level had a post-index DXA scan. A previous administrative claims study showed that 42 % of glucocorticoid users received monitoring, treatment, or both [38]. Other published results are mixed but suggest that only approximately half of glucocorticoid users may receive an osteoporosis-preventing medication and only one quarter may be managed according to guidelines [39–41].
Our results extend previously published evidence, using newer data and a rigorous method for quantifying steroid exposure, that glucocorticoid use has the potential to increase the risk of fracture, particularly at higher daily and cumulative dose levels. While risk decreases within a few months after glucocorticoid discontinuation, it may remain above baseline levels for extended periods of time. Our results suggest that management of steroid-induced bone loss should be an important consideration in RA care, particularly for patients who are on longer-term continuous therapy regardless of age. This stance is consistent with that of the American College of Rheumatology and the European League Against Rheumatism, which recommend monitoring for osteoporosis to ensure safe treatment of patients with rheumatic diseases prescribed glucocorticoids. Our data suggest that there may be opportunities to improve the level of osteoporosis monitoring and treatment in this vulnerable population.
References
Kirwan J, Power L (2007) Glucocorticoids: action and new therapeutic insights in rheumatoid arthritis. Curr Opin Rheumatol 19(3):233–7. doi:10.1097/BOR.0b013e3280d6471a
van Everdingen AA, Jacobs JW (2002) Siewertsz Van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med 136(1):1–12
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res Off J Am Soc Bone Miner Res 15(6):993–1000. doi:10.1359/jbmr.2000.15.6.993
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology 39(12):1383–9
Hoes JN, Jacobs JW, Hulsmans HM, De Nijs RN, Lems WF, Bruyn GA et al (2010) High incidence rate of vertebral fractures during chronic prednisone treatment, in spite of bisphosphonate or alfacalcidol use. Extension of the alendronate or alfacalcidol in glucocorticoid-induced osteoporosis-trial. Clin Exp Rheumatol 28(3):354–9
Pereira RM, Carvalho JF, Canalis E (2010) Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics 65(11):1197–205
Lukert BP, Raisz LG (1994) Glucocorticoid-induced osteoporosis. Rheum Dis Clin N Am 20(3):629–50
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S et al (2006) High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39(2):253–9. doi:10.1016/j.bone.2006.02.005
Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48(11):3224–9. doi:10.1002/art.11283
De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP (2007) Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 56(1):208–14. doi:10.1002/art.22294
Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C et al (2004) The risk and burden of vertebral fractures in Sweden. Osteoporos Int: a J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 15(1):20–6. doi:10.1007/s00198-003-1463-7
Donnan PT, Libby G, Boyter AC, Thompson P (2005) The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf 14(3):177–86. doi:10.1002/pds.1075
Steinbuch M, Youket TE, Cohen S (2004) Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int: a J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 15(4):323–8. doi:10.1007/s00198-003-1548-3
Majumdar SR, Morin SN, Lix LM, Leslie WD (2013) Influence of recency and duration of glucocorticoid use on bone mineral density and risk of fractures: population-based cohort study. Osteoporos Int: a J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 24(9):2493–8. doi:10.1007/s00198-013-2352-3
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54(10):3104–12. doi:10.1002/art.22117
McEvoy CE, Ensrud KE, Bender E, Genant HK, Yu W, Griffith JM et al (1998) Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(3 Pt 1):704–9. doi:10.1164/ajrccm.157.3.9703080
Walsh LJ, Wong CA, Oborne J, Cooper S, Lewis SA, Pringle M et al (2001) Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax 56(4):279–84
Dykman TR, Gluck OS, Murphy WA, Hahn TJ, Hahn BH (1985) Evaluation of factors associated with glucocorticoid-induced osteopenia in patients with rheumatic diseases. Arthritis Rheum 28(4):361–8
Sambrook PN, Cohen ML, Eisman JA, Pocock NA, Champion GD, Yeates MG (1989) Effects of low dose corticosteroids on bone mass in rheumatoid arthritis: a longitudinal study. Ann Rheum Dis 48(7):535–8
Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM (1997) Effects of low dose methotrexate on the bone mineral density of patients with rheumatoid arthritis. J Rheumatol 24(8):1489–94
Tattersfield AE, Harrison TW, Hubbard RB, Mortimer K (2004) Safety of inhaled corticosteroids. Proc Am Thorac Soc 1(3):171–5. doi:10.1513/pats.200402-016MS
Godschalk MF, Downs RW (1988) Effect of short-term glucocorticoids on serum osteocalcin in healthy young men. J Bone Miner Res Off J Am Soc Bone Miner Res 3(1):113–5. doi:10.1002/jbmr.5650030117
Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W et al (2010) American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 62(11):1515–26. doi:10.1002/acr.20295
Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG (2009) Identification and validation of vertebral compression fractures using administrative claims data. Med Care 47(1):69–72. doi:10.1097/MLR.0b013e3181808c05
Ray WA, Griffin MR, Fought RL, Adams ML (1992) Identification of fractures from computerized Medicare files. J Clin Epidemiol 45(7):703–14
Baron JA, Lu-Yao G, Barrett J, McLerran D, Fisher ES (1994) Internal validation of Medicare claims data. Epidemiology 5(5):541–4
Faraone SV (2008) Interpreting estimates of treatment effects: implications for managed care. P & T: a Peer-Rev J Formul Manag 33(12):700–11
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM et al (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res 64(5):625–39. doi:10.1002/acr.21641
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–9
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int: a J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 13(10):777–87. doi:10.1007/s001980200108
Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ 3rd (2014) Trends in fracture incidence: a population-based study over 20 years. J Bone Min Res Off J Am Soc Bone Miner Res 29(3):581–9. doi:10.1002/jbmr.2072
Wade SW, Strader C, Fitzpatrick LA, Anthony MS (2012) Sex- and age-specific incidence of non-traumatic fractures in selected industrialized countries. Arch Osteoporos 7:219–27. doi:10.1007/s11657-012-0100-5
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 43(3):522–30. doi:10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum 43(12):2776–84. doi:10.1002/1529-0131(200012)43:12<2776::AID-ANR18>3.0.CO;2-N
Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH (2014) Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology. doi:10.1093/rheumatology/keu162
Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD et al (2012) Effect of co-morbidities on fracture risk: findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 50(6):1288–93. doi:10.1016/j.bone.2012.02.639
Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, Di Munno O et al (2000) A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol 27(11):2582–9
Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A et al (2005) Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum 52(8):2485–94. doi:10.1002/art.21194
Caplan L, Hines AE, Williams E, Prochazka AV, Saag KG, Cunningham F et al (2011) An observational study of glucocorticoid-induced osteoporosis prophylaxis in a national cohort of male veterans with rheumatoid arthritis. Osteoporos Int: a J Established Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA 22(1):305–15. doi:10.1007/s00198-010-1201-x
Majumdar SR, Lix LM, Yogendran M, Morin SN, Metge CJ, Leslie WD (2012) Population-based trends in osteoporosis management after new initiations of long-term systemic glucocorticoids (1998-2008). J Clin Endocrinol Metab 97(4):1236–42. doi:10.1210/jc.2011-2645
McKeown E, Bykerk VP, De Leon F, Bonner A, Thorne C, Hitchon CA et al (2012) Quality assurance study of the use of preventative therapies in glucocorticoid-induced osteoporosis in early inflammatory arthritis: results from the CATCH cohort. Rheumatology 51(9):1662–9. doi:10.1093/rheumatology/kes079
Acknowledgments
The authors are grateful for the statistical and data analysis contributions of Michael Lane and Lang Chen.
Author contributions
Study design, data interpretation, manuscript revisions, final manuscript approval: all authors. Manuscript drafting, accountability for manuscript content and data integrity: AK and SWW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This work was supported by Amgen, Inc. Dr. Balasubramanian is an employee and stockholder of Amgen, Inc. Drs. O’Malley and Lin also own stock and were employed by Amgen, Inc. during the study. Ms. Wade is a partner in Wade Outcomes Research and Consulting and has received consulting fees from Amgen Inc. Dr. Adler is employed by the Department of Veterans’ Affairs. Dr. Maricic is employed by the Catalina Pointe Rheumatology. Drs. Curtis and Saag are employed by the University of Alabama at Birmingham and have both received consulting fees from Amgen, Inc. Drs. Curtis and Saag have also received research grants from Amgen, Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 14 kb)
Supplementary Table 2
(DOCX 28 kb)
Supplementary Table 3
(DOCX 24 kb)
Supplementary Table 4
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Balasubramanian, A., Wade, S.W., Adler, R.A. et al. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos Int 27, 3239–3249 (2016). https://doi.org/10.1007/s00198-016-3646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3646-z