Abstract
First described by Paul Ehrlich in 1879, who noted its characteristic staining by acidophilic dyes, for many years, the eosinophil was considered to be an end-effector cell associated with helminth infections and a cause of tissue damage. Over the past 30 years, research has helped to elucidate the complexity of the eosinophil’s function and establish its role in host defense and immunity. Eosinophils express an array of ligand receptors which play a role in cell growth, adhesion, chemotaxis, degranulation, and cell-to-cell interactions. They play a role in activation of complement via both classical and alternative pathways. Eosinophils synthesize, store and secrete cytokines, chemokines, and growth factors. They can process antigen, stimulate T cells, and promote humoral responses by interacting with B cells. Eosinophils can function as antigen presenting cells and can regulate processes associated with both T1 and T2 immunity. Although long known to play a role in defense against helminth organisms, the interactions of eosinophils with these parasites are now recognized to be much more complex. In addition, their interaction with other pathogens continues to be investigated. In this paper, we review the eosinophil’s unique biology and structure, including its characteristic granules and the effects of its proteins, our developing understanding of its role in innate and adaptive immunity and importance in immunomodulation, and the part it plays in defense against parasitic, viral, fungal and bacterial infections. Rather than our worst enemy, the eosinophil may, in fact, be one of the most essential components in host defense and immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paul Ehrlich first described the eosinophil and noted its characteristic staining by acidophilic dyes in 1879. He made a note of increased numbers of eosinophils present in the blood of individuals with asthma, helminthic infections, urticaria, and other diseases [1]. Early research on the eosinophil focused primarily on its role in inflammation and anaphylaxis. For some time, the cell was considered as a classic end-stage effector cell which killed parasites, but in the process, also caused harm to host tissues [2]. Over the next several decades, the knowledge about the eosinophil expanded as the functions of its granules and their proteins were characterized, and the ability of the cell to produce and respond to cytokines and chemokines was recognized. However, it has only been over the past 30 years that we have gained a more complete understanding of the eosinophils important role in immunity and host defense. In this review, we will discuss the function of the eosinophil in parasitic diseases as well as bacterial, viral, and fungal infections; review the current understanding of its role in adaptive and innate immunity; and explore the potential interplay between infections and inflammatory diseases.
Differentiation and Development
Eosinophils differentiate from CD34+ antigen pluripotent progenitor stem cells in the bone marrow [3, 4]. They can also develop from these progenitor cells outside the bone marrow, notably in the lung tissue in the setting of airway inflammation [4]. Eosinophils first differentiate into a hybrid precursor common to both eosinophils and basophils before becoming committed to a specific eosinophil lineage [5]. Both transcription factors and cytokines influence the development of eosinophils into mature cells [6]. The transcription factor, PU.1, expressed in hematopoietic cells, works synergistically with GATA-1 and CCAAT-enhancer-binding protein (c/EBP) to regulate the differentiation of eosinophils and the transcription of their granule proteins [5, 6]. Of these, GATA-1 is most important for eosinophil development [5]. These transcription factors are not unique to eosinophils; they also influence other hematopoietic lineages but can have antagonistic properties in those cell lines [4]. The majority of the granule protein production takes place in the final stages of eosinophil maturation [7]. A number of cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, and IL-5 promotes eosinophil maturation from myeloid precursors [2, 5]. IL-5 is the most specific for the eosinophil, influencing its differentiation, activation, migration, and survival in tissues [3–5]. Along with cytokines, eosinophils also respond to a number of chemokines, specifically eotaxin-1, -2, and -3; macrophage inflammatory protein (MIP)-1α, and regulated on activation, normal T cell expressed (RANTES) [5]. Eosinophils are released from the bone marrow in a mature form, but possessing a half-life of approximately 18 h, they only spend a short time in the peripheral blood. Most migrate to the tissues where they can survive up to 2 weeks [2, 5, 8, 9]. At baseline conditions, eosinophils localize to the thymus, gastrointestinal (GI) tract, uterus, and mammary gland [6]. Eosinophils play a role in organ development, metabolism, lymphocyte recruitment, tissue repair, immunomodulation, and tumor immunity as well as antimicrobial and antifungal immunity [10]. Under inflammatory conditions, eosinophils migrate to other body sites including the lungs and skin. Trafficking to inflammatory sites involves the interplay of cytokines, chemokines, and adhesion molecules [2].
Structure
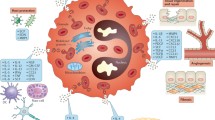
Eosinophils have a distinct appearance containing a bilobed nucleus and large cytoplasmic granules. Two main types of granules are present: primary granules and secondary granules, also called the specific or crystalloid granules. Granules contain basic proteins that characteristically bind eosin giving the cell its unique appearance under microscopic examination. Primary granules contain Charcot-Leyden crystal proteins which have intrinsic lysophospholipase activity [4, 11]. Specific granules are composed of an electron dense crystalline core surrounded by a radiolucent matrix and enclosed by a trilaminar membrane [12–14]. They store and secrete preformed proteins including cationic proteins, cytokines, and chemokines. The specific granule’s core is composed primarily of major basic protein 1 (MBP-1) while the matrix contains eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPO) along with an array of cytokines and chemokines [8, 15]. Specific granules possess a complex system of internal membranous vesiculotubular components in which proteins are sorted prior to transport [15–17]. Eosinophils also contain lipid bodies, the site of synthesis of cysteinyl leukotrienes, thromboxane, and prostaglandins, and cytoplasmic large vesicles, which play a role in secretion [4, 16, 18]. Like other cells, mature eosinophils also possess mitochondria, endoplasmic reticulum, and Golgi bodies [4].
Eosinophils secrete their granule contents through three identified mechanisms: exocytosis, cytolysis, and piecemeal degranulation (PMD). In exocytosis, granule contents are extruded after fusion with the cell membrane. This can occur by classic exocytosis, in which granules are released individually, or by compound exocytosis in which clusters of granules release their contents via pore fusion [19]. In contrast, in cytolysis, granules are released when the cell ruptures or undergoes necrosis [14]. This results in the release of intact granules which can function as extracellular secretory organelles [10]. The mechanism, which is unique to eosinophils, is the piecemeal degranulation. Through a system of small spherical vesicles and larger membrane-bound vesicles, called eosinophil sombrero vesicles, proteins are extruded from the granules, traffic through the cytoplasm, and are then released across the cell membrane [10, 15, 18]. Specific soluble NSF attachment protein (SNAP) receptor (SNARE) proteins coordinate this process [10]. In this manner, preformed mediators are released without the need for de novo synthesis [14]. In addition, various agonists can elicit differential secretion of specific preformed cytokines without the release of others [17]. These characteristics make eosinophils unique among innate and adaptive immune cells.
The four cationic proteins, MBP, ECP, EDN, and EPO, make up most of the content of eosinophilic specific granules. MBP is the most abundant protein and is localized to the crystalloid core of the granule and is expressed as two homologs, MBP-1 and MBP-2. It is small in size, consisting of a single chain of 117 amino acids, and is extremely basic. MBP is toxic to helminths and possesses antibacterial properties. It plays a role in the activation of complement via both the classical and alternative pathways and in the stimulation of signaling pathways involving mast cells, neutrophils, and basophils [5]. ECP and EDN are eosinophil-associated ribonucleases that were first recognized for their neurotoxic properties. They are both members of the ribonuclease (RNase) A superfamily of proteins that possess the ability to hydrolyze RNA [20]. Their homologous genetic sequences have only been detected in primate genomes [21]. ECP is a single chain cationic protein with homology to pancreatic ribonuclease [7]. It is toxic to helminths and possesses antibacterial properties, including the ability to bind lipopolysaccharide and other bacterial cell wall components [7]. ECP also influences the proliferation of T and B lymphocytes, promotes mast cell degranulation, and regulates components of the classical complement pathway [7, 22]. EDN is a single chain polypeptide. It has antiviral properties and is capable of degrading single-stranded RNA (ssRNA) [23]. EPO, a heme-containing haloperoxidase, is associated with bacterial killing and also functions in signaling pathways [5].
Eosinophils can synthesize, store, and secrete multiple cytokines, chemokines, and growth factors, see Table 1. In contrast to T cells, eosinophils store cytokines intracellularly as preformed mediators. Specific granules contain abundant cytokines including IL-2, IL-4, IL-5, IL-6, IL-13, interferon (IFN)-γ, GM-CSF, and tumor necrosis factor (TNF)-α; chemokines, such as RANTES, eotaxins, and MIP-1α; growth factors, such as stem cell factor and transforming growth factor (TGF) α and β; and a variety of other enzymes, see Fig. 1 [4, 24]. Most are stored in the granule matrix, but GM-CSF, IL-2, and IL-4 are also present in the core [24]. When stored in specific granules, mediators can be immediately and selectively released when activated by the piecemeal degranulation [5, 17].
Eosinophils express numerous cell surface receptors which effect growth, adhesion, chemotaxis, degranulation, and cell-to-cell interactions, listed in Table 2 [4]. These include receptors for cytokines, chemokines, adhesion molecules, complement, chemotactic factors, and immunoglobulins [25]. Several types of pattern-recognition receptors (PRPs) are also expressed by eosinophils including those for toll-like receptors (TLRs) [4]. They also possess lipid mediator and proteinase-activated receptors (PARs). The cell surface receptors which set eosinophils apart from other innate and adaptive immune cells include IL-5 receptor subunit-α (IL-5Rα), CC-chemokines receptor 3 (CCR 3), and sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8). The cytokine, IL-5, effects all aspects of eosinophil biology [4]. IL-5 is produced by activated Th2 cells and mast cells, by natural killer cells, and also by eosinophils themselves. It works in synergy with IL-4, IL-13, and the eotaxins, promoting activation and tissue recruitment of eosinophils. In a mouse model of respiratory syncytial virus (RSV) infection, Matthews et al. (2005) showed that blocking eotaxin-1 inhibited eosinophil chemotaxis, altered the recruitment of CD4+ T cells in the lung, and decreased the production of IL-5 [26]. Via CCR 3, chemokines, including the eotaxins, RANTES, and MIP-1α, interact with eosinophils, promoting chemotaxis. SIGLEC-8 is expressed predominantly by eosinophils and promotes selective apoptosis. Remarkably, eosinophil-specific granules have their own cytokine and chemokine receptor sites, suggesting that they may play an independent immunoregulatory role [4, 16, 17].
Migration and Trafficking
Recruitment of eosinophils to tissues is mediated by several interacting components: cytokines, chemokines, and adhesion molecules and their respective receptors [6]. Recruitment to inflammatory sites involves priming, rolling along endothelial cells, and adhesion to the endothelium, transendothelial diapedesis, and chemotaxis [27]. Priming of eosinophils is the result of the effects of numerous inflammatory mediators including cytokines, activating factors, and TLRs [28]. Rolling is thought to be primarily mediated by adhesion molecules such as selectins [11, 29]. Interaction with and adhesion to endothelial cells occurs through the interplay of cytokines and adhesion molecules [11, 30]. Endothelial cell adhesion molecules, such as vascular cell adhesion molecule (VCAM)-1, activate to form passageways between cells allowing for diapedesis [30]. Chemotaxis is primarily governed by chemokines, particularly the eotaxin family [27].
Under inflammatory conditions, activated Th2 lymphocytes produce cytokines including IL-4, IL-5, and IL-13. These cytokines upregulate the production of the chemokines involved in eosinophil trafficking: eotaxins, MCPs, and RANTES [4–6]. IL-5 and eotaxin are specific and selective for eosinophils and also cooperatively promote migration of eosinophils to the tissues [5, 27]. Eotaxin specifically targets eosinophils, with its highest expression found in the GI tract. Proinflammatory and Th2 cytokines induce expression of eotaxin messenger RNA (mRNA) [27]. Eotaxin plays a role in regulating eosinophils both during inflammation and at the baseline [26]. At the baseline conditions, eotaxin-1 directs eosinophils to the thymus, uterus, mammary gland, and GI tract [6]. Eotaxin-1 involved in early recruitment of eosinophils to tissues, while eotaxins 2 and 3 act as chemoattractants and have an effect on later recruitment. RANTES is expressed in eosinophils and both attract and activate them. Adhesion molecules, including selectins and integrins, have been shown to play a role in both inflammatory models and at the baseline particularly in the GI tract. Eosinophils express VCAM-1, mucosal addressin cell adhesion molecule (madCAM)-1, and intracellular adhesion molecule (ICAM)-1, integrins that facilitate endothelial attachment [6]. Other molecules have been implicated in eosinophil trafficking including prostaglandins, histamine, and eoxins, which are proinflammatory arachidonic acid metabolites [6].
Innate Immunity
Eosinophils were first recognized in association with parasitic infections and have long been thought to function as an end-effector cell. Their presence in association with dead or dying parasites was first noted decades ago [14]. Specific granule proteins were noted to possess cytotoxic effects, capable of damaging parasitic pathogens, and, in the process, neighboring host tissues [14]. Eosinophil cationic proteins have now been shown to play a role in host defense mechanisms against not only helminths but also viruses and bacterial organisms. MBP is toxic to helminths, ECP and EDN have neurotoxic properties and ECP has antiviral, antibacterial, and antihelminthic cytotoxicity [14].
There is an increase evidence of the complex role eosinophils play in the host defense and immunity. Similar to neutrophils, eosinophils can phagocytose foreign material including bacteria, yeast, and parasites, although they do so less efficiently [14]. Uniquely, they have been shown to release mitochondrial DNA-containing “traps” into the extracellular space to engulf bacteria in an instantaneous catapult-like fashion [14, 31, 32]. This process appears to require priming by IL-5 suggesting that it is associated with Th2 immune responses [32].
Eosinophils can interact with potential pathogens in other direct and indirect ways. Eosinophils express receptors for complement factors and can respond to the complement cascade. Granule proteins, specifically MBP, ECP, and EPO, regulate both the classical and alternative complement pathways [33]. Eosinophils express pattern recognition receptors (PRRs) which allow them to recognize specific pathogen-associated molecular patterns (PAMPs) of bacteria and fungi including the bacterial lipopolysaccharide (LPS) and fungal beta-glucans, allowing them to be directly activated by these organisms [14, 25]. Eosinophils also express traditional PAMPs such as TLRs which trigger cytokine synthesis and secretion and other host responses. Eosinophils express an array of TLRs including TLRs 1, 2, 4, 5, 6, 7, and 9 [14]. TLR7 is most abundant in eosinophils and is activated by single-stranded RNA found in viruses such as respiratory syncytial virus [34, 35]. Eosinophils also respond to damage-associated molecular patterns (DAMPs) and by doing so are attracted to damaged tissues and necrotic cells [14]. Through these mechanisms, eosinophils play a direct role in innate immune response to a wide variety of pathogens such as helminths, viruses, bacteria, and fungi and contribute to tissue homeostasis, see Fig. 2 [4, 14].
Adaptive Immunity
The role that eosinophils play in adaptive immunity is now more fully appreciated. They can process antigen, stimulate T cells, and promote humoral responses through interactions with B cells. Eosinophils produce T cell polarizing cytokines. In the presence of eosinophils, Th2 lymphocytes become activated producing cytokines such as IL-4 and IL-5. This has been proven for IL-5 in mouse models of RSV infection and Schistosoma infection and for IL-4 in mouse models of Nippostrongylus brasiliensis infection [36]. Sabin and Pearce (1995) demonstrated that eosinophil infiltration occurred soon after egg injection in a mouse model of Schistosoma mansoni infection. Early production of IL-4 predicted a Th2 response [37]. In subsequent work, Sabin et al. (1996) showed that eosinophil recruitment was dependent on IL-5 [38]. Eosinophils function as antigen-presenting cells (APCs) by directly processing and presenting antigen to naïve or primed CD4+ T cells.
Activated eosinophils express major histocompatibility complex (MHC) class II receptors on their cell membranes [36, 39]. This expression has been demonstrated after exposure to tetanus toxoid [36]. Eosinophils have also been shown to function as presenters of Staphylococcal superantigen and as APCs in a mouse model of Strongyloides [36]. Eosinophils can regulate T cell function demonstrating polarization to both Th1 and Th2 pathways and expressing both Th1- and Th2-associated cytokines [6, 36]. Eosinophils may also function as a Th1-promoting immunoregulatory cell. In the presence of rhinovirus, eosinophils have been shown to present antigens to T cells leading to proliferation [36]. Eosinophils also have the potential to indirectly promote Th2 immunity through interactions with other cells such as dendritic cells, B cells, and mast cells. In the granule protein, EDN acts as a chemoattractant and activator for dendritic cells, and in turn, activated dendritic cells enhance Th2 responses [39]. In the setting of Strongyloides stercoralis antigen, eosinophils have been shown to induce antigen-specific IgM and IgG responses demonstrating a possible role as immunoregulators of B cells. Eosinophils regulate and recruit innate immune cells such as mast cells [36]. They produce stem cell factor (SCF), a cytokine which regulates the differentiation, maturation, and survival of mast cells [40].
Parasitic Infections
Helminths are complex, multicellular organisms present worldwide and distinguished by their ability to sustain chronic infections in human beings, see Table 3 [41–43]. Helminthic infections often result in malnutrition, anemia, and increased susceptibility to other infections [43]. Although effective treatments are available, reinfection is common. There are three families of helminths: cestodes, such as Taenia and Echinococcus, commonly known as tapeworms; trematodes (flukes); and nematodes (roundworms). They possess complex life cycles consisting of multiple developmental stages, each of which is antigenically distinct [42]. Most helminths are extracellular with the exception of Trichinella spiralis [41, 42]. Infection by helminth parasites induces immune responses which are characterized by IgE antibody production, tissue and blood eosinophilia, and promote a mast cell response [41, 42]. The immunological role of eosinophils in helminth infections was postulated as early as 1939. Historically, the eosinophil was considered an end-stage cell associated with host defense during helminth infection; however, recent studies have challenged this hypothesis.
Trematodes live in the venous system (e.g., Schistosome species), biliary system (e.g., Clonorchis), gut (e.g., Fasciola), or airway (e.g., Paragonimus). Three main species of schistosomes infect humans are the following: S. mansoni, Schistosoma japonicum, and Schistosoma haematobium. They have a complicated life cycle which involves an intermediate host, fresh water snails, and exist in free living water forms, cercariae, and miracidia. Cercariae penetrate the skin and transform into schistosomula which migrate to the lung. They can elicit blood, tissue, and lung eosinophilia along this course. Eosinophils are able to kill schistosomula via a classical antibody-dependent cellular cytoxicity (ADCC) mechanism [43]. Swartz et al. (2006) studied the S. mansoni infection in two novel mouse models of complete eosinophil lineage ablation. These models showed no difference in worm burden, egg deposition, granuloma size or number, or presence of hepatocellular damage in eosinophil-ablated mice as compared to wild types [44]. However, eosinophils were recruited specifically to granulomata in response to Th2 stimuli, suggesting a role in clearance of cellular debris and tissue remodeling [44]. During S. mansoni infection, adaptive immune responses increase after egg excretion and shift from a predominantly Th1 reaction to a Th2 milieu [37, 38].
Nematodes include intestinal roundworms such as Ascaris lumbricoides, Trichuris trichiura, Necator americanus, and S. stercoralis, tissue nematodes such as Ancylostoma braziliense and Trichinella species and filarial worms such as Wuchereria bancrofti, Onchocerca volvulus, and Brugia malayi. Except for filariae, roundworms reside in the gut. Intestinal nematode infections are among the most common parasitic infections in humans, estimated to affect more than one quarter of the world’s population. They are a significant cause of growth and cognitive delay and malnutrition, particularly in children. Infection occurs either by ingestion of eggs (for A. lumbricoides and T. trichiura) or by skin penetration of infective larvae (for S. stercoralis and N. americanus). The migration of larval and adult worms results in mechanical- and immune-mediated damage. The initial concept of the eosinophil as an end-effector cell was a result of histopathologic tissue evidence of the presence of eosinophils in close proximity to dying parasites [42]. This suggestion has been supported by laboratory demonstration of in vitro killing of worms, such as S. stercoralis, by eosinophils (while in the presence of complement and/or antibodies) as well as their granule products [42]. Early studies using helminth infection models focused on the eosinophil’s ability to facilitate in vitro antibody-dependent cellular toxicity and to aggregate and degranulate in the area of damaged worms in vivo. Eosinophil-mediated parasite expulsion has been observed with S. stercoralis, T. spiralis, and in the mouse model of Trichuris trichiura [43]. Worm expulsion is associated with enhanced Th2 responses such as the production of IL-4, IL-5, and IL-13 [43]. Eosinophils have been shown to be effective at inducing Th2 responses and MHC II-dependent expression to S. stercoralis infection [45]. Murine eosinophils exposed to S. stercoralis antigens demonstrated elevated MHC class II and CD86 and have been shown to drive CD4+ T cells, whether naïve or primed, to generate IL-5 [36]. Padigel et al. (2006) demonstrated that eosinophils, when pulsed with Strongyloides antigen, functioned as antigen-presenting cells and stimulated primed naïve T cells and CD4+ T cells to increase IL-5 production [46]. Unpublished data using a murine airway inflammation model suggests that eosinophils may be as effective as lung dendritic cells in T cell stimulation [36]. This information reinforces the concept that eosinophils are not solely end-stage cells but also play a part in the immunomodulation of the adaptive response [36, 46].
T. spiralis infection occurs after ingestion of raw or undercooked meats which contain a nurse cell-larva complex. After ingestion, larvae migrate to the small intestine and mature into adults and mate. Newborn larvae migrate via the bloodstream to skeletal muscles. The infection results in a pronounced blood and tissue eosinophilia. Despite multiple reports of eosinophil killing of T. spiralis larvae in vitro, studies of eosinophil-depleted mice did not show a difference in parasite survival [47]. Fabre et al. (2009) demonstrated that T. spiralis larvae died in the absence of eosinophils [48]. Huang et al. (2014) further demonstrated a novel mechanism by which the eosinophil provides protection for intracellular, muscle stage T. spiralis infection. Using two strains of eosinophil-ablated mice, their study showed that eosinophils promote the production of IL-10, causing expansion of dendritic cells and CD4+ T-lymphocytes, decreasing the local production of nitric oxide (NO), and thereby enhancing tissue larvae survival [49].
The filarial nematode, O. volvulus, causes river blindness. Infective larvae are transmitted by an insect vector, the black fly. Larval and adult worms reside in subcutaneous and connective tissues creating nodules. Eosinophils infiltrate the subcutaneous nodules and degranulate and release granule proteins including ECP, EDN, and EPO. This has been shown to be dependent on the release of microfilariae from adult worms [43]. The most serious complication of onchocerciasis is ocular disease characterized by keratitis and chorioretinitis. Using a mouse model of O. volvulus keratitis, Pearlman and Hall (2000) demonstrated recruitment of eosinophils to the cornea with a Th2 response and production of IL-4 and IL-5 [50]. Although their role in onchocercal keratitis is unclear, they hypothesize that eosinophils are essential effector cells.
In lymphatic filariasis, caused by W. bancrofti and Brugia species, worms infect lymphatic vessels and lymph nodes resulting in lymphangitis and lymphedema. Hyperresponsiveness to the parasite results in tropical pulmonary eosinophilia, elevated IgE levels, and chronic cough. Using a mouse model, Simons et al. (2005) demonstrated that eosinophil presence is critical for the clearance of primary B. malayi microfilariae infection from both tissue and bloodstream sites. In mice injected intraperitoneally with B. malayi, eosinophils recovered from the peritoneum demonstrated an elevated level of MHC class II expression on their surface [51]. This suggests that eosinophils can be stimulated toward antigen presentation by the local environment [36].
Recent work examining the role of the eosinophil in helminth infections has involved eosinophil-depleted mouse models utilizing knock-out genes or IL-5 neutralization [2, 6, 14]. Animal models using these techniques have suggested the importance of the eosinophil in the reduction of helminth burden and helminth killing, particularly those with tissue-migratory life forms or tissue requirements, including Strongyloides venezuelensis, S. stercoralis, Angiostrongylus, and Onchocerca lienalis [42] However, while IL-5 neutralization models have shown decreased tissue and blood eosinophilia, there is no experimental demonstration of increased susceptibility to infection with certain parasites, including T. spiralis, Trichuris muris, N. brasiliensis, Heligosomoides polygyrus, and Schistosoma [42]. It has also been shown experimentally that IL-5 transgenic mice with persistent eosinophilia display no increased immunity to T. spiralis or S. mansoni [42, 44]. The lack of a human syndrome characterized by eosinophil deficiency has hampered efforts to delineate eosinophil function in defense against helminth infections in vivo [5]. The only eosinophil-specific condition is EPO deficiency which has not been shown to be related to increased susceptibility to or severity of helminth infection in human studies [5]. Human epidemiological studies have correlated a decreased reinfection rates by Schistosoma haematobium and S. mansoni with high eosinophil levels suggesting protective immunity [42]. Despite the body of evidence from animal and human studies, the definitive role of the eosinophil in the parasitic immune response remains uncertain.
Recent studies from several different labs have shifted the paradigm from the traditional perception of the eosinophil as an end-stage cell and provided evidence for one which views the eosinophil as both an initiator and an effector of Th2 immunity [14, 39, 52]. In particular, it has been concluded by several studies that the eosinophil is involved in the early regulation of the immune response through the coordination of cytokines and immature dendritic cells, stimulation of naïve T cells through direct antigen presentation, and consequently, suppression of the Th1 response [36, 39]. There is also some evidence that the eosinophil may provide a role in some helminth species, not in the primary response to acute infection but in the immune response to secondary infections, suggesting a more immunomodulatory role [39, 52].
Viral Infections
There is evidence which suggests that eosinophils may play a role in host response to viral infections, particularly viral respiratory infections. Eosinophils and their granule proteins have been detected in lung tissue and washings following severe infection due to RSV [3]. The ribonuclease activity of eosinophil granules in animals was identified by Archer and Hirsch in 1963 [53]. The potential antiviral properties of granules and their proteins, specifically EDN and ECP, were recognized more recently [9]. EDN and ECP are both members of the ribonuclease (RNase) A gene superfamily [9, 54].
During trials of a formalin-inactivated RSV vaccine in the 1960s, it was found that recipients developed a hypersensitivity reaction with bronchoconstriction and severe pneumonia when subsequently exposed to wild-type RSV infection [3]. Antibody-virus complexes and pronounced eosinophilia were noted at autopsy in the lung tissue of children who died. Gene and cytokine depletion studies highlight the role of Th2 cytokines, IL-4, IL-5, and IL-13, as critical to eliciting eosinophilia in response to formalin-inactivated RSV [55]. It is still not clear whether or not eosinophils were responsible for the pathogenic effects seen following these vaccines trials.
Although eosinophil granules were identified as possessing ribonuclease activity, their antiviral properties were not recognized until more recently [9]. Using a modified quantitative shell vial assay, Domachowske et al. (1998) demonstrated a dose-dependent decrease in infectivity of RSV-A and -B and to a lesser degree, parainfluenza virus-1, -2, and -3, when the viruses were exposed to eosinophils in vitro [56]. The eosinophil secretory ribonuclease, specifically EDN, was shown to possess an antiviral effect [56].
Rosenberg and Domachowske (2001) examined the role of eosinophils in antiviral host defense in vivo using a mouse model [21]. They measured murine immune response to pneumovirus of mice (PVM), a respiratory virus causing an illness similar to severe RSV disease in humans. Infection with PVM resulted in an inflammatory response. They found that eosinophils, along with neutrophils, were recruited to the lung tissue early in the course of infection and following infection and preceded the developments of respiratory symptoms. Following infection, the levels of MIP-1α were found to be increased [20]. There were no changes noted in eotaxin and RANTES in response to infection. Rosenberg, Dyer, and Domachowske (2009) further examined this species-matched pathogen model for RSV infection [55]. They described an ex vivo culture system for generating eosinophils from mouse bone marrow progenitors. PVM was able to replicate in these cultures, and its replication was accompanied by the release of IL-6. The generated cells produced characteristic cytokines and responded to mouse eotaxin-1 [54]. More recently, Percopo et al. (2014) found that eosinophils were antiviral and promoted survival in lethal PVM infection using a mouse model of Th2 cytokine-driven asthmatic inflammation [57].
Davoine et al. (2008) presented a novel in vitro system which they used to study the virus-induced eosinophil mediator release. They demonstrated that human eosinophils were unable to release granule proteins in response to a challenge with respiratory viruses (parainfluenza virus, RSV, or rhinovirus) without interaction with CD4+ T cells and antigen presenting cells. Antigen presenting stimulated the release of EPO or leukotriene from eosinophils when T cells were co-cultured with virus [58]. This finding suggests that eosinophils may play a role in the adaptive host defense to viral pathogens [4]. Phipps et al. (2007) demonstrated eosinophil-dependent viral clearance in a mouse model of RSV infection. They showed that eosinophils express surface and intracellular TLRs associated with antiviral immunity and respond to TLR ligands [34]. Following stimulation with ssRNA, a TLR-7 ligand, eosinophils became functionally activated demonstrated by degranulation and expression of CD11b. This suggests a role for eosinophils in antiviral immunity and viral clearance [5, 34].
In addition to respiratory viruses, eosinophils have been shown to play a role in other viral infections. Hypereosinophilia is often seen in late-stage human immunodeficiency virus (HIV) infection [4]. EDN has been shown to possess inhibitory activity against HIV [4]. Macrophages containing eosinophilic intracytoplasmic inclusions bodies have been noted within hepatic, alveolar, and splenic histopathological samples infected with Ebola virus [59, 60]. Immunohistochemical staining by Wyers et al. (2015) suggests that these eosinophil inclusions are the major sites of viral replication [60]. Other than these observations, the knowledge of the role of the eosinophil in the immune response of Ebola virus remains limited.
Fungal Infections
Eosinophils may play a role in fungal immunity. Fungi are ubiquitous in the environment; they contribute to the development of airway diseases such as asthma and chronic rhinosinusitis and can cause invasive disease. Fungal cell wall components are recognized by cognate receptors and membrane bound receptors such as TLRs. Killing of fungal organisms and host immunity to these pathogens often depends on multiple TLRs and related pathways [61]. Innate immune cells in the airway have been shown to recognize chitins and β-glucans. Yoon et al. (2008) demonstrated that eosinophils react to the environmental fungus, Alternaria alternata, in vitro. Eosinophils appeared to interact with Alternaria by contact-dependent killing, adhering to the surface of the fungus and releasing cytotoxic granule proteins, EDN and MBP-1. They showed that eosinophils utilize a β2-integrin, CD11b, to recognize and adhere to β-glucan, but do not seem to express other fungal receptors or react to chitin [62]. Eosinophils appear to interact with fungal pathogens by contact-dependent killing. They have been shown to release cytotoxic granule proteins such as EDN and MBP-1 into the extracellular milieu and on to the surface of fungal organisms. Kita et al. demonstrated that eosinophils released EDN in response to exposure to Alternaria and Penicillium [61]. Via proteinase-activated receptors (PARs), proteases activate cells and induce production of proinflammatory mediators. Human eosinophils transcribe mRNA for PAR-2 and -3. PAR-2 has been shown to recognize a protease produced by Alternaria [61]. Fungal proteases may also play a role in fungal-mediated eosinophilic inflammation. Inoue et al. (2005) showed that fungal products induced activation and degranulation of human eosinophils in vitro [63]. The interplay between eosinophils and environmental fungi may play a detrimental role in T2-mediated airway diseases such as allergic bronchopulmonary aspergillosis and severe asthma associated with fungal sensitivity [61].
Garro et al. (2010) and Piehler et al. (2011) demonstrated that eosinophils contribute to the inflammatory response to Cryptococcus neoformans infection. Using a rat model, Garro et al. showed that eosinophils phagocytosed the opsonized form of C. neoformans, increased the expression of MHC Class 1 and increased the production of Th1 cytokines IFN-γ, TNF-α, and IL-12p40 [64]. Piehler et al. (2011) showed that eosinophils play an immunoregulatory role in contributing to IL-4 production and modifying T-helper cytokine profiles and the inflammatory response to C. neoformans pulmonary infection in mice [65].
Eosinophils may play a role in the response to other fungal infections. Eosinophilia has been documented to occur during human infection due to Coccidioides immitis [61, 64]. In human infection due to Paracoccidiodes brasiliensis, eosinophils infiltrate lesions and deposit MBP on the organism [61, 64]. Further study is needed to elucidate the role of eosinophils in fungal immunity and response to fungal infection.
Bacterial Infections
Eosinophils have been shown to interact with bacterial pathogens in a variety of ways. Similar to neutrophils, but less effectively, they can engulf bacterial organisms by phagocytosis [13, 66]. This has been demonstrated in vitro with Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes. Eosinophil granule proteins have also been implicated in the killing of another Gram-negative bacterium, Pseudomonas aeruginosa [67]. Eosinophil granule proteins, ECP, EPO, and MBP, possess antibacterial properties [5, 7, 68]. Lehrer et al. (1989) showed that both ECP and MBP-1 exhibit bactericidal activity against S. aureus and E. coli in vitro. Both proteins were able to permeabilize the inner and outer membranes of E. coli, but ECP required the presence of nutrients to be effective [69]. High levels of ECP decreased the number of colony-forming units by 72 % for E. coli and almost 100 % for S. aureus [69]. ECP interacts with artificial lipid membranes; tryptophan residues, W10 and W35, have been associated with this interaction [7]. W35 was found to be necessary for killing both Gram-negative and Gram-positive bacteria [7]. Subsequent studies demonstrated that ECP has an affinity for bacterial lipopolysaccharide and peptidoglycan [70]. Elevated levels of serum ECP have been associated with cases of bacterial sinusitis, tuberculosis, and other bacterial infections [7].
EPO is also associated with bacterial killing. Perrson et al. (2001) found that, under aerobic conditions, eosinophils contributed to the rapid in vitro killing of E. coli and that this activity was related to granule proteins, specifically EPO [68]. In combination with EPO, eosinophil-derived reactive oxygen species can destroy E coli [67]. This bactericidal activity was dependent on the interaction between superoxide generated by NADPH oxidase and EPO [69]. EPO catalyzes the peroxidative oxidation of halides and pseudohalides in plasma along with hydrogen peroxide to form bactericidal hypohalous acids [5].
Interestingly, eosinophils have also been shown to produce extracellular mitochondrial DNA traps in response to bacteria. Yousefi et al. (2008) demonstrated catapult-like ejection of mitochondrial DNA by eosinophils in response to bacteria such as E. coli [31, 71]. This was not related to cell death or apoptosis. The researchers localized extracellular mitochondrial DNA in association with eosinophils in the colons of subjects with Crohn’s disease and in an individual who had a bacterial GI infection. Both ECP and MBP were found to be localized with the extracellular DNA suggesting that bacterial killing was mediated by eosinophil granule proteins [31, 71]. This antibacterial characteristic was confirmed in vivo by studies using IL-5 transgenic, hypereosinophilic mice [31]. In vitro studies were performed which showed that eosinophils required priming by Il-5 or IFN-γ followed by stimulation with lipopolysaccharide, C5a, or eotaxin to release extracellular DNA [31]. The release of mitochondrial DNA by eosinophils was dependent on the respiratory burst, the production of reactive oxygen species. Eosinophils from individuals with chronic granulomatous disease are deficient in NADPH oxidase function and respiratory burst which may contribute to their susceptibility to certain infections [32].
Discussion
Our understanding of the role of the eosinophil in host defense and immunity has greatly expanded over the past few decades, yet many questions remain. Eosinophilia has long been recognized as an indicator of helminth infection. Early on eosinophils were thought to function solely as end-effector cells, degranulating and releasing granule products to kill parasites, but in the process causing tissue damage. The complexity of the host-parasite relationship is becoming better recognized through studies using eosinophil-free transgenic mouse models. Evidence supports a more permissive role for eosinophils in the life cycle of helminths. It has been suggested that helminths recruit eosinophils to help repair the damage they cause in host tissues and that eosinophils may provide protection for certain parasites and enhance their survival. Mouse models have helped to further our understanding, but the lack of a human eosinophil deficiency syndrome has posed challenges.
We now know that eosinophils play a role in the host response to infections due to viruses, fungi, and bacteria as well as parasites. The eosinophil secretory ribonucleases, EDN and ECP, both possess antiviral effects. In addition, eosinophils express surface and intracellular receptors such as TLRs and bind TLR ligands [34]. Eosinophils appear to react to both environmental and pathogenic fungi by a variety of mechanisms including contact-dependent killing, release of cytotoxic granule proteins, and production of proinflammatory mediators [61, 63]. In interactions with bacterial organisms, eosinophils are able to phagocytose, release their granule proteins, and produce extracellular mitochondrial DNA traps [68, 69, 71].
In their interaction with pathogens, eosinophils play a role in both innate and adaptive immune responses. They not only have direct effects on pathogenic organisms but also interact with complement factors and recognize PAMPs and DAMPs. Eosinophils communicate with, have effects on, and are affected by other innate immune cells. The role of the eosinophil in adaptive immunity is now better recognized. Eosinophils can process antigen by functioning as antigen-presenting cells, stimulate T cells by producing T cell polarizing cytokines, and promote humoral responses by interacting with B cells. It is clear that eosinophils possess immunomodulatory capabilities.
Over the past several years, a hypothesis has been put forth in an attempt to explain the increase in allergic and chronic inflammatory disorders in the developed world. The “hygiene hypothesis” suggests that the increased prevalence of these disorders may be the result of dysregulation of the immune system due to decreased exposure to microorganisms and particularly helminths. Helminths have coexisted with human beings and our ancestors for over a million years [72]. Helminths are complex multicellular organisms which have achieved the ability to cause chronic infections by modulating the host immune response [73–76]. Epidemiological data suggests that helminth infections may play a protective role in various inflammatory diseases such as allergic disorders, asthma, inflammatory bowel disease, and multiple sclerosis, and therapeutic applications are being investigated [77]. Given the intimate and long standing relationship between eosinophils and helminths, it is likely that eosinophils are also involved in this shifting epidemiology, particularly in light of the role of eosinophils in tissue remodeling and homeostasis and their anti-inflammatory effects [75].
Conclusions
Once thought to function solely as an end-effector cell in host defense against helminth infections and implicated as a cause of inflammation and tissue damage, the true role of the eosinophil in human health and disease is undoubtedly much more complex. Eosinophils possess many unique characteristics which set them apart from other immune cells. They are able to store preformed mediators such as cytokines and chemokines for selective and specific release by the piecemeal degranulation. They have numerous and unique cell surface receptors allowing them to play a key role in immune regulation. Eosinophils interact with a variety of human pathogens including viruses, fungi, and bacteria as well as helminths. Although they function in a defensive capacity in most circumstances, there is now evidence that eosinophils may interact with helminths in a more symbiotic or collaborative fashion. It may be that the role the eosinophil plays in infection is more immunomodulatory. The research has demonstrated that the eosinophil both responds to and regulates a wide variety of innate and adaptive immune cells, responding to infections and inflammation and acting to maintain homeostasis. Future research will hopefully help to further elucidate the multitude of roles this fascinating cell plays.
References
Gleich GJ (2013) Historical overview and perspective on the role of the eosinophil in health and disease. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 1–11
Rothenberg ME, Hogan SP (2006) The eosinophil. Annu Rev Immunol 24:147–174
Rosenberg HF, Dyer KD, Domachowske JB (2009) Respiratory viruses and eosinophils: exploring the connections. Antivir Res 83(1):1–9
Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22
Hogan SP, Rosenberg HF, Moqbel R et al (2008) Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 38(5):709–750
Blanchard C, Rothenberg ME (2009) Biology of the eosinophil. Adv Immunol 101:81–121
Bystrom J, Amin K, Bishop-Bailey D (2011) Analysing the eosinophil cationic protein—a clue to the function of the eosinophil granulocyte. Respir Res 12:10
Wardlaw AJ (1994) Eosinophils in the 1990s: new perspectives on their role in health and disease. Postgrad Med J 70:536–552
Rosenberg HF, Domachowske JB (1999) Eosinophils, ribonucleases and host defense: solving the puzzle. Immunol Res 20(3):261–274
Spencer LA, Bonjour K, Melo RCN, Weller PF (2014) Eosinophil secretion of granule-derived cytokines. Front Immunol 5:496. doi:10.3389/fimmu.2014.00496
Giembycz MA, Lindsay MA (1999) Pharmacology of the eosinophil. Pharmacol Rev 51(2):213–339
Gleich GJ, Loegering DA (1984) Immunobiology of eosinophils. Annu Rev Immunol 2:429–459
Kay AB (1974) The eosinophil in infectious diseases. J Infect Dis 129(5):606–613
Shamri R, Xenakis JJ, Spencer LA (2011) Eosinophils in innate immunity: an evolving story. Cell Tissue Res 343(1):57–83
Melo RCN, Dvorak AM, Weller PF (2013) Eosinophil ultrastructure. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 20–30
Muniz VS, Weller PF, Neves JS (2012) Eosinophil crystalloid granules: structure, function and beyond. J Leukoc Biol 92(2):281–288
Neves JS, Weller PF (2009) Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21:694–699
Weller PF (2013) Eosinophil structure and cell surface receptors. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 19–20
Lacy P, Moqbel R (2013) Signaling and degranulation. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 206–219
Rosenberg HF (2008) RNase a ribonucleases and host defense: an evolving story. J Leukoc Biol 83(5):1079–1087
Rosenberg HF, Domachowske JB (2001) Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol 70(5):691–698
Rosenberg HF (1998) The eosinophil ribonucleases. Cell Mol Life Sci 54:795–803
Rosenberg HF, Domachowske JB (2001) Eosinophil-derived neurotoxin. Methods Enzymol 341:273–286
Davoine F, Lacy P (2014) Eosinophil cytokines, chemokines and growth factors: emerging roles in immunity. Front Immunol 5:570. doi:10.3389/fimmu.2014.00570
Driss V, Legrand F, Capron M (2013) Eosinophil receptor profile. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 30–38
Matthews AN, Friend DS, Zimmerman N et al (1998) Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A 95(11):6273–6278
Zhu X, Zimmerman N (2013) Eosinophil chemotaxis. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 121–131
Koenderman L (2013) Priming: a critical step in the control of eosinophil activation. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 170–179
Dombrowicz D, Capron M (2001) Eosinophils, allergy and parasites. Curr Opin Immunol 13:716–720
Cook-Mills JM (2013) Eosinophil-endothelial cell interactions during inflammation. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 139–153
Yousefi S, Gold JA, Andina N et al (2008) Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14(9):949–953
Nizet V, Rothenberg ME (2008) Mitochondrial missile defense. Nat Med 14(9):910–912
Weiler JM, Edens RE, Bell CS, Gleich GJ (1995) Eosinophil granule cationic proteins regulate the classical pathway of complement. Immunol 84:213–219
Phipps S, Lam CE, Mahalingam S et al (2007) Eosinophils contribute to innate immunity and promote clearance of respiratory syncytial virus. Blood 110(5):1578–1586
Kvarnhammar AM, Cardell LO (2013) Eosinophil responses to pathogen-associated and damage-associated molecular patterns. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 219–227
Akuthota P, Wang HB, Spencer LA, Weller PF (2008) Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy 38:1254–1263
Sabin EA, Pearce EJ (1995) Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol 155:4844–4853
Sabin EA, Kopf MA, Pearce EJ (1996) Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med 184:1871–1878
Spencer LA, Weller PF (2010) Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol 88:250–256
Teplinsky A, Elishmereni M, Katz HR, Levi-Schaffer F (2013) Eosinophil-mast cell interactions. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 361–367
Nutman TB (2013) Immune responses in helminth infections. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 312–320
Klion AD, Nutman TB (2004) The role of eosinophils in host defense against helminths parasites. J Allergy Clin Immunol 113:30–37
Gentil K, Hoerauf A, Layland LE (2013) Eosinophil-mediated responses toward helminths. (2013). In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 303–312
Swartz JM, Dyer KD, Cheever AW et al (2006) Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood 108:2420–2427
Jacobsen EA, Taranova AG, Lee NA, Lee JJ (2007) Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol 119:1313–1320
Padigel UM, Lee JJ, Nolan TJ et al (2006) Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun 74(6):3232–3238
Gebreselassie NG, Appleton JA (2013) Eosinophils as facilitators of helminth infection. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 321–327
Fabre V, Beitling DP, Bliss SK et al (2009) Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol 182:1577–1583
Huang L, Gebreselassie NG, Garliardo LF et al (2014) Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol 193:4178–4187
Pearlman E, Hall LR (2000) Immune mechanisms in Onchocerca volvulus-mediated corneal disease (river blindness). Parasite Immunol 22:625–631
Simons JE, Rothenberg ME, Lawrence RA (2005) Eotaxin-1-regulated eosinophils have a critical role in innate immunity against experimental Brugia malayi infection. Eur J Immunol 35:189–197
Cadman ET, Lawrence RA (2010) Granulocytes: effector cells or immunomodulators in the immune response to helminth infections? Parasite Immunol 32:1–19
Archer GT, Hirsch JG (1963) Isolation of granules from eosinophil leucocytes and study of their enzyme content. J Exp Med 118:277–291
Rosenberg HF, Dyer KD, Domachowske JB (2009) Eosinophils and their interactions with respiratory virus pathogens. Immunol Res 43(1–3):128–137
Rosenberg HF, Dyer KD, Domachowske JB (2013) Interactions of eosinophils with respiratory virus pathogens. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 281–290
Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF (1998) Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis 177(6):1458–1464
Percopo CM, Dyer KD, Ochkur SI et al (2014) Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123:743–752
Davoine F, Cao M, Wu Y et al (2008) Virus-induced eosinophil mediator release requires antigen presenting and CD4+ T cells. J Allergy Clin Immunol 122:69–77
Martines RB, Ng DL, Greer PW et al (2015) Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 235:153–174
Wyers M, Formenty P, Cherel Y et al (1999) Histopathological and immunohistochemical studies of lesions associated with Ebola virus in a naturally infected chimpanzee. J Infect Dis 179(Suppl 1):S54–S59
Kita H (2013) Antifungal immunity by eosinophils. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 291–299
Yoon J, Ponikau JU, Lawrence CB, Kita H (2008) Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol 181(4):2907–2915
Inoue Y, Matsuwaki Y, Shin S-H et al (2005) Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol 175:5439–5447
Garro AP, Chiapello LS, Baronetti JL, Masih DT (2010) Rat eosinophils stimulate the expansion of Cryptococcus neoformans-specific CD4+ and CD8+ T cells with a T-helper 1 profile. Immunology 132:174–187
Piehler D, Stenzel W, Grahnet A et al (2011) Eosinophils contribute to IL-4 production and shape the T-helper cytokine profile and inflammatory response in pulmonary Cryptococcosis. Am J Pathol 179:733–744
Weller PF, Goetzel EJ (1980) The human eosinophil: roles in host defense and tissue injury. Am J Pathol 100:791–820
Simon H-U, Yousefi S (2013) Eosinophil-mediated antibacterial host defense. In: Lee JJ, Rosenberg HF (eds) Eosinophils in health and disease. Academic Press, Waltham, pp 279–281
Persson T, Andersson P, Bodelsson M et al (2001) Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect Immun 69:3591–3596
Lehrer RI, Sklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ (1989) Antibacterial properties or eosinophil major basic protein and eosinophil cationic protein. J Immunol 142(12):4428–4434
Torrent M, Navarro S, Moussaoui M et al (2008) Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and petidoglycans. Biochemistry 47:3544–3555
Von Kockritz-Blickwede M, Nizet V (2009) Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med 87(8):775–783
Rook GAW (2008) Review series on helminthes, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126:3–11
Versini M, Jeandel P-Y, Bashi T et al (2015) Unraveling the hygiene hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med 13:81–96
Shepherd C, Navarro S, Wangchuk P et al (2015) Identifying the immunomodulatory components of helminthes. Parasite Immunol 37:293–303
Nutman TB (2015) Looking beyond the induction of Th2 responses to explain immunimodulation by helminths. Parasite Immunol 37:304–313
Loke P, Lim YA (2015) Helminths and the microbiota: parts of the hygiene hypothesis. Parasite Immunol 37:314–323
Weinstock JV, Elliott DE (2009) Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis 15:128–133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravin, K.A., Loy, M. The Eosinophil in Infection. Clinic Rev Allerg Immunol 50, 214–227 (2016). https://doi.org/10.1007/s12016-015-8525-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8525-4