Abstract
Autoantibodies specific for idiopathic inflammatory myopathy (myositis-specific autoantibodies (MSAs)) are clinically useful biomarkers to help the diagnosis of polymyositis/dermatomyositis (PM/DM). Many of these are also associated with a unique clinical subset of PM/DM, making them useful in predicting and monitoring certain clinical manifestations. Classic MSAs known for over 30 years include antibodies to Jo-1 (histidyl transfer RNA (tRNA) synthetase) and other aminoacyl tRNA synthetases (ARS), anti-Mi-2, and anti-signal recognition particle (SRP). Anti-Jo-1 is the first autoantibodies to ARS detected in 15–25 % of patients. In addition to anti-Jo-1, antibodies to seven other aminoacyl tRNA synthetases (ARS) have been reported with prevalence, usually 1–5 % or lower. Patients with any anti-ARS antibodies are associated with anti-synthetase syndrome characterized by myositis, interstitial lung disease (ILD), arthritis, Raynaud’s phenomenon, and others. Several recent studies suggested heterogeneity in clinical features among different anti-ARS antibody-positive patients and anti-ARS may also be found in idiopathic ILD without myositis. Anti-Mi-2 is a classic marker for DM and associated with good response to steroid treatment and good prognosis. Anti-SRP is specific for PM and associated with treatment-resistant myopathy histologically characterized as necrotizing myopathy. In addition to classic MSAs, several new autoantibodies with strong clinical significance have been described in DM. Antibodies to transcription intermediary factor 1γ/α (TIF1γ/α, p155/140) are frequently found in DM associated with malignancy while anti-melanoma differentiation-associated gene 5 (MDA5; CADM140) are associated with clinically amyopathic DM (CADM) complicated by rapidly progressive ILD. Also, anti-MJ/nuclear matrix protein 2 (NXP-2) and anti-small ubiquitin-like modifier-1 (SUMO-1) activating enzyme (SAE) are recognized as new DM-specific autoantibodies. Addition of these new antibodies to clinical practice in the future will help in making earlier and more accurate diagnoses and better management for patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specific autoantibodies in systemic autoimmune rheumatic diseases (SARD) are clinically useful biomarkers associated with a particular disease and/or clinical manifestations. Some of them, such as anti-Sm and dsDNA antibodies in systemic lupus erythematosus (SLE), anti-topoisomerase I (topo I; Scl-70) and RNA polymerase III in scleroderma (SSc; systemic sclerosis), and anti-Jo-1 in polymyositis/dermatomyositis (PM/DM) are highly specific for a particular diagnosis and called disease marker antibodies and included in classification criteria of each disease [1, 2]. These autoantibodies are detectable years before clinical manifestation or diagnosis and thus have predictive value for the development of the disease [3]. A majority of disease-associated autoantibodies in SLE and SSc have been known for decades and there are a few classic PM/DM-specific autoantibodies such as anti-Jo-1 [4] and Mi-2 [5, 6]. In recent years, there has not been any identification of new autoantibodies in SLE and SSc with significant impact on clinical medicine although many known classic autoantibodies are continuingly used as standard clinical tests. In contrast, several new and important autoantibody specificities with strong clinical impact, such as antibodies to transcription intermediary factor 1γ (TIF1γ) that are frequently found in cancer-associated DM [7, 8] and anti-melanoma differentiation-associated gene 5 (MDA5) associated with clinically amyopathic DM (CADM) with rapidly progressive interstitial lung disease (ILD) [9–12], have been identified in PM/DM. Autoantibodies that are found in PM/DM are often classified into myositis-specific autoantibodies (MSA) and myositis-associated autoantibodies (MAA) [6, 13, 14]. MSAs are almost exclusively found in PM/DM among SARD, although some antibodies such as anti-aminoacyl transfer RNA (tRNA) synthetases (ARS) are also found in patients classified as idiopathic ILD [15–17]. In this article, we will focus on recent updates on clinical significance and discuss other issues related to MSA.
Myositis-Specific Antibodies and Myositis-Associated Antibodies
Autoantibodies found in patients with PM/DM have been classified into MSA and MAA [6, 13, 18, 19] (Table 1). These are clinically relevant well-accepted concepts. However, there are differences in opinion on antibodies to be included in MSA vs. MAA, in particular to the latter. MSA is defined as autoantibody specificities that are considered relatively specific for PM/DM [6, 13, 18, 21]. The disease specificity is usually defined based on comparison of the prevalence of autoantibodies within various SARD and data on screening of MSA in non-SARD patients are often limited. Detection of certain anti-ARS, such as antibodies to PL-12 [15] and KS [16] in patients with idiopathic ILD, has been reported. One recent study reported that 10 % of patients with idiopathic ILD had anti-ARS [17]. This is not a surprise because the anti-ARS has a strong association with ILD and ILD may precede myositis in some cases. Nevertheless, some patients appear to stay as idiopathic ILD for many years and may not develop myositis. Despite detection of some MSA in non-PM/DM patients, their specificity for PM/DM among SARD is consistent and has clear clinical significance.
There are several classic MSA known for years including antibodies to Jo-1, PL-7, PL-12, EJ, OJ, Mi-2, and SRP [6]. Perhaps, there is not much disagreement to classify them as MSA as they are specific for PM/DM among SARD. In addition, each of them is associated with certain clinical manifestation such as anti-ARS with anti-synthetase syndrome and anti-SRP with treatment-resistant necrotizing myopathy. “Which new autoantibodies should be classified as MSA” is not well established and arguable. Among new antibodies, perhaps anti-TIF1γ/α and anti-MDA5/CADM-140 are the two that have the best evidence to be considered MSA and also both are associated with distinct clinical subsets of DM; anti-TIF1γ/α with cancer-associated DM [7, 8, 22] and anti-MDA5 with ADM or CADM often complicated by rapidly progressive ILD (RPILD) [9, 10]. Other antibodies such as anti-MJ/nuclear matrix protein 2 (NXP-2) and small ubiquitin-like modifier-1 (SUMO-1) activating enzyme (SAE) are less prevalent and limited data are available [14].
Definition of MAA is more vaguely defined than MSA as “autoantibody specificity found in PM/DM but not specific for this diagnosis and may be found in other SARD” [21]. MAA include anti-PM-Scl, anti-Ku, anti-U1ribonucleoprotein (RNP), and U1/U2RNP, which are all associated with a subset of PM/DM-overlap syndrome. They also are associated with muscular involvement in SLE and SSc even if they are not considered PM/DM-overlap syndrome. Anti-Ro52 is classified as MAA in some studies [14]; however, the significance of anti-Ro52 appears to be different from others in this category. Anti-Ro52 is frequently detected in patients with anti-Jo-1, PL-7, PL-12, and others but also found frequently in patients with SLE, SSc, SjS, and other diseases [23].
Anti-Ro60 and anti-La [24–26] and anti-U3RNP [27, 28] are also included in MAA in some articles. Significance of anti-U3RNP may be somewhat similar to that of anti-Ku and PM-Scl as it is sometimes found in SSc-PM-overlap syndrome.
Distribution of anti-Ro52 appears to be more similar to that of anti-Ro60 and anti-Su/Argonaute2 (Ago2) [29] and its association with a subset of PM/DM or muscle involvement is not clear other than the data that it often coexists with anti-ARS [23]. Classifying these antibodies as MAA may be confusing because these three antibodies are also commonly found in SSc and SLE. If these antibodies are classified as MSA, they also may need to be called scleroderma-associated and SLE-associated autoantibodies and would be confusing. In addition, anti-Ro60, Ro52, and anti-Su/Ago2 are the specificities found in unselected populations or healthy individuals relatively frequently (~0.5–1 %) in contrast to rare occurrence (<0.1 %) of MSA and other MAA that is associated with overlap syndrome [20].
Spectrum of Muscle and Skin Involvement in Inflammatory Myopathy and Myositis-Specific Autoantibodies
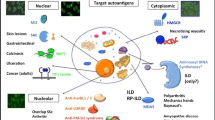
Spectrum of muscle and skin involvements in inflammatory myopathy varies from muscle disease without skin disease (Fig. 1, PM, left), involvement of both muscle and skin with different degree of each (DM, middle) to skin disease with minimal muscle involvement (CADM), or no muscle involvement (ADM). Anti-ARS is detected in both PM and DM and occasionally in ADM. Most anti-SRP-positive patients have PM and a majority of anti-Mi-2, TIF1γ/α, MJ/NXP-2, and SAE are found in DM. Anti-MDA5 is mainly found in CADM/ADM and prominent muscle disease is uncommon. Anti-Jo-1 and other ARS, anti-SRP, and anti-Mi-2 are considered classic MSA. The several new MSA described recently including anti-TIF1γ/α, anti-MJ/NXP-2, anti-SAE, and anti-MDA5, are all mainly detected in DM and each of them is associated with a unique subset. Although all MSA are specific for PM or DM, presence of more than one MSA in each patient is uncommon for unknown reasons [6, 14]. Non-MSA autoantibodies associated with PM/DM overlap syndrome such as anti-Ku, PM-Scl, U1RNP, and U1/U2RNP are not shown here, but they can be found in both PM and DM (Table 1). Coexistence of anti-U1RNP with anti-Ku or anti-ARS is relatively common.
Myositis-Specific Antibodies
MSA specificities and characteristics of target autoantigens are summarized in Table 2. Prevalence and association with subset of PM/DM and clinical association are summarized in Table 3. This section will mainly focus on clinical significance of MSA in adult PM/DM patients because the prevalence and clinical association of MSA are quite different in juvenile DM (JDM).
Anti-ARS
Anti-ARS are a group of autoantibodies that recognize the cytoplasmic amino acid-charging enzymes, aminoacyl tRNA synthetases. So far, autoantibodies to eight of them including histidyl (Jo-1), threonyl (PL-7), alanyl (PL-12), glycyl (EJ), isoleucyl (OJ), asparaginyl (KS), phenylalanyl (ZO), and tyrosyl (YRS/HA) tRNA synthetases have been reported [14]. Anti-Jo-1 antibodies are the first MSA and anti-ARS described in 1980, defined by DID using calf thymus extract as antigen [4]. Anti-Jo-1 was detected in 30.8 % (8/26) of PM, 4.5 % each of DM (1/22), and overlap syndrome (1/22, 1/11 of PM-SSc overlap) but not in SLE, SSc, rheumatoid arthritis (RA), and other diseases. Arnett et al. confirmed disease specificity of anti-Jo-1 (30 % (6/20) in PM and 13 % (2/16) of DM) but did not find clinical features associated with anti-Jo-1 [31]. Yoshida et al. confirmed disease specificity of anti-Jo-1 in Japanese patients, finding in 28 % of PM/DM (9/32, 8 PM and 1 DM) and 2/28 overlap syndrome but none in SLE, SSc, and RA [32]. Importantly, they for the first time noted unique clinical features associated with anti-Jo-1, later known as anti-synthetase syndrome. All nine cases of anti-Jo-1 (+) PM/DM had ILD and polyarthritis, and hypocomplementemia and rheumatoid factor were also more common compared with anti-Jo-1-negative patients.
Following studies confirmed an association of anti-Jo-1 antibodies with a unique clinical subset characterized by myositis, ILD, arthritis, mechanic’s hands, and Raynaud’s phenomenon [13]. Autoantibodies to the other seven ARS have been described using immunoprecipitation (IP) technique [14]. Interestingly, autoantibodies to all other aminoacyl tRNA synthetases are associated with the same syndrome, which has been designated anti-synthetase syndrome [13]. Anti-Jo-1 antibodies, usually found in 15–25 % of PM/DM patients, are by far the most common among anti-ARS antibodies; all others are usually found only in 0.5–6 % of patients [13, 18, 33–36]. The reported frequency of various anti-synthetase antibodies is very similar in all previous studies regardless of the race, ethnicity, or nationality of the subjects [18, 34–36], except for a report suggesting clustering of anti-PL-12 in southern USA [37] and high prevalence of anti-PL-7 in a Japanese cohort [38]. Therefore, whether there is any difference in clinical manifestations between patients with different anti-ARS autoantibodies has not been studied extensively. Nevertheless, some differences in clinical manifestations between different anti-synthetase antibodies have been described. Reports on anti-PL-12 and anti-KS suggested that they are common in ILD without myositis [15, 16, 37].
A high frequency (5/7, 71 %) of PM/DM-SSc overlap in anti-PL-7-positive Japanese patients was reported [39]. However, it was not confirmed by another Japanese study [38] and also was unusual in other studies [33]. Yamasaki et al. found anti-PL-7 in 17 % of their PM/DM cohort and reported an association with milder muscular involvement [38]. More recently, several studies reporting on differences in clinical features of different anti-ARS have been published. Kalluri et al. analyzed clinical features of 31 anti-PL-12-positive patients and reported that anti-PL-12 is strongly associated with ILD but less so with myositis and arthritis and 3/31 cases were idiopathic ILD [40], consistent with an earlier study [15, 37]. Labirua-Iturburu et al. reviewed their 18 cases of anti-PL-7-positive patients and found 50 % of patients had pericardial effusion, in addition to common features of anti-synthetase syndrome [41]. Marie et al. compared 75 anti-Jo-1 (+) patients vs. 20 anti-PL-7 (n = 15)/PL-12 (n = 5) (+) patients and reported that the latter had milder muscle involvement and less recurrence of muscle disease [42, 43]. In contrast, anti-PL-7/PL-12 was associated with early and severe ILD and gastrointestinal manifestations. Aggarwal et al. compared 122 anti-Jo-1 vs. 80 non-Jo-1 anti-ARS and reported that anti-Jo-1 (+) patients had more myositis (83 vs. 40 %) and less overlap/undifferentiated connective tissue disease (UCTD; 17 vs. 47.5 %) [44]. The non-Jo-1 group had delayed diagnosis and low survival rate. Hamaguchi et al. compared clinical features of patients with different anti-ARS and reported that while most patients with anti-Jo-1, EJ, and PL-7 had a diagnosis of PM/DM, it was CADM or ILD for anti-PL-12 and ILD for anti-KS and anti-OJ [45].
In summary, patients with any anti-ARS have a similar clinical syndrome known as anti-synthetase syndrome; however, several recent studies suggest that antibodies to non-Jo-1 ARS are associated with earlier and more severe ILD and poor prognosis compared with anti-Jo-1 (+) patients. Also, non-Jo-1 anti-ARS (+) patients are more likely to have ILD without typical myositis.
Anti-SRP
SRP antigen is a complex of 7SL RNA and several proteins including 72, 68, 54, 19, 14, and 9 kD, playing a role in regulating the translocation of proteins across the endoplasmic reticulum. Anti-SRP antibodies were originally described by IP in a patient with PM [46]. Another study also identified anti-SRP antibodies in two patients (a Japanese patient with typical PM and a patient with non-destructive arthritis). The former serum was described in an earlier study as immunoprecipitating 7.5S RNA [47], which was identified as 7SL RNA of the SRP complex [48]. The first large case series of anti-SRP-positive cases by Targoff et al. reported 4 % in PM/DM and 18 % of PM/DM patients with anti-cytoplasmic antibodies other than anti-Jo-1 from the analysis of 265 cases of PM/DM [49]. All 13 cases were PM and anti-SRP was associated with classic PM and some were unusually severe and/or rapid onset. Low prevalence of ILD, arthritis, and Raynaud’s phenomenon were associated with this subset [49]. Hirakata et al. found anti-SRP in 4 % (3/52) of PM/DM and a case was DM [36]. Kao et al. reported 19 cases of anti-SRP from analysis of 134 PM, 129 DM, and other SARD (predominantly SSc, n = 790). Anti-SRP was found in 16 cases of pure PM (12 % in PM, 16/134) and not in DM (0/129); however, three were without myositis (two with SSc and one with anti-synthetase syndrome) [50]. Hengstman et al. described 23 anti-SRP-positive cases, 20 PM and 3 DM. Muscle pathology of anti-SRP-positive patients was characterized by the presence of necrotic muscle fibers and no inflammatory infiltrates [51] similar to the findings by Miller et al. [52]. Takada et al. analyzed 23 anti-SRP-positive patients, 21 were PM (2 with RA), 3 DM, and 2 RA without myositis [53]. Clinical and pathological characteristics were consistent with other studies. Benveniste et al. reported correlation of anti-SRP antibody levels determined by a newly developed addressable laser bead assay (ALBIA) to one of the SRP components SRP54 and creatine kinase (CK) levels [54]. Other studies validated anti-SRP54 enzyme-linked immunosorbent assay (ELISA) vs. IP and showed a parallel change of anti-SRP54 levels and serum CK levels [55, 56]. Since anti-SRP antibody itself is not considered directly pathogenic, it is possible to consider the simple result of treatment with steroid, rituximab, and immunosuppressive agents for myositis have reduced both CK levels and anti-SRP antibody levels.
Although coexistence of more than one MSA in each patient is uncommon, there are a few reports on coexistence of anti-SRP and anti-ARS; two cases of anti-SRP with anti-Jo-1 [57, 58] and cases with anti-PL-12 were reported [50, 59]. MSA generally recognize protein components of the target antigen; however, one study reported that 50 % (5/10) of Japanese and 5 % (1/22) of North American patients with anti-SRP had antibodies directed against 7SL RNA [60].
Several recent studies focused on the association of anti-SRP antibodies with a unique histological subset of PM/DM, necrotizing myopathy [51, 55, 56]. Prevalence of necrotizing myopathy in patients with anti-SRP antibodies in the literature is summarized (Table 4). Suzuki et al. analyzed clinical features of 100 anti-SRP antibody-positive cases, selected based on IP of 7SL RNA [56]. Eighteen of them were IP positive but negative for anti-SRP54 ELISA. Histologically, 84 % had necrotizing myopathy while 14 % had non-specific myositis and one each had PM or DM pathology, supporting findings in other studies that a majority of anti-SRP-positive cases had necrotizing myopathy.
Necrotizing myopathy is a heterogeneous pathological category including autoimmune (autoantibody associated), drug-induced, paraneoplastic, viral infections, and others. A few studies started from necrotizing myopathy and examined the sensitivity of anti-SRP antibodies [52, 55, 61, 62]. Prevalence of anti-SRP antibodies in patients with necrotizing myopathy is summarized (Table 5). Anti-SRP antibody was the most frequent etiology that 53 % (34/64) of patients with necrotizing myopathy had [63]. In contrast, none of sera from 23 patients with necrotizing myopathy were tested positive for anti-SRP by line immunoassay (LIA) in another study [61]. In Chinese patients with PM/DM, 16/123 cases (13 %) with necrotizing myopathy had anti-SRP antibodies detected by LIA [62]. Thus, two studies using IP to detect anti-SRP antibodies showed prevalence of 41–52 % [55, 63] while the other two studies using LIA showed low prevalence of 0–13 % [62, 64] in patients with necrotizing myopathy. Whether the difference is due to genetic or environmental factors, selection bias of patients, or different immunoassay remains to be clarified.
In summary, the majority of literature support that anti-SRP is specific for PM and associated with treatment-resistant severe myopathy, which is histologically characterized by necrotizing myopathy [50–53, 62].
Anti-Mi-2
Autoantibodies to Mi-2 were originally defined by DID using calf thymus extract as antigen and reported as the first specific serologic marker of DM [5]. Eleven of 52 DM patients were positive by DID but none detected in PM though a few more sera were detected positive by ELISA in other diseases [5]. Love et al. reported anti-Mi-2 in 13 % (10/79) DM and 8 % (1/13) cancer-associated myositis but again none in PM, confirming specificity for DM [18]. Anti-Mi-2 was associated with classic DM skin rash, good response to steroid, and good prognosis. Components of Mi-2 antigen were characterized by IP and western blot (WB) [65] and later identified as nucleosome remodeling deacetylase complex (NuRD) [66].
All previous studies confirmed that anti-Mi-2 antibodies are nearly specific for DM when tested by DID or IP, though positive sera in PM may also be found in particular by ELISA [5, 67, 68] (Table 6). Clinical studies are consistent in showing that anti-Mi-2 is associated with classic features of DM including Gottron’s papules, heliotrope rash, shawl sign, and V-sign, but a risk to develop clinically significant ILD is low and cancer is uncommon [5, 18, 68, 69]. This subset of patients responds well to steroid therapy and has a good prognosis [18]. However, clinical characteristics associated with anti-Mi-2 have not been studied extensively due to limited availability of the immunoassays and relatively low prevalence in PM/DM. One study from Mexico compared clinical features of anti-Mi-2 positive patients vs. others as the prevalence of anti-Mi-2 in this cohort was high (35 % in PM/DM, 45 % in DM) [77]. High CK level before treatment was noted in anti-Mi-2 (+) DM vs. anti-Mi-2 (−) DM (initial CK, >1000 IU/L, 100 vs. 52 %, P < 0.0001; initial CK, >5000 IU/L, 54 vs. 14 %, P < 0.005). However, anti-Mi-2 (+) patients responded well to steroid therapy and prevalence of normal CK at last visit was comparable between groups (45 vs. 69 %).
Although all studies showed the specificity of anti-Mi-2 for DM, reported prevalence of anti-Mi-2 in different studies is quite different even in the same country [77] as summarized in Table 6. Prevalence of anti-Mi-2 in DM varies 5–27 % in Italy and 2–19 % in Japan. In a study that examined the role of environmental factors in the production of anti-Mi-2, prevalence was as low as 3.2 % (Montreal, Canada), 3.7 % (Warsaw, Poland), and 5.4 % (Bethesda, USA) in some areas while it was 60 % (Guatemala City, Guatemala), 36.1 % (Mexico City, Mexico), and 23.1 % (Santiago, Chile) in Central and South American countries [78]. Another study also showed 59 % prevalence of anti-Mi-2 in DM patients in Mexico City, but it was only 12 % in Guadalajara [77], suggesting a role of factors other than ultraviolet (UV). Correlation of UV radiation of the area with development of DM and production of anti-Mi-2 antibodies was suggested [78, 79]. However, roles of genetic vs. environmental factors responsible for the production of anti-Mi-2 will need further studies because the majority of countries with very high prevalence of anti-Mi-2 are in Central and South America [77, 78].
Anti-MDA5/CADM140
It has been known for years that a subset of DM patients may have typical DM skin rash but have little or no muscle involvement, and these patients are called ADM or CADM [80, 81]. A subset of CADM patients may also develop RPILD, resistant to treatment and with poor prognosis. DM patients with these uncommon features did not express the classic MSAs or marker antibodies until Sato et al. described a new autoantibody called anti-CADM140 that is associated with CADM and ILD [10]. In this report, Sato et al. described 53 % (8/15) of Japanese CADM patients with anti-CADM140 antibodies, but none in 61 PM, 27 classic DM, other SARD, or idiopathic pulmonary fibrosis, thus this antibody is considered specific for CADM. Furthermore, 50 % (4/8) of anti-CADM140-positive DM had RPILD vs. 6 % (2/34) in anti-CADM140-negative DM. The target antigen was later identified as a cytoplasmic viral double-stranded RNA (dsRNA) receptor involved in innate immune response, called MDA5 or interferon induced with helicase C domain 1 (IFIH1) [9]. The possible mechanism leading to the autoimmune response is the binding of the viral dsRNA to MDA5 and the consequent induction of type I interferon responses. Another study from different institutes in Japan confirmed the strong association of anti-MDA5/CADM140 with CADM, RPILD, poor prognosis, high prevalence of liver dysfunction, and increased serum levels of ferritin [11, 12, 82–84]. In particular, Gono et al. reported that high ferritin levels are associated with poor prognosis for ferritin levels >1600 ng/ml [83]. No overlap with other SARD is present in DM patients with anti-MDA5/CADM140-positive antibodies, as shown by Hoshino et al., who reported that 65 % (20/31) of anti-MDA5/CADM140 positives were CADM and only one had another diagnosis (SSc) [11].
Anti-MDA5/CADM140 was also associated with ILD in JDM [85], and a few cases of apparent myositis were reported in anti-MDA5/CADM140-positive patients [12, 85].
Overall, studies on anti-MDA5/CADM140 in DM showed a prevalence ranging from 3 to 58 %, and this percentage increases up to 100 % when only CADM patients are considered. Moreover, prevalence and specificity of autoantibodies in PM/DM are quite different between studies from different countries or even within the same countries [38, 77], and this is true also for anti-MDA5/CADM140 antibodies. All earlier reports on anti-MDA5/CADM140 antibodies were on cohorts of DM patients in Asian countries, mainly Japan and South Korea [9–12, 83, 84, 86–88], except for some recent studies from the USA [89, 90]. In one of the two reports on anti-MDA5/CADM140 antibodies in cohorts of American DM patients, the authors also identified a unique cutaneous phenotype characterized by skin ulcerations, tender palmar papules, or both, and by severe arthritis [89]. In another US study, 6.9 % (11/160) of DM had anti-MDA5/CADM140 but 6/11 had overt clinical myopathy and 8/11 had ILD [90]. Their anti-MDA5/CADM140 patients were similar to anti-synthetase syndrome and were not associated with RPILD, in contrast to Asian studies.
There have been two recent studies from Europe. In a study from Italy, anti-MDA5/CADM140 antibodies were detected in 7 % (5/76) of adult European Caucasian patients with PM/DM, and they were the second most frequent specificity after anti-MJ antibodies (8/76, 11 %) [91]. All five anti-MDA5/CADM140 (+) patients had a diagnosis of DM, with CADM and normal CPK levels similar to reports from Asian countries [9], and a significantly higher prevalence of ILD compared with anti-MDA5/CADM140 (−) DM patients. Another study from Spain reported 12 % (14/117, 8 were CADM) prevalence of anti-MDA5/CADM140 in 117 DM patients [92]. Eight of 14 anti-MDA5/CADM140-positive patients had RPILD, similar to Asian cohorts [10] but different from US cohorts [90]. Anti-MDA5-positive patients with ILD had lower survival rates vs. anti-ARS-positive ILD. Among cutaneous manifestations, only panniculitis was significantly associated with anti-MDA5/CADM140. These differences in the prevalence and clinical features associated with anti-MDA5/CADM140 suggest the importance of accumulating data on prevalence and clinical association of MSAs from different ethnicities.
Muro et al. showed that anti-MDA5/CADM140 antibody levels can be related to disease activity, as they decrease and become negative by ELISA in nine of ten patients considered in remission after treatment, whereas the level of control anti-diphtheria toxoid DT antibodies did not change [87]. Sato et al. reported that anti-MDA5/CADM140 antibody levels in patients who responded to therapy and survived was significantly lower than the patients who did not respond and died [93]. These are very interesting findings as they suggest that anti-MDA5/CADM140 antibody levels may be used as a biomarker of disease activity and to predict response to therapy.
In summary, most reports describe that anti-MDA5/CADM140 antibodies are specific for DM and a majority of patients have CADM and high prevalence of RPILD leading to poor prognosis [9–11].
Anti-TIF1γ/α and β
Targoff et al. identified a new autoantibody called anti-p155/140 that immunoprecipitated a set of 155 and 140 kD proteins and has a striking association with cancer-associated DM [7]. P155 was identified as TIF1γ and published in an abstract [94]. Fujimoto et al. later confirmed p155 as TIF1γ and identified p140 as TIF1α [8] and anti-p155/140 has now been called anti-TIF1γ/α. In addition, anti-TIF1β was also identified in combination with anti-TIF1γ/α in some cases [8, 95]. TIF1α, TIF1β, and TIF1γ belong to the TIF family of transcription cofactors and are part of a tripartite motif superfamily (TRIM24, TRIM28, and TRIM33, respectively) [96].
In the original study, anti-p155/140 was found in 75 % (6/8) of patients with cancer-associated myositis though it was also detected in 29 % of JDM, 33 % of overlap syndrome, and 21 % of adult DM [7]. When the clinical features of anti-p155/140 patients were compared with those with anti-ARS, prevalence of fever, Raynaud’s phenomenon, arthritis, ILD, and mechanic’s hand was lower, in particular none of the 16 anti-p155/140 patients had ILD. V-sign rash, shawl sign rash, and malignancy was significantly higher in anti-p155/140 patients [94]. The association of anti-TIF1γ/α with DM, in particular with cancer-associated DM has been confirmed in many reports from the USA [7], UK [97], Spain [98], Japan [8, 11, 82, 99], and South Korea [100]. Almost all anti-TIF1γ/α-positive patients have DM with typical skin rash but low prevalence of ILD. Prevalence of anti-TIF1γ/α antibodies in adult inflammatory myopathy (Table 7) and association of anti-TIF1γ/α antibodies with malignancy (Table 8) in the literature are summarized. Prevalence of anti-TIF1γ/α in cancer-associated DM is 22–100 %, and all reported statistically significant associations of anti-TIF1γ/α with malignancy in DM (Table 7) except one study [101] that reported high prevalence of malignancy in overall DM. Prevalence of malignancy in anti-TIF1γ/α antibody-positive patients was 42–100 % (Table 8). Meta-analysis indicated sensitivity of anti-TIF1γ/α for cancer-associated DM as 0.50 to 1.00, combined 0.78 (95 % confidence interval (CI), 0.45–0.94), whereas specificity was 0.79–1.00, combined 0.89 (95 % CI, 0.82–0.93) [22]. However, it should be noted that association of anti-TIF1γ/α with cancer does not seem to apply to children [97] or young adults affected by DM [8].
Anti-MJ/NXP-2
Anti-MJ antibodies recognize a ~140-kD nuclear protein called nuclear matrix protein 2 (NXP-2; also known as MORC3) [102–104], which plays an important role in diverse nuclear functions such as RNA metabolism and maintenance of nuclear architecture [102]. NXP-2 localizes in the promyelocytic leukemia (PML) nuclear bodies, where it recruits and activates p53 to induce cellular senescence [103, 105]. Anti-MJ/NXP-2 antibodies were originally described in 1997 in a subset of patients with JDM, who were characterized by severe refractory DM with polyarthritis, joint contractures, severe calcinosis, and intestinal vasculitis [106]. More than 10 years later, two studies in JDM were published [107, 108]. In the first study of a cohort of Argentine pediatric myositis patients, anti-MJ/NXP-2 antibodies were the most prevalent specificity (25 % of cases), associated with muscle contracture, atrophy, and significant compromise of the functional status [107]. In the other study, based on the JDM National Registry and Repository for UK and Ireland, 23 % prevalence of anti-MJ/NXP-2 in juvenile myositis patients was reported, and they were all JDM with significantly higher prevalence of calcinosis (54 vs. 15 % in anti-MJ-negative patients) [108].
Anti-MJ/NXP-2 antibodies in adult patients with myositis was first reported in a British cohort [109]. Anti-MJ/NXP-2 were found in 3 % (11/393) of PM/DM and 6 % in DM and none in PM. In anti-MJ/NXP-2-positive patients, typical DM skin rash was common and higher prevalence of ILD (64 vs. 28 % in anti-MJ/NXP-2-negative DM) was noted, but only one had calcinosis and none had cancer. This is in contrast to what was reported in another study of anti-MJ/NXP-2 antibodies in adult myositis patients in Japan. The authors estimate 1.6 % (8/507) prevalence of anti-MJ/NXP-2 [110], and they show higher prevalence (four of eight cases) of malignancy associated with this autoantibody [110]. A US study also reported anti-MJ/NXP-2 in 17 % (37/213) of adult DM patients and anti-MJ/NXP-2 was specifically associated with cancer in males (odd ratio, 5.78; 95 % confidence interval, 1.35–24.7) [111]. No similar finding on the association between anti-MJ/NXP-2 antibodies and higher risk of cancer was detected in other cohorts. A French-Canadian study reported anti-MJ/NXP-2 in 8 % (2/26) of adult DM cases but none in PM [112]. Another recent study in adult DM patients in the USA reported anti-MJ/NXP-2 in 13 % (16/126) of cases and its association with calcinosis (odds ratio, 15.52; 95 % CI, 2.01–119.90) in this adult cohort [113]. In an Italian study [75], anti-MJ/NXP-2 antibodies were the most prevalent specificity (30 % in DM and 17 % in PM/DM) detected at a prevalence similar to the one observed in JDM Argentinian [107] and UK/Ireland [108] cohorts but much higher than other adult DM studies [109, 110]. Lack of malignancy in anti-MJ/NXP-2-positive patients in the Italian cohort [75] may be related to their young age compared with anti-MJ/NXP-2 (+) patients with malignancy in other cohorts [110]. This may be similar to a strong association of anti-TIF1γ/α antibodies with malignancy in middle to old age DM but not in children or young adults [8, 97].
As shown by these reports, prevalence of anti-MJ/NXP-2 antibodies can be very different in studies performed worldwide, and this could be due to different ethnic background, influence of environmental factors on autoantibody production, or simply for technical reasons. Genetic and/or environmental factors within Caucasians may be important variables, since the prevalence of anti-MJ/NXP-2 within Caucasians seems different.
Anti-SAE
Antibodies to SAE were first identified by Betteridge et al. in 2007 in two DM patients [114]. The target antigens of 40 and 90 kD heterodimer proteins were identified as small ubiquitin-like modifier-activating enzyme A subunit (SAE1) and the SUMO-1 activating enzyme B subunit (SAE2), respectively. These are enzymes involved in the post-translational modification of specific proteins known as SUMOylation. Anti-SAE was found in 10 % (2/20) DM but none in 24 PM patients. In the following study in patients recruited to the Adult Onset Myositis Immunogenetic Collaboration, anti-SAE was found in 4 % (11/266) of PM/DM and 8 % in DM as all anti-SAE positives were DM patients [115]. Among 11 patients with anti-SAE, a high frequency of cutaneous lesions including heliotrope (82 %) and Gottron rash (82 %) were identified. Nine of the 11 patients had systemic features (82 %), and dysphagia was noted in 78 % (seven of nine). A majority (78 %) of them presented with skin disease prior to onset of myositis. There are only a few reports from other countries. A study from Italy also found anti-SAE in 7 % (5/73) of DM patients [116]. Prevalence appears low in Japanese as Fujimoto et al. found 1.5 % (7/456) [117] and Muro et al. reported 1.8 % (2/110) prevalence [118] in Japanese DM patients. Clinical features in seven Japanese anti-SAE-positive patients were similar to those in a UK study except that ILD was common in Japanese patients (71 %) [117] vs. 18 % in the UK study (P < 0.05) [115].
Immunoprecipitation Detection of Myositis-Specific Autoantibodies
IP analysis of protein components of autoantigens using 35S-methionine-labeled cell extract is a very powerful technique that allows screening for almost all known PM/DM autoantibodies in a single assay. Combining the protein analysis with the analysis of RNA components of the autoantigens by urea-PAGE and silver staining is useful for confirmation of RNA-protein complex. Protein IP of MSA and other autoantibodies seen in PM/DM is shown (Fig. 2). Molecular weights of the target antigens are summarized in Table 2. Many myositis autoantibodies can be interpreted almost conclusively while some may require additional techniques for confirmation [1]. Identifying a multiprotein or multiprotein-nucleic acid complex characterized by a set of proteins, such as anti-OJ, SRP, Mi-2, TIF1γ/α, and SAE is usually not so difficult though IP of unrelated proteins is relatively common in certain molecular weight ranges.
Immunoprecipitation analysis of protein components of autoantigens recognized by autoantibodies in PM/DM. 35S-methionine-labeled K562 cell extract was immunoprecipitated by sera from patients with PM/DM. Main components of each autoantigen are indicated by arrowheads. Jo-1 histidyl tRNA synthetase, EJ glycyl tRNA synthetase, PL-7 threonyl tRNA synthetase, PL-12 alanyl tRNA synthetase, OJ isoleucyl tRNA synthetase, multienzyme complex, KS asparaginyl tRNA synthetase, SMN survival of motor neuron, SRP signal recognition particle, PM-Scl polymyositis-scleroderma, Mi-2, Mi-2 + , TIF1α, TIF1γ/α transcription intermediary factor 1α, TIF1β transcription intermediary factor 1β, MJ/NXP-2 nuclear matrix protein 2, MDA5 melanoma differentiation-associated gene 5, SAE small ubiquitin-like modifier 1 (SUMO-1) activating enzyme, Ku, NHS normal human serum
Among MSA listed in Table 3, anti-Jo-1 and Mi-2 were originally defined by DID while all others were defined by IP analysis of proteins, in combination with analysis of RNA components by IP for anti-ARS and anti-SRP. IP is still considered a gold standard for most of them. An apparent weakness of IP is anti-Jo-1, as the Jo-1 antigen is seen as a relatively thin uncharacteristic band in IP, in contrast to other ARS. In addition, it comigrates with IgG heavy chain, making it more difficult to clearly observe. IP analysis of RNA component to confirm the presence of tRNA is helpful; however, it does not tell the tRNA specificity; it only tells immunoprecipitation of “some” tRNA and cannot confirm if it is histidyl tRNA or not. Practically, IP of ~50 kD protein consistent with Jo-1 and the presence of tRNA by RNA analysis is reasonable to strongly suggest it is Jo-1. IP of ~50 kD protein and positive anti-Jo-1 ELISA also is practical.
IP of ~140 kD protein that exactly comigrates with MDA5 or MJ/NXP-2 immunoprecipitated by a reference serum is reasonable and correctly interpreted in most cases. Nevertheless, it will be ideal to confirm the specificity of an uncharacteristic single band such as MDA5 and MJ/NXP-2 with additional test such as ELISA, western blot, or IP-western blot. IP and ELISA using biotinylated in vitro transcription/translation product [11] and IP of 35S-methionine-labeled in vitro transcription/translation product [113] have also been used for these antibody specificities.
SRP72/68, SRP54, SAE1 (40 kD), and SAE2 (90 kD) are confirmed by IP in most cases but there are many proteins of similar size recognized by human sera, and confirmation of exact comigration of the proteins compared with a reference serum may be necessary. For anti-SRP, confirmation by coimmunoprecipitation of small subunits of 19, 14, and 9 kD appears more characteristic than identifying SRP72/68 and SRP54.
Immunofluorescence ANA Staining by Myositis Autoantibodies
Some of the representative immunofluorescence ANA-staining patterns by autoantibodies seen in PM/DM using HEp-2 cells are shown (Fig. 3). Anti-U1RNP antibodies show a coarse speckled pattern (Fig. 3a). Anti-Mi-2 (Fig. 3b), anti-TIF1γ/α (Fig. 3c), and anti-TIF1β (Fig. 3d) all show fine speckled nuclear staining, and the difference in staining pattern is not apparent though anti-Mi-2-positive sera show brighter staining in general. Anti-SAE also shows fine speckled nuclear staining (Fig. 3e). PML body staining is clearly observed by some anti-MJ/NXP-2-positive sera (Fig. 3f) [75], but it may not be always clear and only nuclear fine speckled staining may be observed by some sera (Fig. 3g). Anti-SMN stains a few nuclear dots known as Cajal bodies (Fig. 3h). Anti-PM-Scl antibodies show homogenous nucleolar staining with fine speckled nuclear staining (Fig. 3i, j) though nucleolar staining may be less clear in some cases (Fig. 3j). Anti-U3RNP antibodies show clumpy nucleolar staining (Fig. 3k). Antibodies to Jo-1 (Fig. 3l), PL-7 (Fig. 3m), PL-12 (Fig. 3n), and other ARS stain cytoplasm in a fine speckled pattern but the staining may be weak or absent in some cases. Anti-SRP antibodies show fine speckled cytoplasmic staining (Fig. 3o). MDA5 antigen localizes to the cytoplasm and positive sera may stain the cytoplasm but it is often very weak or negative (Fig. 3p)
Immunofluorescence antinuclear antibodies using sera from patients with PM/DM. HEp-2 ANA slides were stained using sera from patients with PM/DM. a Anti-U1RNP, b anti-Mi-2, c anti-TIF1γ/α, d anti-TIF1β, e anti-SAE, f, g anti-MJ/NXP-2, h anti-SMN, i, j anti-PM-Scl, k anti-U3RNP, l anti-Jo-1, m anti-PL-7, n anti-PL-12, m anti-SRP, p anti-MDA5
Immunofluorescence ANA Pattern and Autoantibody Specificities in PM/DM
IP is a gold standard for detection of the majority of MSA; however, this technique is available only at limited research laboratories. ELISA for anti-Jo-1 and U1RNP are widely available and anti-ARS ELISA that detects anti-Jo-1, EJ, PL-7, PL-12, and KS is available in Japan; mixed antigens are used, and individual specificity is not available [17]. Line immunoassay and other new types of assays have been used for some of the MSA; however, validation of the assay compared with a gold standard is limited and discrepancies in test results have been reported [119]. Thus, being familiar with the localization of the target antigens and immunofluorescence ANA pattern can be useful in two ways. First, specificities of autoantibodies that have higher chance of positivity for a particular patient can be considered when clinicians order specific autoantibody tests selected based on immunofluorescence pattern. Second, when specific autoantibody test result is inconsistent with the ANA pattern, false-positive test result may be considered, e.g. positive anti-Mi-2 with cytoplasmic staining can be considered inconsistent [1].
ANA pattern and corresponding myositis autoantibody specificities are summarized (Fig. 4). Anti-U1RNP, anti-U1/U2RNP, and anti-Ku are considered for a serum with coarse nuclear speckled pattern. For fine nuclear speckled pattern, anti-Mi-2, TIF1γ/α, and anti-SAE may be considered. Staining patterns by anti-Mi-2 and TIF1γ/α are indistinguishable though anti-Mi-2 sera usually show brighter staining. Multiple nuclear dots (PML) pattern is consistent with anti-MJ/NXP-2 staining though this may not be apparent in all cases. Some sera may show nuclear fine speckled pattern without PML body staining [75]. Cajal body staining can be seen by anti-SMN (survival of motor neuron) antibodies found in PM or PM overlap syndrome [120]. Nucleolar stainings associated with myositis are homogeneous staining by anti-PM-Scl and clumpy staining by anti-U3RNP antibodies. Strong cytoplasmic staining is consistent with the presence of anti-ARS or SRP. Although ARS localize in the cytoplasm, staining may be weak or absent. MDA5 antigen localizes to the cytoplasm but cytoplasmic staining is often weak or absent.
Future Direction of MSA Testing
IP is a very powerful and reliable technique that has been used and considered a reliable assay over 30 years, but it has been performed only at a limited number of laboratories and never become a routine assay in clinical practice. Currently, anti-Jo-1 ELISA is the only widely available testing for most clinicians. A new ELISA of anti-ARS to detect antibodies to Jo-1, EJ, PL-7, PL-12, and KS in a single ELISA using a mixture of these antigens has been validated compared with IP and released recently in Japan [17]. Line immunoassay is available in certain countries but has not been used extensively. ELISA and beads assays for several MSAs, in particular the ones with high clinical significance such as anti-TIF1γ and MDA5 are currently under development. They will be widely available in the near future and tests for these autoantibodies will become a part of standard tests in clinical practice of inflammatory myopathy.
While wide availability of new autoantibody immunoassays will be definitely welcomed, performance of new immunoassays without validation is a concern [119]. New immunoassays should be validated against a gold standard such as IP and DID before releasing to the market as performed for some new assays [17] to avoid confusion among clinicians and researchers [119].
Cryptic Epitopes and Mechanisms of MSA Production
Autoantibodies in each PM/DM patient target only a few proteins or protein-RNA complexes. Several autoantibodies are considered MSA; however, presence of more than one MSA in the same patient is uncommon. Patients with antibodies to any ARS could have similar clinical features, known as anti-synthetase syndrome; however, detection of antibodies to more than one ARS in each patient is rare for unknown reasons. Thus, it is assumed that there are mechanisms, which select the target antigens of MSA out of thousands of proteins in cells in each patient. We would like to discuss a possibility that certain MSAs, in particular cancer-associated autoantibodies, are triggered by formation of cryptic epitopes resulting from a mutation of the target antigens.
It has been speculated that quantitative (e.g., upregulation or reduced degradation of certain proteins) or qualitative changes (e.g., mutation, aberrant post-translational modification, unusual interaction with other proteins, etc.) or unusual location (e.g., translocation of nuclear proteins to the cell surface) may trigger specific autoimmune responses. A concept of “cryptic epitopes” may be important to understand this idea [121]. Extracellular antigens are typically processed via endosomes and the resulting peptides are presented on MHC class II at the surface of antigen-presenting cells (APCs), whereas intracellular antigens are processed via the proteasomes and the peptides presented on MHC class I. Whether the MHC-peptide complex is recognized as non-self is a critical step toward triggering autoimmune response. Mutation of amino acids can change the pattern of protein digestion and create cryptic epitopes presented on the cell surface MHC. Autoreactive T cells are deleted during T cell development; however, immunological tolerance to epitopes that have little or no expression during this process may be incomplete. When the cryptic epitopes are expressed on APCs in the body in certain conditions, it could trigger autoimmune response. It is not a new concept that changes in the structure or expression levels of certain self-proteins, occurring during tumorigenesis for example, may be associated with triggering autoimmune responses [122].
Perhaps the best-known example is an autoimmune response to a tumor suppressor gene p53 in patients with malignancy. In individuals with a mutation of the tumor suppressor gene p53, loss of normal p53 functions due to amino acid replacement may lead to a development of cancer while the mutated p53 can create cryptic epitopes (Fig. 5), which is recognized as non-self by the immune system and triggers autoantibodies to p53. The immune system is sensitive to detect a single amino acid change and accompanying conformational changes resulting from a point mutation in p53. Autoantibodies to p53 have been reported in patients with various types of cancer that are associated with certain p53 mutations [123, 124]. Recently, an association of POL3A mutation and production of anti-RNA polymerase III antibodies in SSc patients with malignancy was reported [125]. Six of 8 anti-RNAPIII-positive cancer-SSc patients had somatic mutation or loss of heterozygosity, which suggest the presence of mutation. In contrast, these changes were not observed in SSc patients with cancer in six anti-centromere and six anti-topo I-positive patients. Thus, the mechanism of production of anti-RNAPIII in these patients may be exactly same as that of anti-p53 antibody.
Formation of cryptic epitopes via a somatic mutation. A somatic mutation that causes amino acid replacement may create cryptic epitopes, which can be recognized as non-self and trigger autoimmune response. Top, normal protein digested by trypsin makes normal peptides that are supposed to have immunological tolerance. Bottom, if arginine is replaced by other amino acid, trypsin digestion may create cryptic epitopes that have no or incomplete immunological tolerance and trigger autoimmune response
Autoantibodies to TIF-1γ/α have been described recently in strong association with cancer-associated DM [7, 97–99]. Based on the effects of TIF-1γ as a tumor suppressor reported in murine models and humans [96, 126, 127], it seems reasonable to hypothesize that the mechanisms of production of anti-TIF-1γ in patients with cancer-associated DM could be similar to what is speculated in patients with p53-mutated cancer; TIF-1γ mutation could be the primary event that leads to the development of cancer and production of autoantibodies to TIF-1γ (Fig. 6). DM in these patients may be considered as a paraneoplastic syndrome. Roles of TIF-1γ in viral infection and its interactions with viral proteins have been reported [128, 129]. TIF-1α, TIF-1β, and TIF-1γ are identified as adenoviral E1B-55K-binding proteins. TIF-1γ is shown to be a target for degradation by human adenoviruses and it possesses anti-viral activity and limits adenovirus early and late gene product expression during infection [128]. In a mouse model of autoimmune response to p53, it was shown that immunization of viral SVT/self p53 complex induced anti-p53 autoimmune response while immunization of p53 alone did not [130]. One possible explanation was that interaction of viral protein with self p53 modified antigen processing, leading to production of cryptic epitopes. A possible role of viral infection in the pathogenesis of JDM has been discussed for many years without solid and universal evidence. It is tempting to speculate that viral infection, which may create cryptic epitopes of TIF-1γ/α, is the primary event in certain cases of JDM or young adult patients with anti-TIF-1γ/α.
Hypothesis on the production of anti-TIF1γ/α antibodies based on mutation of TIF1γ or interaction of viral proteins with TIF1. In old adult DM patients with malignancy, TIF1γ mutation may allow development of malignancy while the mutated protein may also trigger autoimmune response to TIF1γ. In JDM or young adult DM patients, interaction of viral proteins with TIF1 proteins may create cryptic epitopes, leading to the autoimmune response
It is also possible that de novo mutations of a gene for other MSA antigens, which occur somewhere in the body, lead to production of autoantibodies. A difference from the p53 model and difficulty when trying to prove or disprove this hypothesis will be the location to test for the mutations in MSA antigens not associated with malignancy. While locating the p53 mutation in the body is relatively easy in a majority of cases, usually at the site of cancer, it will not be easy to locate the site of mutation of genes for MSA antigens in PM/DM without malignancy [131].
Conclusions
MSA are highly specific for the diagnosis of PM/DM, and many of them are also associated with a unique clinical subset of PM/DM, making them clinically useful biomarkers. There has been significant progress in MSA in the last 10 years and several new MSA with strong clinical significance have been identified. New immunoassays for these new MSA as well as classic MSA will become widely available for clinical practice in the near future. Clinicians are expected to know and understand the significance of MSA and proper use in clinical practice.
ARS, aminoacyl tRNA synthetases;
ALBIA, addressable laser bead assay;
CIE, counter immunoelectrophoresis;
CK, creatine kinase;
CTD, connective tissue disease;
DID, double immunodiffusion;
DM, dermatomyositis;
ELISA, enzyme-linked immunosorbent assay;
ENA, extractable nuclear antigen;
ILD, interstitial lung disease ;
IP, immunoprecipitation;
JDM, juvenile dermatomyositis;
LIA, line immunoassay;
MAA, myositis-associated autoantibodies;
MCTD, mixed connective tissue disease;
MDA5, melanoma differentiation associated gene 5;
MSA, myositis-specific autoantibodies;
NHS, normal human serum;
NXP-2, nuclear matrix protein 2;
PM, polymyositis;RNP, ribonucleoprotein;
PML, promyelocytic leukemia;
RA, rheumatoid arthritis;
RPILD, rapidly progressive interstitial lung disease;
SAE, small ubiquitin-like modifier activating enzyme;
SARD, systemic autoimmune rheumatic diseases;
SRP, signal recognition particle;
SSc, scleroderma;
SUMO, small ubiquitin-like modifier;
TIF1, transcription intermediary factor 1;
UCTD, undifferentiated connective tissue disease;
UsnRNPs, U small nuclear ribonucleoproteins;
References
Satoh M, Chan EKL, Sobel ES, Kimpel DL, Yamasaki Y, Narain S, Mansoor R, Reeves WH (2007) Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert Rev Clin Immunol 3:721–738
Satoh M, Vazquez-Del Mercado M, Chan EK (2009) Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol 19:219–228
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349:1526–1533
Nishikai M, Reichlin M (1980) Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum 23:881–888
Targoff IN, Reichlin M (1985) The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum 28:796–803
Targoff IN (1992) Autoantibodies in polymyositis. Rheum Dis Clin N Am 18:455–482
Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP, Miller FW, Rider LG (2006) A novel autoantibody to a 155-kD protein is associated with dermatomyositis. Arthritis Rheum 54:3682–3689
Fujimoto M, Hamaguchi Y, Kaji K, Matsushita T, Ichimura Y, Kodera M, Ishiguro N, Ueda-Hayakawa I, Asano Y, Ogawa F et al (2012) Myositis-specific anti-155/140 autoantibodies target transcriptional intermediary factor 1 family proteins. Arthritis Rheum 64:513–522
Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Kuwana M (2009) RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 60:2193–2200
Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, Nishikawa T, Oddis CV, Ikeda Y (2005) Autoantibodies to a 140-kD polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52:1571–1576
Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T (2010) Anti-MDA5 and anti-TIF1-{gamma} antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 49:1726–1733
Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, Kawabata D, Ohmura K, Usui T, Fujii T et al (2010) The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 49:433–440
Targoff IN (2000) Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 12:475–481
Nakashima R, Mimori T (2010) Clinical and pathophysiological significance of myositis-specific and myositis-associated autoantibodies. Int J Clin Rheumatol 5:523–536
Targoff IN, Arnett FC (1990) Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase). Am J Med 88:241–251
Hirakata M, Suwa A, Nagai S, Kron MA, Trieu EP, Mimori T, Akizuki M, Targoff IN (1999) Anti-KS: identification of autoantibodies to asparaginyl-transfer RNA synthetase associated with interstitial lung disease. J Immunol 162:2315–2320
Nakashima R, Imura Y, Hosono Y, Seto M, Murakami A, Watanabe K, Handa T, Mishima M, Hirakata M, Takeuchi T et al (2014) The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS ONE 9:e85062
Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, Miller FW (1991) A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 70:360–374
Targoff IN, Miller FW, Medsger TA Jr, Oddis CV (1997) Classification criteria for the idiopathic inflammatory myopathies. Curr Opin Rheumatol 9:527–535
Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ et al (2012) Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 64:2319–2327
Rider LG, Shah M, Mamyrova G, Huber AM, Rice MM, Targoff IN, Miller FW (2013) The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore) 92:223–243
Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O’Callaghan A, Martinez-Gomez X, Bosch X, Labrador-Horrillo M, Grau-Junyent JM, Vilardell-Tarres M (2012) Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum 64:523–532
Frank MB, McCubbin V, Trieu E, Wu Y, Isenberg DA, Targoff IN (1999) The association of anti-Ro52 autoantibodies with myositis and scleroderma autoantibodies. J Autoimmun 12:137–142
Selva-O’Callaghan A, Labrador-Horrillo M, Solans-Laque R, Simeon-Aznar CP, Martinez-Gomez X, Vilardell-Tarres M (2006) Myositis-specific and myositis-associated antibodies in a series of eighty-eight Mediterranean patients with idiopathic inflammatory myopathy. Arthritis Rheum 55:791–798
Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, Briani C, Rondinone R, Sarzi-Puttini P, Todesco S, Doria A (2006) Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 39:217–221
Vancsa A, Gergely L, Ponyi A, Lakos G, Nemeth J, Szodoray P, Danko K (2010) Myositis-specific and myositis-associated antibodies in overlap myositis in comparison to primary dermatopolymyositis: relevance for clinical classification: retrospective study of 169 patients. Joint Bone Spine 77:125–130
Chinoy H, Fertig N, Oddis CV, Ollier WE, Cooper RG (2007) The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis 66:1345–1349
Gunawardena H, Betteridge ZE, McHugh NJ (2009) Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 48:607–612
Satoh M, Chan JY, Ceribelli A, Del-Mercado MV, Chan EK (2013) Autoantibodies to argonaute 2 (su antigen). Adv Exp Med Biol 768:45–59
Satoh M, Ceribelli A, Hirakata M, Chan EKL (2015) Immunodiagnosis of autoimmune myopathies. In: Detrick B, Hamilton RG, Schmitz JL (eds) Manual of molecular and clinical laboratory immunology, 8th edn. ASM Press, Washington, D. C, In Press
Arnett FC, Hirsch TJ, Bias WB, Nishikai M, Reichlin M (1981) The Jo-1 antibody system in myositis: relationships to clinical features and HLA. J Rheumatol 8:925–930
Yoshida S, Akizuki M, Mimori T, Yamagata H, Inada S, Homma M (1983) The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. Arthritis Rheum 26:604–611
Targoff IN, Arnett FC, Reichlin M (1988) Antibody to threonyl-transfer RNA synthetase in myositis sera. Arthritis Rheum 31:515–524
Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD (1996) Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 39:1507–1518
Bernstein RM, Bunn CC, Hughes GRV, Francoeur AM, Mathews MB (1984) Cellular protein and RNA antigens in autoimmune disease. Mol Biol Med 2:105–120
Hirakata M, Mimori T, Akizuki M, Craft J, Hardin JA, Homma M (1992) Autoantibodies to small nuclear and cytoplasmic ribonucleoproteins in Japanese patients with inflammatory muscle disease. Arthritis Rheum 35:449–456
Friedman AW, Targoff IN, Arnett FC (1996) Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 26:459–467
Yamasaki Y, Yamada H, Nozaki T, Akaogi J, Nichols C, Lyons R, Chin Loy A, Chan EK, Reeves WH, Satoh M (2006) Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 54:2004–2009
Sato S, Hirakata M, Kuwana M, Nakamura K, Suwa A, Inada S, Mimori T, Ikeda Y (2005) Clinical characteristics of Japanese patients with anti-PL-7 (anti-threonyl-tRNA synthetase) autoantibodies. Clin Exp Rheumatol 23:609–615
Kalluri M, Sahn SA, Oddis CV, Gharib SL, Christopher-Stine L, Danoff SK, Casciola-Rosen L, Hong G, Dellaripa PF, Highland KB (2009) Clinical profile of anti-PL-12 autoantibody. Cohort study and review of the literature. Chest 135:1550–1556
Labirua-Iturburu A, Selva-O'Callaghan A, Vincze M, Danko K, Vencovsky J, Fisher B, Charles P, Dastmalchi M, Lundberg IE (2012) Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Medicine (Baltimore) 91:206–211
Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C, Roblot P, Jouneau S, Hatron PY, Tiev KP et al (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11:739–745
Marie I, Josse S, Decaux O, Diot E, Landron C, Roblot P, Jouneau S, Hatron PY, Hachulla E, Vittecoq O et al (2013) Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med 24:474–479
Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, Oddis CV (2014) Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 73:227–232
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M, Kodera M, Muroi E, Fujikawa K, Seishima M et al (2013) Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS ONE 8, e60442
Reeves WH, Nigam SK, Blobel G (1986) Human autoantibodies reactive with the signal-recognition particle. Proc Natl Acad Sci U S A 83:9507–9511
Nakao Y, Mukai R, Kabashima T, Ohshima Y, Hamaguchi H, Kashiwagi H, Okada N (1982) A novel antibody which precipitates 7.5S RNA is isolated from a patient with autoimmune disease. Biochem Biophys Res Commun 109:1332–1338
Okada N, Mimori T, Mukai R, Kashiwagi H, Hardin JA (1987) Characterization of human autoantibodies that selectively precipitate the 7SL RNA component of the signal recognition particle. J Immunol 138:3219–3223
Targoff IN, Johnson AE, Miller FW (1990) Antibody to signal recognition particle in polymyositis. Arthritis Rheum 33:1361–1370
Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV (2004) Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 50:209–215
Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J, Doria A, Mosca M, van Venrooij WJ, van Engelen BG (2006) Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis 65:1635–1638
Miller T, Al-Lozi MT, Lopate G, Pestronk A (2002) Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73:420–428
Takada T, Hirakata M, Suwa A, Kaneko Y, Kuwana M, Ishihara T, Ikeda Y (2009) Clinical and histopathological features of myopathies in Japanese patients with anti-SRP autoantibodies. Mod Rheuamtol 19:156–164
Benveniste O, Drouot L, Jouen F, Charuel JL, Bloch-Queyrat C, Behin A, Amoura Z, Marie I, Guiguet M, Eymard B et al (2011) Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum 63:1961–1971
Aggarwal R, Oddis CV, Goudeau D, Fertig N, Metes I, Stephens C, Qi Z, Koontz D, Levesque MC (2015) Anti-signal recognition particle autoantibody ELISA validation and clinical associations. Rheumatology (Oxford) 54:1194–1199
Suzuki S, Nishikawa A, Kuwana M, Nishimura H, Watanabe Y, Nakahara J, Hayashi YK, Suzuki N, Nishino I (2015) Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphan J Rare Dis 10:61
Vincze M, Molnar PA, Tumpek J, Szollosi L, Gyetvai A, Kapitany A, Danko K (2010) An unusual association: anti-Jo1 and anti-SRP antibodies in the serum of a patient with polymyositis. Clin Rheumatol 29:811–814
Sugie K, Tonomura Y, Ueno S (2012) Characterization of dermatomyositis with coexistence of anti-Jo-1 and anti-SRP antibodies. Intern Med 51:799–802
Malkan A, Cappelen-Smith C, Beran R, Griffith N, Toong C, Wang MX, Cordato D (2015) Anti-synthetase syndrome associated with anti PL-12 and anti-signal recognition particle antibodies and a necrotizing auto-immune myositis. J Clin Neurosci 22:396–398
Satoh T, Okano T, Matsui T, Watabe H, Ogasawara T, Kubo K, Kuwana M, Fertig N, Oddis CV, Kondo H et al (2005) Novel autoantibodies against 7SL RNA in patients with polymyositis/dermatomyositis. J Rheumatol 32:1727–1733
Ellis E, Ann Tan J, Lester S, Tucker G, Blumbergs P, Roberts-Thomson P, Limaye V (2012) Necrotizing myopathy: clinicoserologic associations. Muscle Nerve 45:189–194
Wang L, Liu L, Hao H, Gao F, Liu X, Wang Z, Zhang W, Lv H, Yuan Y (2014) Myopathy with anti-signal recognition particle antibodies: clinical and histopathological features in Chinese patients. Neuromuscul Disord 24:335–341
Suzuki S, Yonekawa T, Kuwana M, Hayashi YK, Okazaki Y, Kawaguchi Y, Suzuki N, Nishino I (2014) Clinical and histological findings associated with autoantibodies detected by RNA immunoprecipitation in inflammatory myopathies. J Neuroimmunol 274:202–208
Ellis AE, De Sousa M (1974) Phylogeny of the lymphoid system. I. A study of the fate of circulating lymphocytes in plaice. Eur J Immunol 4:338–343
Nilasena DS, Trieu EP, Targoff IN (1995) Analysis of the Mi-2 autoantigen of dermatomyositis. Arthritis Rheum 38:123–128
Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D (1998) The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95:279–289
Roux S, Seelig HP, Meyer O (1998) Significance of Mi-2 autoantibodies in polymyositis and dermatomyositis. J Rheumatol 25:395–396
Brouwer R, Hengstman GJ, Vree Egberts W, Ehrfeld H, Bozic B, Ghirardello A, Grondal G, Hietarinta M, Isenberg D, Kalden JR et al (2001) Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 60:116–123
Hausmanowa-Petrusewicz I, Kowalska-Oledzka E, Miller FW, Jarzabek-Chorzelska M, Targoff IN, Blaszczyk-Kostanecka M, Jablonska S (1997) Clinical, serologic, and immunogenetic features in Polish patients with idiopathic inflammatory myopathies. Arthritis Rheum 40:1257–1266
Komura K, Fujimoto M, Matsushita T, Kaji K, Kondo M, Hirano T, Orito H, Horikawa M, Hamaguchi Y, Hasegawa M et al (2005) Prevalence and clinical characteristics of anti-Mi-2 antibodies in Japanese patients with dermatomyositis. J Dermatol Sci 40:215–217
Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo R, Gambari PF, Doria A (2005) Anti-Mi-2 antibodies. Autoimmunity 38:79–83
Ronnelid J, Barbasso Helmers S, Storfors H, Grip K, Ronnblom L, Franck-Larsson K, Nordmark G, Lundberg IE (2009) Use of a commercial line blot assay as a screening test for autoantibodies in inflammatory myopathies. Autoimmun Rev 9:58–61
Ghirardello A, Rampudda M, Ekholm L, Bassi N, Tarricone E, Zampieri S, Zen M, Vattemi GA, Lundberg IE, Doria A (2010) Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology (Oxford) 49:2370–2374
Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, Komura K, Nakamura M, Kodera M, Suga N et al (2011) Clinical correlations with dermatomyositis-specific autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 147:391–398
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, Tincani A, Ross SJ, Chan JY, Pauley BA et al (2012) Anti-MJ/NXP-2 autoantibody specificity in a cohort of adult Italian patients with polymyositis/dermatomyositis. Arthritis Res Ther 14:R97
Muro Y, Sugiura K, Akiyama M (2013) A new ELISA for dermatomyositis autoantibodies: rapid introduction of autoantigen cDNA to recombinant assays for autoantibody measurement. Clin Dev Immunol 2013:856815
Petri MH, Satoh M, Martin-Marquez BT, Vargas-Ramirez R, Jara LJ, Saavedra MA, Cruz-Gonzalez C, Andrade-Ortega L, Vera-Lastra OL, Salazar-Paramo M et al (2013) Implications in the difference of anti-Mi-2 and -p155/140 autoantibody prevalence in two dermatomyositis cohorts from Mexico City and Guadalajara. Arthritis Res Ther 15:R48
Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW (2003) Global surface ultraviolet radiation intensity may modulate the clinical and immunologic expression of autoimmune muscle disease. Arthritis Rheum 48:2285–2293
Love LA, Weinberg CR, McConnaughey DR, Oddis CV, Medsger TA Jr, Reveille JD, Arnett FC, Targoff IN, Miller FW (2009) Ultraviolet radiation intensity predicts the relative distribution of dermatomyositis and anti-Mi-2 autoantibodies in women. Arthritis Rheum 60:2499–2504
Euwer RL, Sontheimer RD (1991) Amyopathic dermatomyositis (dermatomyositis sine myositis). Presentation of six new cases and review of the literature. J Am Acad Dermatol 24:959–966
Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD (2006) A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol 54:597–613
Fujikawa K, Kawakami A, Kaji K, Fujimoto M, Kawashiri S, Iwamoto N, Aramaki T, Ichinose K, Tamai M, Kamachi M et al (2009) Association of distinct clinical subsets with myositis-specific autoantibodies towards anti-155/140-kDa polypeptides, anti-140-kDa polypeptides, and anti-aminoacyl tRNA synthetases in Japanese patients with dermatomyositis: a single-centre, cross-sectional study. Scand J Rheumatol 38:263–267
Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, Masuda I, Tochimoto A, Baba S, Okamoto Y et al (2010) Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49:1713–1719
Gono T, Kawaguchi Y, Ozeki E, Ota Y, Satoh T, Kuwana M, Hara M, Yamanaka H (2011) Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol 21:223–227
Kobayashi I, Okura Y, Yamada M, Kawamura N, Kuwana M, Ariga T (2011) Anti-melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr 158:675–677
Chen F, Wang D, Shu X, Nakashima R, Wang G (2012) Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 32:3909–3915
Muro Y, Sugiura K, Hoshino K, Akiyama M (2011) Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology (Oxford) 51:800–804
Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, Shi R, Lou J, Zhou M, Kuwana M et al (2012) Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res (Hoboken) 64:1602–1610
Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L (2011) The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65:25–34
Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, Christopher-Stine L (2013) Anti-MDA5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res (Hoboken) 65:1307–1315
Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Tincani A, Selmi C, Chan JY, Chan EK, Satoh M, Franceschini F (2014) Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol 32:891–897
Labrador-Horrillo M, Martinez MA, Selva-O'Callaghan A, Trallero-Araguas E, Balada E, Vilardell-Tarres M, Juarez C (2014) Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res 2014:290797
Sato S, Kuwana M, Fujita T, Suzuki Y (2013) Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 23:496–502
Targoff IN, Trieu EP, Levy-Neto M, Prasertsuntarasai T, Miller FW (2006) Autoantibodies to transcriptional intermediary factor 1-gamma (TIF1-γ) in dermatomyositis. Arthritis Rheum 54(Suppl):S518
Satoh M, Chan JY, Ross SJ, Li Y, Yamasaki Y, Yamada H, Mercado MV, Petri MH, Jara LJ, Saavedra MA et al (2012) Autoantibodies to transcription intermediary factor (TIF)1beta associated with dermatomyositis. Arthritis Res Ther 14:R79
Herquel B, Ouararhni K, Khetchoumian K, Ignat M, Teletin M, Mark M, Bechade G, Van Dorsselaer A, Sanglier-Cianferani S, Hamiche A et al (2011) Transcription cofactors TRIM24, TRIM28, and TRIM33 associate to form regulatory complexes that suppress murine hepatocellular carcinoma. Proc Natl Acad Sci U S A 108:8212–8217
Gunawardena H, Wedderburn LR, North J, Betteridge Z, Dunphy J, Chinoy H, Davidson JE, Cooper RG, McHugh NJ (2008) Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology (Oxford) 47:324–328
Trallero-Araguas E, Labrador-Horrillo M, Selva-O'Callaghan A, Martinez MA, Martinez-Gomez X, Palou E, Rodriguez-Sanchez JL, Vilardell-Tarres M (2010) Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine (Baltimore) 89:47–52
Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, Matsushita T, Orito H, Hamaguchi Y, Yanaba K et al (2007) Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford) 46:25–28
Kang EH, Nakashima R, Mimori T, Kim J, Lee YJ, Lee EB, Song YW (2010) Myositis autoantibodies in Korean patients with inflammatory myositis: anti-140-kDa polypeptide antibody is primarily associated with rapidly progressive interstitial lung disease independent of clinically amyopathic dermatomyositis. BMC Musculoskelet Disord 11:223
Ikeda N, Takahashi K, Yamaguchi Y, Inasaka M, Kuwana M, Ikezawa Z (2011) Analysis of dermatomyositis-specific autoantibodies and clinical characteristics in Japanese patients. J Dermatol 38:973–979
Targoff IN, Trieu EP, Levy-Neto M, Fertig N, Oddis CV (2007) Sera with autoantibodies to the MJ antigen react with NXP2. Arthritis Rheum 56:S787
Mimura Y, Takahashi K, Kawata K, Akazawa T, Inoue N (2010) Two-step colocalization of MORC3 with PML nuclear bodies. J Cell Sci 123:2014–2024
Kimura Y, Sakai F, Nakano O, Kisaki O, Sugimoto H, Sawamura T, Sadano H, Osumi T (2002) The newly identified human nuclear protein NXP-2 possesses three distinct domains, the nuclear matrix-binding, RNA-binding, and coiled-coil domains. J Biol Chem 277:20611–20617
Takahashi K, Yoshida N, Murakami N, Kawata K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Zinn AR, Shime H, Inoue N (2007) Dynamic regulation of p53 subnuclear localization and senescence by MORC3. Mol Biol Cell 18:1701–1709
Oddis CV, Fertig N, Goel A, Espada G, Confalone Gregorian M, Maldonado Cocco JA, Londino AV (1997) Clinical and serological characterization of the anti-MJ antibody in childhood myositis. Arthritis Rheum 40:S139
Espada G, Maldonado Cocco JA, Fertig N, Oddis CV (2009) Clinical and serologic characterization of an Argentine pediatric myositis cohort: identification of a novel autoantibody (anti-MJ) to a 142-kDa protein. J Rheumatol 36:2547–2551
Gunawardena H, Wedderburn LR, Chinoy H, Betteridge ZE, North J, Ollier WE, Cooper RG, Oddis CV, Ramanan AV, Davidson JE et al (2009) Autoantibodies to a 140-kD protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum 60:1807–1814
Betteridge ZE, Gunawardena H, Chinoy H, Venkovsky J, Allard SA, Gordon PA, Cooper RG, McHugh NJ (2009) Autoantibodies to the p140 autoantigen NXP-2 in adult dermatomyositis. Arthritis Rheum 60:S304
Ichimura Y, Matsushita T, Hamaguchi Y, Kaji K, Hasegawa M, Tanino Y, Inokoshi Y, Kawai K, Kanekura T, Habuchi M et al (2012) Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis 71:710–713
Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, Rosen A, Casciola-Rosen L (2013) Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum 65:2954–2962
Troyanov Y, Targoff IN, Payette MP, Raynauld JP, Chartier S, Goulet JR, Bourre-Tessier J, Rich E, Grodzicky T, Fritzler MJ et al (2014) Redefining dermatomyositis: a description of new diagnostic criteria that differentiate pure dermatomyositis from overlap myositis with dermatomyositis features. Medicine (Baltimore) 93:318–332
Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D (2014) Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol 150:724–729
Betteridge Z, Gunawardena H, North J, Slinn J, McHugh N (2007) Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum 56:3132–3137
Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG, McHugh NJ (2009) Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann Rheum Dis 68:1621–1625
Tarricone E, Ghirardello A, Rampudda M, Bassi N, Punzi L, Doria A (2012) Anti-SAE antibodies in autoimmune myositis: identification by unlabelled protein immunoprecipitation in an Italian patient cohort. J Immunol Methods 384:128–134
Fujimoto M, Matsushita T, Hamaguchi Y, Kaji K, Asano Y, Ogawa F, Yamaoka T, Fujikawa K, Tsukada T, Sato K et al (2013) Autoantibodies to small ubiquitin-like modifier activating enzymes in Japanese patients with dermatomyositis: comparison with a UK Caucasian cohort. Ann Rheum Dis 72:151–153
Muro Y, Sugiura K, Akiyama M (2013) Low prevalence of anti-small ubiquitin-like modifier activating enzyme antibodies in dermatomyositis patients. Autoimmunity 46:279–284
Satoh M, Tanaka S, Chan EK (2015) The uses and misuses of multiplex autoantibody assays in systemic autoimmune rheumatic diseases. Front Immunol 6:181
Satoh M, Chan JY, Ross SJ, Ceribelli A, Cavazzana I, Franceschini F, Li Y, Reeves WH, Sobel ES, Chan EK (2011) Autoantibodies to survival of motor neuron (SMN) complex in patients with polymyositis—immunoprecipitation of D-E-F-G without other components of small nuclear ribonucleoproteins. Arthritis Rheum 63:1972–1978
Lanzavecchia A (1995) How can cryptic epitopes trigger autoimmunity? J Exp Med 181:1945–1948
Tan EM (2001) Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest 108:1411–1415
Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y (2001) Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis 60:433–441
Reuschenbach M, von Knebel DM, Wentzensen N (2009) A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother 58:1535–1544
Joseph CG, Darrah E, Shah AA, Skora AD, Casciola-Rosen LA, Wigley FM, Boin F, Fava A, Thoburn C, Kinde I et al (2014) Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343:152–157
Vincent DF, Yan KP, Treilleux I, Gay F, Arfi V, Kaniewski B, Marie JC, Lepinasse F, Martel S, Goddard-Leon S et al (2009) Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet 5, e1000575
Aucagne R, Droin N, Paggetti J, Lagrange B, Largeot A, Hammann A, Bataille A, Martin L, Yan KP, Fenaux P et al (2011) Transcription intermediary factor 1gamma is a tumor suppressor in mouse and human chronic myelomonocytic leukemia. J Clin Invest 121:2361–2370
Forrester NA, Patel RN, Speiseder T, Groitl P, Sedgwick GG, Shimwell NJ, Seed RI, Catnaigh PO, McCabe CJ, Stewart GS et al (2012) Adenovirus E4orf3 targets transcriptional intermediary factor 1gamma for proteasome-dependent degradation during infection. J Virol 86:3167–3179
Herquel B, Ouararhni K, Martianov I, Le Gras S, Ye T, Keime C, Lerouge T, Jost B, Cammas F, Losson R et al (2013) Trim24-repressed VL30 retrotransposons regulate gene expression by producing noncoding RNA. Nat Struct Mol Biol 20:339–346
Dong X, Hamilton KJ, Satoh M, Wang J, Reeves WH (1994) Initiation of autoimmunity to the p53 tumor suppressor protein by complexes of p53 and SV40 large T antigen. J Exp Med 179:1243–1252
Satoh M, Ceribelli A, Chan EK (2012) Common pathways of autoimmune inflammatory myopathies and genetic neuromuscular disorders. Clin Rev Allergy Immunol 42:16–25
Acknowledgment
This work was supported by JSPS KAKENHI (Grants-in-Aid for Scientific Research) grant number 15K08790.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, M., Tanaka, S., Ceribelli, A. et al. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clinic Rev Allerg Immunol 52, 1–19 (2017). https://doi.org/10.1007/s12016-015-8510-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8510-y