Abstract
Peripheral nerve injuries (PNIs) are common and debilitating, cause significant health care costs for society, and rely predominately on autografts, which necessitate grafting a nerve section non-locally to repair the nerve injury. One possible approach to improving treatment is bolstering endogenous regenerative mechanisms or bioengineering new nervous tissue in the peripheral nervous system. In this review, we discuss critical-sized nerve gaps and nerve regeneration in rats, and summarize the roles of adipose-derived stem cells (ADSCs) in the treatment of PNIs. Several regenerative treatment modalities for PNI are described: ADSCs differentiating into Schwann cells (SCs), ADSCs secreting growth factors to promote peripheral nerve growth, ADSCs promoting myelination growth, and ADSCs treatments with scaffolds. ADSCs’ roles in regenerative treatment and features are compared to mesenchymal stem cells, and the administration routes, cell dosages, and cell fates are discussed. ADSCs secrete neurotrophic factors and exosomes and can differentiate into Schwann cell-like cells (SCLCs) that share features with naturally occurring SCs, including the ability to promote nerve regeneration in the PNS. Future clinical applications are also discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injury (PNI) results from trauma or surgical complications. Despite improvements in microsurgical techniques, the repair of PNI remains a challenging clinical problem. PNI causes demyelination of the distal stump with subsequent degradation. Without innervation, the target muscle atrophies and loses its ability to function.
Graft necessity is determined by the size of the nerve gap. Tensionless suturing is sufficient for small gaps, but autografts, epineural sheaths, veins, and skeletal muscle tissue are used for large gaps >5-mm. Among these bridging options, autografts have yielded the most favorable results and remain the “gold standard” treatment for large nerve defects despite significant disadvantages, such as limited graft supply, secondary deformities, loss of sensation at the donor site, potential differences in tissue structure, and difficulty finding suitable donor sites in diabetic or chronically sick patients [1,2,3]. Alternative conduit structures have emerged as a potential solution to these limitations. However, few viable alternatives to nerve grafts exist, and those available do not adequately reproduce the biological properties of nerve grafts [4]. Transplantation of cells, including Schwann cells (SCs) and stem cells, has been a suggested augmentation to improve success to alternative conduit structures [2]. Adipose-derived stem cells (ADSCs) are a promising option because they have shown the potential to differentiate into SCs and are easily accessible in large numbers.
This review paper focuses on the application of ADSCs as a potential alternative treatment for PNI and summarizes the current knowledge of ADSCs in the regeneration of peripheral nerves. We aim to summarize the work completed thus far, investigating administration routes, dosages, the fate of ADSCs after administration, and therapeutic mechanisms of ADSCs in promoting nerve regeneration. We will analyze this disconnection between preclinical and clinical outcomes, focusing on future directions for the clinical translation of this therapeutic intervention to promote its clinical application after PNI.
PNI
Normally, following PNI, the axon and enveloping myelin undergo anterograde and retrograde degeneration in the first few days. In the next few days or weeks, Wallerian degeneration of the distal nerve stump proceeds as macrophages remove debris. The cell body experiences chromatolytic changes that cause the proximal axonal stump to develop axonal sprouts, which penetrate the endoneurial tube and follow the guiding bands of Büngner. If the axonal sprout reaches the target organ and resumes neural transmission, the other axonal sprouts degenerate [5]. This occurs in the first few weeks to months. Complications to this process arise if the axonal nerve sprouts are unable to penetrate the endoneurial tube; even with successful penetration, axonal misguidance due to antibodies targeting neurotrophic factors could lead to inappropriate or absent target organ innervation [6, 7]. The likelihood of these complications rises given a longer nerve gap. The following sections will discuss critical-sized nerve gaps, evaluation time periods of PNIs, and nerve regeneration rates; these topics have not been addressed in previous reviews so we have collected relevant information and synthesized it below.
Critical-Sized Nerve Gaps
Unlike the central nervous system (CNS), the peripheral nervous system (PNS) has the regenerative capacity, but is limited in cases of neurotmesis with large gaps. The main factors that cause poor nerve regeneration are the duration of chronic axotomy, chronic denervation, and the slow growth of axons [8]. In chronic axotomy and chronic denervation, the inadequate concentration of neurotrophic factors and gradual loss of SC and neuronal repair capacity lead to poor outcomes. The peripheral nerve is unable to regenerate in humans if the residual gap following injury is greater than 4 cm [9].
This threshold is known as the critical-sized nerve gap and requires nerve grafting or bridging to repair. It varies by species: rats have a critical-sized nerve gap of ~1.5 cm [9,10,11], mice ~1 cm [12], rabbits ~3 cm, and pigs and humans ~4 cm [9]. It can also vary by nerve, evidenced by nonhuman primates having different critical-sized nerve gaps for their median nerve (2 cm) and their ulnar nerve (3 cm) [13]. Recently, neural guidance conduits (NGCs) have gained notoriety in the literature as a possible alternative to autologous nerve grafts for large gap neurotmesis, but a critical-sized nerve gap greater than 1.3 cm in rats has not been overcome by conduits alone [13].
Evaluation Time Periods
There has been little reported on standardized evaluation time periods for in vivo peripheral nerve regeneration studies. Many studies collect data at 4, 6, 8, and 12 weeks or similar variations in the model of sciatic nerve injury, but these time points ranged from 2 weeks to 4 months [6, 7, 14,15,16]. At two weeks, debris is cleared from the site of injury by macrophages and axonal sprouts should initiate recovery. The four-week time point is usually indicative of the early electrophysiological changes commonly seen after nerve repair [16]. At weeks 6 and 8, recovery is in its intermediate stages, as axonal sprouts will have penetrated the endoneurial tube and other axonal sprouts will have degenerated. At 12 weeks after the operation, axonal sprouting and muscle reinnervation have been completed. This time point should generally be considered as the endpoint of the recovery [7, 15]. Standardization of data collection time points would help researchers generalize results and ensure efficient collaboration.
Nerve Regeneration Rates
Peripheral nerve regeneration rates vary. If left untreated, a peripheral nerve axon will regenerate 1-mm/day in humans, a number based on the advancing Tinel sign seen in surgery and widely accepted in the literature [17,18,19,20]. Growth factors and electrical stimulation (ES) have also been reported to increase the growth rate of axons [21, 22]. However, conflicting reports have claimed this rate to be 0.5–9-mm/day [23]. This wide range can be attributed to decreasing axonal regeneration rates with increasing distance from the cell body, variable severity of the injury, and different methods of measurement (i.e., Tinel sign vs. functional recovery) [23]. Menorca et al. believe that proximal segments may see an increase of 2–3-mm/day while more distal segments may progress at a rate of 1–2-mm/day [24]. It is assumed that animal peripheral nerves regenerate 1-mm/day as well. Those reporting higher measurements tended to use the crush model and measured rates earlier in the regeneration process. Rates measured in animals vary because of the inconsistency of measurement location, varying models of determination, and the extrapolation technique that is often employed to identify rate as a secondary endpoint. For example, if a 10-mm nerve gap is bridged in 4 weeks, then the treatment will have an estimated regeneration rate of 0.35-mm/day [25, 26]. This technique does not consider differing rates at different phases of recovery. Further experiments with regeneration rate as a primary outcome would produce a more comprehensive understanding of the different phases of regeneration and identify the characteristics of the environment in which regeneration is fastest and translate that into a new model. When a nerve gap exceeds its critical size, grafting or bridging treatments supplant the injured nerve and provide an environment advantageous for regrowth, which happens at a rate of 1-mm/day in human autografts, 2-mm/day in animal autografts, and 0.2–1.0-mm/day in human and animal bridging treatment with NGC’s [25,26,27,28,29].
Several methods have been shown to increase the regeneration rate in vivo and in vitro. Transcutaneous ultrasound application to the PNI site has shown the potential to enhance regeneration rate in a poly(lactic‐co‐glycolic acid) (PLGA) and Pluronic F127 NGC model from 0.48-mm/day in controls to 0.71-mm/day. These rates were measured using a functional recovery extrapolation technique [30]. Further, SC exosomes have been shown to increase the growth rate of dorsal root ganglion cell axons in vitro from 0.44-mm/day in controls to 0.61-mm/day in the experimental group [31].
ADSCs as a Surrogate to SCs After PNI
SCs play a pivotal role in peripheral nerve regeneration by producing various neurotrophic factors (NTFs), cytokines, extracellular matrix (ECM), and adhesion molecules that promote axonal regeneration [32]. However, cultured SCs have limited clinical application and are imperfect resources for cell therapy because of their slow proliferation rates and loss of function [33]. In addition, the requirement for nerve donor material induces additional morbidity, and at least 2 weeks are required to culture and expand the cells, which delays treatment [34]. The maintenance of SCs in culture has also been difficult [33, 35, 36]. Culturing high-purity, abundant SCs requires 19 (from either embryonic or neonatal tissue) to 45 days [33, 37,38,39]. Ideally, a transplantable cell would be easy to harvest, proliferate rapidly in culture, and withstand or avoid host immunological defenses. ADSCs could potentially fulfill these requirements.
ADSCs Compared to Mesenchymal Stem Cells (MSCs), a Popular Alternative
MSCs, which are similar to ADSCs in that they also proliferate rapidly and are immunologically tolerable, are an appealing source for nerve regeneration because of their rapid self-renewal and multi-potent differentiation capabilities. Friedenstein et al. first isolated MSCs from rodent bone marrow in 1976 [40]. Recently, the application of MSCs has been seen as a promising adjunct during the initial process of peripheral nerve regeneration [40,41,42], primarily due to their ability to differentiate into adipocytes, chondrocytes, osteoblasts, myoblasts, hepatocytes, and phenotypically neurogenic cells. Dezawa et al. demonstrate that MSCs can be induced to differentiate into cells with SC characteristics in 11 days that are capable of eliciting peripheral nerve regeneration in adult rats [43]. In this study, beta-mercaptoethanol-treated MSCs subsequently treated with retinoic acid differentiated into cells morphologically similar to primary SC’s expressing p75, S-100, GFAP, and O4 [43]. Lavorato et al. [44] recently reviewed and highlighted MSC’s potential to produce paracrine effects that are stimulated in a targeted fashion, and their ability to differentiate into SC-like cells and neuronal type cells.
However, there are important differences between MSCs and ADSCs. Zuk reported in 2001 that ADSCs were obtainable in large quantities with little discomfort from patients under local anesthesia [45]. Like MSCs, ADSCs are adult stem cells that can differentiate into different cell types [40], so their use is seen as an attractive alternative to the use of autologous SCs. The advantage of ADSCs compared to MSCs is that they are easy to harvest, are available in large amounts [46]. ADSCs proliferate faster than MSCs in culture. Quantified differentiation into SCs using immune cytochemical staining showed that the BMSC–SCs positive for S100 (84.23 ± 5.65%) were less than ADSC–SCs positive for S100 (88.6 ± 4.0%), though the difference was not significant. ADSC- and BMMSC transplantation were shown to be similar in their positive functionality in nerve regeneration [47]. More importantly, ADSCs retain regenerative potential as donor age increases [48].
Administration Routes of ADSCs
Local transplantation of ADSCs in injured nerves has been the most common route of administration. In cases of multiple nerve injuries, the systemic administration of ADSCs capable of reaching damaged nerves is advisable. Several studies examined the efficacy of local transplantation. One such study reported that perineural transplantation of canine ADSCs expedited functional motor recovery assessed by sciatic nerve functional index (SFI) analysis two weeks after axonotmesis and improved electrophysiological recovery three weeks crush injury [49]. Tremp et al. [50] used ultrasound guidance with clip removal to inject ADSCs distal to the lesion and found accelerated sciatic nerve regeneration after crush injury. Not all studies have yielded positive results. Kappos injected 5 × 106 ADSCs with a 30G needle in the epineurium of the sciatic nerve in a rodent model and found no differences in functional gait evaluations, imaging analysis, histomorphometric analyses, and muscle weight between the ADSC treated group and control group treated with culture medium [51]. Furthermore, ADSCs have been shown to have a beneficial effect on myelin thickness, but no more than a control group treated with a re-sutured nerve segment autograft [52]. While most studies demonstrated beneficial effects, the epineurium injection of ADSC in the chronic nerve crush injury model needs further analyses.
Previous studies aiming to restore sciatic nerve function after nerve injury have employed artificial nerve conduits and scaffolds containing ADSCs or have delivered the ADSCs directly into the lesion site. Nerve scaffolds can help bridge a peripheral nerve gap, especially when combined with ADSCs, by restoring and regenerating damaged tissues. [53,54,55]. Various synthetic catheters can be used for nerve repair. ADSC delivery with poloxamer hydrogel in Poly(caprolactone) (PCL) based guides showed the longest axonal regrowth in an experiment assessing critical-sized nerve gaps (1.5 cm) in rat sciatic nerves six weeks following transection and repair. The qPCR results showed the inclusion of ADSCs promoted the expression of factors that aid in muscle tissue reinnervation [56]. Santiago LY tested the PCL catheter on the 6-mm nerve gap model and found that by the third week, the sciatic nerve index of the experimental group was significantly better than that of the control group; however these differences were not observed at 12 weeks. The regenerated sciatic nerve transplanted with ADSCs was also thicker than those transplanted with catheter alone in 12 weeks, suggesting that the PCL catheter containing ADSCs has a positive effect on promoting nerve regeneration [57].
Klein used a U.S. Food and Drug Administration (FDA) approved type I collagen conduit to carry autologous ADSCs [58]. Transplantation of ADSCs significantly improved motor and sensory nerve conduction velocity in peripheral nerve gaps after 6 months. When compared to nerve conduits alone, pre-seeded conduits showed a more organized axon arrangement inside. Fibrin catheters were also used in the 10-mm sciatic nerve gap model of rats and compared with SCs, ADSCs, and bone marrow mesenchymal stem cells (BMMSCs). The results showed that the regeneration distance of SCs was significantly higher than that of ADSCs and BMMSCs. There was no significant difference between ADSCs and BMMSCs [59]. All the models containing ADSCs significantly promote axonal regeneration [54, 60,61,62]. Because axons grow only a short distance outside their repair matrix, and a complete intima is related to better results, there has been a significant research focus on bridging this gap through scaffolds. ADSCs combined with acellular nerve allografts (ANAs) effectively promote the regeneration and repair of peripheral nerves [63].
Nonbiodegradable nerve conduits are composed of synthetic materials. The conduit might trigger an immune response in the implantation site which results in fibrous scar tissue formation. Biodegradable nerve guides serve as acute structural support for regenerating axons, and degrade over time, thus avoid future surgery to remove the conduit. Acellular nerve grafts, a type of biological tubular graft, have an internal structure that resembles an autograft and provides support and mechanical strength. However, they are limited by availability and immunological rejection. Therefore, the compatibility of ADSC therapy with conduit biomaterials, as well as the properties of the materials themselves, are particularly important to the therapy efficacy.
The limitations of using an epineurium injection are the additional trauma while accessing the injection site and the multiple or diffuse sites of injury. A systemic administration of cells that can reach the PNS would address these limitations. ADSCs display a multitude of adhesion molecules that facilitate their localization to damaged tissues, making them excellent candidates for systemic administration. Marconi injected 2 × 106 human ADSC through the tail vein of rats 7 days after sciatic nerve crush injury [64]. Mice sciatic nerves treated with ADSCs demonstrated improved fiber sprouting and decreased inflammation for three weeks after surgery, as well as a small number of undifferentiated ADSCs (uADSCs) at the site of injury. Schweizer et al. transected and repaired sciatic nerves in rat models and intravenously administered allogeneic ADSCs on postoperative day 1. The group treated with ADSCs demonstrated better functional recovery measured by the swim test at two, four, and six weeks when compared with the repair-only group. Both voluntary and involuntary motions measured by static and dynamic functional tests improved following early, single-dose systemic administration of ADSCs [65].
Intracavernous (IC) injection of stem cells improves erectile function in several animal models. In Lin et al.’s experiment, 1 million autologous or allogeneic ADSCs labeled with 5-thynyl-2-deoxyuridine (EdU) were injected into the cavernous nerve after crush injury and then compared with the sham operation, and the ADSCs migrated from the penis to bone marrow within days of injection; allogenicity did not affect ADSC appearance. However, cavernous nerve injury had a diminishing effect on the quantity of ADSCs in the bone marrow at 7 days [66]. The authors believe one possible explanation for these results is that ADSCs that migrate to bone marrow congregate and form a repository of repair cells that can subsequently migrate to injury sites [66].
ADSC Dosage
The efficiency of cell transplantation into the desired tissue destination significantly impacts the likelihood of success. Rodríguez Sánchez [49] performed perineural transplantation of 1 × 106 cells in suspension using the Hamilton microsyringe in an experimental sciatic nerve crush injury. Transplantation of canine ADSCs demonstrated pro-regenerative effects two to four weeks after sciatic nerve crush injury in rats. Rbia and colleagues used a dynamic bioreactor rotating system to seed 1 × 106 ADSCs into their Sprague Dawley decellularized rat allografts in vitro and reported a seeding efficiency of 89.2% at 72 h, suggesting that almost 900,000 cells were attached to the surface of the 10-mm nerve segment before in vivo implementation [67]. Marconi et al. systematically injected 2 × 106 human ADSC through the tail vein of rats after sciatic nerve crush. Researchers found a significant acceleration of functional motor recovery lasting at least 5 weeks, evaluated by SFI analysis. The improvement was confirmed by histopathological analysis [64]. Despite the wide variety of delivery efficiencies, most studies indicated that 1 × 106 ADSCs are needed to generate tracible, therapeutic biological effects after transplantation. However, no studies on the optimal dosing of ADSCs have been officially reported. Tumorigenicity might be a potential concern in stem cell therapies, and the risk is positively correlated with the number of stem cells used [68, 69]. Thus, a lower dose of ADSCs might be considered to achieve equal positive effects. Wu et al. [70] report a positive effect on erectile function after decreasing intracavernous injection dosage from 1 × 106 to 2 × 105 cells. Functional evaluation still showed an effective improvement in erectile function in erectile dysfunction rats.
The Fate of ADSCs After Administration
ADSCs are reported to have low survival after transplantation. Although there is no quantitative data about ADSC, it should be similar to other types of cell transplantations. In general, fewer than 5% of transplanted stem cells persist at the site of transplantation [71, 72]. Ischemia, extracellular matrix degradation, oxidative stress, inflammation, and immune rejection are the most likely causes of in vivo apoptosis of transplanted cells [73]. The majority of transplanted cells apoptose before differentiating and integrating into their environment [74]. Future quantitative ADSC data is needed to prevent these pathways of apoptosis, since the current conditions for augmenting peripheral nerve regeneration with ADSCs appear very nebulous. Though the survival rate of cells is low (about 106 transplanted cells), data suggests that surviving cells contribute to improved outcomes as long as ≥106 cells survive. The detailed therapeutic mechanisms remain unelucidated because of the limited differentiation of surviving transplanted cells, but local secretion of growth factors might contribute to the improved outcomes.
Where Do ADSCs Migrate?
Homing and migration of the transplanted ADSCs were studied by Masgutov [75], who used fibrin glue contained 1 × 106 of ADSCs transduced with lentivirus coding the e-green fluorescent protein (GFP) gene to cover anastomosis of nerve graft. ADSCs locally transplanted fourteen days after autologous nerve graft using the In Vivo Imaging System (IVIS) Spectrum system and green fluorescent protein were found to remain predominately local, although some cells underwent retrograde migration. Another study showed local transplantation had no ADSC migration from the site to other locations in bioluminescence imaging and noted that cells were detected for up to 29 days after the surgery with diminishing quantity [76]. However, the ADSCs did have the ability to migrate into damaged tissues after intravenous, intraperitoneal, or subcutaneous injection in sublethally irradiated nonobese diabetic/severe combined immunodeficient/MOSVII immunodeficient mice regardless of administration route [77].
How Long Do ADSCs Survive After Transplantation?
The number of days post-surgery that transplanted ADSCs survive varies according to the literature depending on how the cells are tracked. Tracking technologies include bioluminescent labeling and immunofluorescence labeling. Erba et al. [78] reported that ADSCs transplanted in an artificial nerve conduit were no longer viable by day 14 post-surgery using a rat sciatic nerve model and tracking the fate of the transplanted cells using green fluorescent protein labeling and polymerase chain reaction. In another study, non-invasive imaging systems were able to track bioluminescently labeled ADSCs in live nude rats with nerve defects and found that gene expression was tracible 3 weeks post-surgery [79]. In a study that implanted 3-D collagen scaffolds with bromodeoxyuridine (BrdU)-labeled human and porcine ADSCs, the cells were identifiable 30 days post-surgery [80]. In a study using the sciatic nerve crush model, labeled uADSCs were still detected 3 months post-surgery and expressed SC proteins [81]. The temporal pattern of luciferase-positive labeled ADSCs in acellular nerves could be detected by an in vivo imaging system for up to 29 days in vivo, but one week after transplantation, many of the cells underwent apoptosis [76]. When human 1 × 106 ADSCs labeled with PKH26 were transplanted into a 6-mm unilateral sciatic nerve injury in athymic rats, the cells stayed alive in the injury site for up to 12 weeks. Colocalization was not observed between the glial fibrillary protein and anti-human lamin A/C (assessed via immunostaining); thus, adipose precursor cells did not differentiate into SCs in the lumen of the nerve [57]. Human ADSCs systemically injected in the sciatic crush mouse model showed the cells alive for up to forty days, without expression of SC-markers, indicating ADSCs may use complex paracrine and autocrine mechanisms to achieve their therapeutic effects, but do not directly participate in the regenerative process [64].
What Happens to ADSCs After Transplantation?
In the existing literature, the fate of the transplanted ADSCs remains unclear. Liao et al. showed that ADSCs indirectly co-cultured with SCs could realize neural trans-differentiation [82]. The cells most likely died one month after transplantation. With electrical stimulation, transplanted neural crest stem cells were able to be observed up to 12 weeks post-transplantation [22]. Another in vitro study examined the survival of ADSCs in a chemically extracted acellular nerve autograft and showed ADSCs survived 5 days after transplantation and had comparable beneficial effects as BMSCs in sciatic nerve injury up to 16 weeks after transplantation [47]. It appeared that differentiated ADSCs (dADSCs) have a greater propensity for survival compared with uADSCs [83]. In vivo survival of ADSCs is not well studied, but the survival of other transplanted cell types ranges from 0.5–38% [78, 84,85,86]. Therefore, the therapeutic effects of cell-based therapies depend not on cell survival at the site of injury, but rather on the survivorship, localization, and identity of administered cells over time, all of which need more future studies.
ADSCs Differentiation into Schwann-Like Cells (SCLCs)
A variety of in vitro studies have shown that the morphology of ADSCs changes under a series of stimuli, forming a tissue similar to nerve tissue [61, 87,88,89]. SCLCs differentiation of ADSCs, induced either with chemical factors, co-culture, cell-cell contact with SCs, or SC-conditioned medium, has been obtained by multiple groups [90, 91]. Kingham et al. first differentiated ADSCs into an SC phenotype in two weeks using the same method [92] as Dezawa [43], who differentiated BMMSCs into an SC phenotype. ADSCs were incubated in alpha-Modified Eagle Medium (α-MEM) containing 1 mM beta-mercaptoethanol (BME). Then, after 24 h, the media was replaced with alpha-MEM, 10% FBS and 35 ng/mL all-trans-retinoic acid (RA) for 3 days. Cells washed with PBS were transferred to α-MEM that contained 10% FBS, 5 mM forskolin (FSK), 10 ng/mL recombinant human basic-fibroblast growth factor (bFGF), 5 ng/mL recombinant human platelet-derived growth factor-AA (PDGF) and 200 ng/mL recombinant human heregulin-beta 1 (HRG) and incubated for 7 days [43]. The SCLCs spindle, similar to that of SCs. The p75, S-100, and glial fibrillary acidic protein (GFAP) markers were co-expressed and up-regulated in SCLCs [92]. ADSCs can also be induced to form neurospheres, which have the potential to become glial cells that is a promising candidate for future clinical translation in nerve regeneration [93]. Most of the methods were modified according to Dezawa and were reported to be more efficient [91, 94]. The intermittent induction method has been cited as the most efficient to induce ADSCs into SCLCs; the SCLCs induced by this method were also more capable of neurotrophin secretion and promotion of DRG axon regeneration in vitro, more similarly like mature myelinating SCs did [18]. Interestingly, fetal bovine serum (FBS) can support ADSCs differentiation better than human serum (HS), and HS puts great stress on differentiating ADSCs, demonstrated by enormous cell detachment and deformation [95].
The time span of induction during the sustaining period following pre-induction is not consistent in the Dezawa and Kingham methods. Cells were incubated from ten days to 2 weeks under these conditions with fresh medium added approximately every 72 h [92, 95] [43, 92, 95]. Long induction times affect the clinical application. For example, induction of BMSCs into SCLCs was dependent on how long the sustaining induction period was, with the optimal length of time being 5–6 days [96, 97]. Tse et al. demonstrated high amounts of BDNF were secreted by native ADSC 48 h after stimulation with a mixture of growth factors (forskolin, bFGF, PDGF-AA, and glial growth factor 2 (GGF-2)). Previously reported pre-differentiation of the ADSCs for 2 weeks might not be necessary for initiating their potential to produce neurotrophic factors, at least not for brain-derived neurotrophic factor (BDNF) [98].
ADSCs can differentiate into SCLCs in terms of morphology, phenotype, and functional capacities. In primary SCs, notch ligand plays a critical role in myelination. Inhibition of notch ligand has no effect on myelination by differentiated ADSCs, but M2 receptor stimulation may strengthen the dADSCs spindle-like phenotype and promote differentiation to an SC-like phenotype [99]. The signaling pathways mediating the neurotrophic activity and myelination capacity of ADSCs remain to be elucidated [100]. Withdrawal of differentiation media from dADSCs resulted in a rapid reversion of the dADSC phenotype to a cell with stem cell-like characteristics [101]. Thus, this process is reversible. It seems more likely that dADSCs are stimulated whilst in a permissive environment rather than truly transdifferentiated. Cells lacking fibroblast growth factor did not sustain a Schwann-like morphology, and those lacking forskolin downregulated BDNF production. Thus, multiple growth factors most likely act synergistically to sustain a Schwann-like phenotype in dADSCs [102]. Further refinements to the cell therapy may be addressed by evaluating alternative protocols providing validated differentiation of stem cells, perhaps by sustained delivery of growth factors to the cells, including in the post-transplant stage. Additionally, these protocols may identify neurotrophic subpopulations of the heterogenous uADSC population that can generate more stable SC-like cells.
Direct differentiation of MSCs to SCs is reported using substrates with imprinted SC-like topographies and geometry. The SC-specific shapes and plasma membrane topographies could be mimicked by imprinted substrates that aid in controlling cell differentiation and fate using a shape-dependent mechanism. Induction of stem cells into target cells is highly efficient using this method, and it is safe and economical [103]. However, the process is time-consuming. This method also has the potential for ADSC application, as ADSCs and MSCs share numerous properties, outlined above.
Examining the Mechanisms of Differentiated vs. Undifferentiated ADSCs
The in vitro capabilities of dADSCs in peripheral nerve repair have been extensively examined. Kingham et al. [104] found increased mRNA levels correlated with enhanced secretion of nerve growth factor (NGF), BDNF, glial cell-derived neurotrophic factor (GDNF), vascular endothelial growth factor A (VEGF-A), and angiopoietin-1 in differentiated MSC compared to uADSCs. In another in vitro evaluation, Kingham et al. [92] found that dADSCs significantly extended the number and the length of formed neurites by motor neuron-like cells compared to uADSCs. Tomita and colleagues also showed that differentiated human ADSCs (dhADSCs) generated more neurotrophic factors like BDNF, NGF, and GDNF compared to uADSCs. Importantly, the neurotrophin levels from dhADSCs did not appear to be affected by the increased donor age [105]. Undifferentiated and dADSCs with a processed nerve allograft showed persistent enhanced expression of neurotrophic genes and neurotrophic growth factor secretion, which results in increased neurite outgrowth in in vitro studies [79, 80]. These results reinforce the hypothesis that dADSCs have a neurotrophic function in nerve regeneration.
In vivo research showed dADSCs transplantation can promote more significant regeneration [81, 83, 104, 106]. dADSCs transplanted into the injured area can supplement and enhance the role of the remaining SCs, thus helping the regeneration process. In addition to their paracrine effects, ADSCs can differentiate into neurons in vitro and in vivo [81, 107]. Scholz et al. bridged a 13-mm sciatic nerve gap with silastic conduits in 64 athymic nude rats, and human dADSCs were transplanted into the nerve gap. Four months after nerve injury, the sciatic nerve function of animals transplanted with dADSCs combined with differentiation medium was similar to that of nerve isograft transplantation. dADSCs alone could not form synaptic connections, but compared with nerve transplantation, the diameter of axons increased significantly after differentiation of ADSCs co-existing with culture medium [106]. Therefore, the beneficial role of ADSC in PNI and regeneration may be mainly achieved through the interaction of nutrient growth factors between nerves [57].
dADSCs’ role in PNI has also been extensively studied. When seeded on a conduit and transplanted in a rat model, Kingham found the dADSCs evoked more total outgrowth and enhanced vascularity in nerve conduits compared to uADSCs [92]. Hence, dADSC-produced neurotrophic and angiogenic factors improve the recovery of injured nerves by vascularizing the area and bolstering nerve regeneration. Kappos et al. [52] showed that in a rat sciatic nerve gap model, the addition of human dADSCs to a nerve conduit led to less atrophy and superior functional results when compared to uADSCs. Tomita et al. [83] showed in a rat tibial nerve crush model, in vivo differentiated human ADSC transplantation improved myelin formation rate (tenfold increase) and nerve survival (sevenfold increase) when compared to undifferentiated human ADSCs. Orbay et al. [81] studied the effects of seeding dADSCs and uADSCs in silicone tubes and comparing outcomes to unseeded silicone tubes and nerve grafts and found that the functional outcomes of both ADSC groups were significantly better than control groups, but that there were no significant differences between the two ADSC groups. Watanabe et al. [108] compared uADSCs, differentiated ADSCs, and SCs in a rat facial nerve injury model and concluded that each group had comparable nerve regeneration, and that cell-based therapies gave functional results commensurate with autografts.
In vitro and in vivo research on differentiated and undifferentiated MSCs in peripheral nerve regeneration experiments yielded similar results [109] to those of ADSCs, supplying additional evidence for the promising future of ADSCs given the similarities between the two cell types. Future clinical study designs should take into consideration that the extra cost and preparation time (three weeks) are required for MSC differentiation when choosing between differentiated and undifferentiated cells. However, dADSCs reduce scar tissue formation and promote nerve regeneration efficiently [110].
Therapeutic Mechanisms of ADSCs in Promoting Nerve Regeneration (Fig. 1)
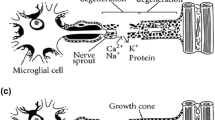
Therapeutic Mechanisms of ADSCs in promoting nerve regeneration. ADSCs are transplanted to fill the gap between the damaged peripheral nerves. ADSCs secrete a variety of neurotrophic factors, such as brain derived neurotrophic factor (BDNF), glial cell derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), nerve growth factor (NGF), and neurotrophin (NT)-3 and NT-4, which are critical signals that Schwann cells utilize to direct axons to the distal nerve stump. ADSCs may also trans-differentiate into Schwann cell like cells (SCLCs), which can assist bridging the nerve gap, similar to SC. SCLCs ensheath the regenerating axons and are positive for MBP and P0 myelin proteins
In vivo Trans-Differentiation into SCs
ADSCs establish tissue regeneration by structural support of tissue and in vivo differentiation in injured tissue and subsequent growth factor secretion and other paracrine product releases by surrounding tissue, which induces differentiation into the requisite type of cell. Tomita and colleagues reported that a small fraction of their GFP-labeled ADSCs were present eight weeks after rat sciatic nerve injury and expressed myelin protein, suggesting trans-differentiation into SCs [83]. Transplanted, uADSCs visualized in gelatin hydrogel conduit using Cre-loxP-mediated fate tracking, a more suitable tool monitor system for SC differentiation, can promote peripheral nerve regeneration in vivo without differentiation into SCs [111]. This is consistent with most previous studies, in which no evidence of in vivo transdifferentiation of uADSCs into SCs was observed [57, 64]. While cellular differentiation is crucial for neuronal development, it is unclear if ADSC therapies replicate the normal SC differentiation.
ADSCs Secrete Growth Factors to Promote Peripheral Nerve Growth
The paracrine function involved in neural regeneration depends on soluble growth factors that induce vascularization, protect tissue, or suppress host immune defenses [112]. Increasing the expression of neurotrophic factors could promote axonal germination and nerve regeneration, increasing the muscle mass of target organs, and thus accelerating the recovery of motor function [64, 112, 113]. The positive effects of ADSCs for peripheral nerve regeneration are thought to primarily occur because of the secretion of a variety of neurotrophic factors. BDNF, GDNF, ciliary neurotrophic factor (CNTF), bFGF, insulin-like growth factor 1 (IGF-1), nerve growth factor (NGF), neurotrophin (NT)-3, and NT-4 were expressed in ADSCs in vitro and in vivo [60, 114]. Each of these growth factors had a clear effect on the peripheral nervous system.
Neurotrophic factor expression profiles of ADSCs and BMMSCs showed similar gene expression characteristics in the two groups [115]. Both of them secrete several growth factors, such as insulin-like growth factor 1 (IGF-1), VEGF, FGF-2, PDGF, and BDNF [114, 116]. In addition, it has been shown that after systematic injection of ASDCs, some cells have been shown to migrate to nerve injury sites, helping to reduce inflammation and release nerve growth factors to promote nerve regeneration [64]. It was also shown that systemically induced ADSCs played an immunomodulatory role by increasing the production of BDNF and GDNF by host SCs. This was associated with increased sprouting and decreased inflammatory infiltrate [64].
The neurotrophic potential of ADSCs is determined by anatomical sites [117, 118], the depth of the fat layer [113, 119], and the age of the donor [120, 121]. Both ADSCs and ADSCs stimulated with growth factors increased the vascularity of the fibrin nerve conduits. Thus, ADSC produces functional neurotrophic and angiogenic factors, creating a more desirable microenvironment for nerve regeneration [104].
On the other hand, different types of growth factors can also promote the differentiation of ADSCs. In Mallappa et al.’s study, they showed that ADSCs stimulated by glial growth factor produce a differentiated SC-like phenotype (dADSCs), and the induced cells assume a spindle shape similar to that of SCs. The neurotrophic factors were up-regulated [92]. Treated rat ADSCs [92] and human ADSCs [83, 122] also showed increased expression of growth factors, which could promote neurite growth in vitro. The phenotype of differentiated cells increases the production of nerve growth factor, brain-derived neurotrophic factor, and glial cell-derived neurotrophic factor, which is a molecule that promotes regeneration and neuronal survival [123].
ADSCs Promote Myelination Growth
In PNI, the myelin sheath is formed by the differentiation of the SC plasma membrane. The compact structure of the myelin sheath is the premise of electrical signal transmission, and myelin formation is another important factor to determine the quality of PNI regeneration and functional recovery. In vitro, SCLCs induced from ADSCs were able to form myelin structures with PC12 cells [89]. The synthesis of a large number of myelin basic proteins (Myelin Basic Protein, MBP) by SCs plays an important role in the recovery of myelin structure and function [124, 125]. Similar to other stem cells, ADSC that differentiate into SC-like cells in vivo has shown the ability to support myelin formation in regenerated nerves. dADSCs were ensheathing the regenerating axons and were positive for MBP and P0 myelin proteins as early as 10 weeks after transplantation, suggesting that the presence of in vivo environmental factors could provide dADSCs enough cues for myelination, indicating glial fate commitment, thus implying their potential for clinical use [105]. dADSCs are able to express the myelin proteins found in the PNS, thus there is evidence that these cells are morphologically and functionally similar to SCs [52].
ADSC Exosomes on Nerve Regeneration
Exosomes are small extracellular nano-sized (30 ~ 100 nm) vesicles with a lipid bilayer membrane released by all cell types. Exosomes have been shown to improve the nerve regeneration process [126]. The role of secreted exosomes in cell-to-cell communication as an alternative to the traditional paracrine signaling processes has recently been elucidated [126]. In addition to conventional secreted paracrine molecules, exosomes, constitutively produced by ADSCs or dADSCs, are involved in peripheral nerve regeneration [127, 128]. Bucan et al. [127] found that ADSC exosomes promoted the proliferation of SCs 4 days after incubation and there is a tendency for exosomes to enhance the neurite length of dorsal root ganglion (DRG) neurons. Moreover, the researchers demonstrated the presence of neural growth factors in the ADSC- exosomes, such as BDNF, IGF-1, NGF, FGF-1, and GDNF. These results suggest a possible mechanism by which exosomes bolster axon regeneration in vivo. Ching found that dADSC exosomes replicated SC exosomes’ effect on neurite outgrowth and transferred RNA molecules play an important role in the process [128]. In addition, low doses of ADSC- exosomes increased the viability of and exerted antiapoptotic effects on neural cells by inhibiting the apoptotic cascade after those cells were exposed to oxidative damage with H2O2. ADSC‐exosomes may reduce the apoptosis of SCs after PNI by upregulating the anti-apoptotic Bcl‐2 mRNA expression and downregulating the pro‐apoptotic Bax mRNA expression [129]. Further, it also improved the proliferation rate of SCs [129]. ADSC exosomes could increase the process of remyelination and activate nestin-positive oligodendroglia precursors to exert their neuro-regeneration functions [130].
Conclusions
ADSCs are easy to access, derive and expand. Furthermore, these cells can be successfully differentiated into SCLCs. Therefore, ADSCs, particularly ADSCs differentiated into SCLCs, have been broadly studied to improve the outcomes of peripheral nerve repair/reconstruction. The surviving cells may promote nerve regeneration by secreting NGF or with paracrine crosstalk to SCs. Relevant literature has been summarized in Table 1.
Future Perspective
ADSC transplantation has broad prospects for repairing PNI, but it is still at an early stage. Several issues need to be addressed. Differentiated adipose-derived stem cells have significant advantages, but the induction and differentiation time of current methods is too long, which affects the clinical application. Maintaining differentiation requires additional research. The survival rate of ADSCs transplantation remains low. Mechanisms to reduce ischemia, inflammation, immune rejection, and apoptosis to improve cell survival rate are a prerequisite and must be elucidated in future studies. The mechanism of successful ADSC transplantation for nerve regeneration is still unclear. Future research must elucidate how to further induce SCs from ADSCs.
Further studies examining the advantages of dADSCs vs. uADSCs must be conducted. In vitro studies demonstrate dADSCs increased neurotrophic gene expression and neurotrophic factor secretion that led to increased neurite outgrowth compared to uADSCs. The advantages of dADSCs are yet to be confirmed by in vivo studies. Current induction methods are time-consuming and need chemicals to maintain phenotype. More effective methods, such as physical induction, are needed without chemical maintenance. Furthermore, the ideal method of delivery and dosage of dADSCs and uADSCs must be established to elucidate the potential for regenerative peripheral nerve reconstruction.
Stem cell transplantation after PNI is still in the early stage of research, has not yet made significant progress in clinical practice. No clinical ADSC application after PNI has been reported yet. Although the simple application of stem cell transplantation in experimental animals has shown promising results, there are still genetic manipulation, cell instability, and tumorigenesis. The expression of stem cells in vivo after homing and migration is still a matter of concern. At present, the traditional nerve repair technology is still the main clinical nerve repair treatment, and has not yet been used in large-scale stem cell therapy. Preclinical and final clinical studies are needed, and other factors, such as optimal differentiation, exact potential mechanisms, signal transduction between ADSCs and injured neurons, potential interaction mechanisms, and the interaction between cytokines all need to be taken into account. Of course, age is also a very important factor. Several clinical studies have demonstrated that younger age is associated with a more favorable prognosis comparing to the elder after PNI [131]. However, there is no clear literature on the specific differentiation and growth of ADSCs by age. A lot of research is still needed to come to a clear conclusion.
Abbreviations
- PNIs:
-
Peripheral nerve injuries
- ADSCs:
-
Adipose-derived stem cells
- uADSCs:
-
Undifferentiated ADSCs
- dADSCs:
-
Differentiated ADSCs
- SCs:
-
Schwann cells
- SCLCs:
-
Schwann cell-like cells
- NGCs:
-
Neural guidance conduits
- MSCs:
-
Mesenchymal stem cells
- BMMSCs:
-
Bone marrow mesenchymal stem cells
References
Jiang, L., Jones, S., & Jia, X. (2017). Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. International Journal of Molecular Science, 18, 1.
Jones, S., Eisenberg, H. M., & Jia, X. (2016). Advances and Future applications of augmented peripheral nerve regeneration. International Journal of Molecular Science, 17, 9.
Hallgren, A., et al. (2013). Subjective outcome related to donor site morbidity after sural nerve graft harvesting: A survey in 41 patients. BMC Surgery, 13, 39.
Kehoe, S., Zhang, X. F., & Boyd, D. (2012). FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury, 43(5), 553–572.
Deumens, R., et al. (2010). Repairing injured peripheral nerves: Bridging the gap. Progress in Neurobiology, 92(3), 245–276.
Guntinas-Lichius, O., et al. (2005). Factors limiting motor recovery after facial nerve transection in the rat: Combined structural and functional analyses. European Journal of Neuroscience, 21(2), 391–402.
Boriani, F., et al. (2017). A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. Journal of Biomedical Materials and Research A, 105(8), 2228–2240.
Willand, M. P., et al. (2016). Electrical stimulation to promote peripheral nerve regeneration. Neurorehabilitation and Neural Repair, 30(5), 490–496.
Kaplan, H. M., Mishra, P., & Kohn, J. (2015). The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. Journal of Materials Science: Materials in Medicine, 26(8), 226.
Georgiou, M., et al. (2015). Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials, 37, 242–251.
Gaudin, R., et al. (2016). Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Research International, 2016, 3856262.
Labroo, P., et al. (2019). Drug-delivering nerve conduit improves regeneration in a critical-sized gap. Biotechnology and Bioengineering, 116(1), 143–154.
Berrocal, Y. A., et al. (2013). Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. Journal of Neurosurgery, 119(3), 720–732.
Ursu, D., et al. (2017). Adjacent regenerative peripheral nerve interfaces produce phase-antagonist signals during voluntary walking in rats. Journal of Neuroengineering Rehabilation, 14(1), 33.
Xu, F., et al. (2017). NECL1 coated PLGA as favorable conduits for repair of injured peripheral nerve. Materials Science and Engineering C: Materials in Biology Application, 70(Pt 2), 1132–1140.
Lewitus, D., et al. (2011). Designing tyrosine-derived polycarbonate polymers for biodegradable regenerative type neural interface capable of neural recording. IEEE Transaction on Neural System Rehabilation Engineering, 19(2), 204–212.
Sullivan, R., et al. (2016). Peripheral nerve injury: Stem cell therapy and peripheral nerve transfer. International Journal of Molecular Science, 17, 12.
Sun, X., et al. (2018). Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Research Theraphy, 9(1), 133.
Walocko, F. M., et al. (2016). The potential roles for adipose tissue in peripheral nerve regeneration. Microsurgery, 36(1), 81–88.
Houschyar, K. S., et al. (2016). The role of current techniques and concepts in peripheral nerve repair. Plastic Surgery International, 2016, 4175293.
Du, J., et al. (2018). Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomaterials and Science, 6(6), 1299–1311.
Du, J., et al. (2018). Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials, 181, 347–359.
Burnett, M. G., & Zager, E. L. (2004). Pathophysiology of peripheral nerve injury: A brief review. Neurosurgery Focus, 16(5), E1.
Menorca, R. M., Fussell, T. S., & Elfar, J. C. (2013). Nerve physiology: Mechanisms of injury and recovery. Hand Clinic, 29(3), 317–330.
Girard, C., et al. (2008). Etifoxine improves peripheral nerve regeneration and functional recovery. Proceedings of the Natational Academic Science USA, 105(51), 20505–20510.
Oh, S. H., et al. (2008). Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials, 29(11), 1601–1609.
Young, R. C., Wiberg, M., & Terenghi, G. (2002). Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. British Journal of Plastic Surgery, 55(3), 235–240.
Fawcett, J. W., & Keynes, R. J. (1986). Muscle basal lamina: A new graft material for peripheral nerve repair. Journal of Neurosurgery, 65(3), 354–363.
Humphrey, M. F., et al. (1989). Peripheral nerve repair across a gap studied by repeated observation in a new window implant chamber. Brain Research, 497(1), 132–137.
Park, S. C., et al. (2010). Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Journal of Biomedical Material Research B Applied Biomaterials, 94(2), 359–366.
Lopez-Verrilli, M. A., Picou, F., & Court, F. A. (2013). Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia, 61(11), 1795–1806.
Yuan, Y. C., et al. (2005). Axon and schwann cell partnership during nerve regrowth. Journal of Neuropathology and Experimental Neurology, 64(7), 613–622.
Kaewkhaw, R., Scutt, A. M., & Haycock, J. W. (2012). Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nature of Protocol, 7(11), 1996–2004.
Levi, A. D., et al. (2016). The use of autologous schwann cells to supplement sciatic nerve repair with a large gap: First in human experience. Cell Transplantation, 25(7), 1395–1403.
Rutkowski, J. L., et al. (1995). Purification and expansion of human Schwann cells in vitro. Nature of Medicine, 1(1), 80–83.
Kim, H. S., et al. (2017). Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Reports, 8(6), 1714–1726.
Palomo Irigoyen, M., et al. (2018). Isolation and purification of primary rodent schwann cells. Methods Molecular Biology, 1791, 81–93.
Weiss, T., et al. (2018). Detailed protocols for the isolation, culture, enrichment and immunostaining of primary human Schwann cells. Methods Molecular Biology, 1739, 67–86.
Anderson, K. D., et al. (2017). Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. Journal of Neurotrauma, 34(21), 2950–2963.
Reichenberger, M. A., et al. (2016). ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery, 36(6), 491–500.
Hsieh, S. C., et al. (2016). Effect of an epineurial-like biohybrid nerve conduit on nerve regeneration. Cell Transplantation, 25(3), 559–574.
He, X., et al. (2016). Transplantation of miRNA-34a overexpressing adipose-derived stem cell enhances rat nerve regeneration. Wound Repair and Regeneration, 24(3), 542–550.
Dezawa, M., et al. (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. European Journal of Neuroscience, 14(11), 1771–1776.
Lavorato, A., et al. (2021). Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: systematic review. International Journal of Molecular Science, 22, 2.
Zuk, P. A., et al. (2001). Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering, 7(2), 211–228.
Gimble, J. M., Katz, A. J., & Bunnell, B. A. (2007). Adipose-derived stem cells for regenerative medicine. Circulation Research, 100(9), 1249–1260.
Zhou, L. N., et al. (2020). A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Research Theraphy, 11(1), 153.
Beane, O. S., et al. (2014). Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE, 9(12), e115963.
Rodriguez Sanchez, D. N., et al. (2019). Canine adipose-derived mesenchymal stromal cells enhance neuroregeneration in a rat model of sciatic nerve crush injury. Cell Transplantation, 28(1), 47–54.
Tremp, M., et al. (2018). Regeneration of nerve crush injury using adipose-derived stem cells: A multimodal comparison. Muscle and Nerve, 58(4), 566–572.
Kappos, E. A., et al. (2018). Epineural adipose-derived stem cell injection in a sciatic rodent model. Brain Behaviour, 8(7), e01027.
Kappos, E. A., et al. (2015). Peripheral nerve repair: Multimodal comparison of the long-term regenerative potential of adipose tissue-derived cells in a biodegradable conduit. Stem Cells and Development, 24(18), 2127–2141.
Li, Y. C., et al. (2014). A neural stem/precursor cell monolayer for neural tissue engineering. Biomaterials, 35(4), 1192–1204.
Lasso, J. M., et al. (2015). Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. Journal of Plastic Reconstruction Aesthetics Surgery, 68(12), e189–e197.
Luo, H., et al. (2015). Tissue-engineered nerve constructs under a microgravity system for peripheral nerve regeneration. Tissue Engineering Part A, 21(1–2), 267–276.
Allbright, K. O., et al. (2018). Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle and Nerve, 58(2), 251–260.
Santiago, L. Y., et al. (2009). Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplantation, 18(2), 145–158.
Klein, S. M., et al. (2016). Peripheral motor and sensory nerve conduction following transplantation of undifferentiated autologous adipose tissue-derived stem cells in a biodegradable US food and drug administration-approved nerve conduit. Plastic Reconstruction Surgery, 138(1), 132.
di Summa, P. G., et al. (2010). Adipose-derived stem cells enhance peripheral nerve regeneration. Journal of Plastic, Reconstructive and Aesthetic Surgery: JPRAS, 63(9), 1544–1552.
Kim, D. Y., et al. (2014). In vivo effects of adipose-derived stem cells in inducing neuronal regeneration in Sprague-Dawley rats undergoing nerve defect bridged with polycaprolactone nanotubes. Journal of Korean Medical Science, 29, S183–S192.
Han, I. H., et al. (2015). Cultures of Schwann-like cells differentiated from adipose-derived stem cells on PDMS/MWNT sheets as a scaffold for peripheral nerve regeneration. Journal of Biomedical Materials Research Part A, 103(11), 3642–3648.
Kolar, M. K., & Kingham, P. J. (2014). Regenerative effects of adipose-tissue-derived stem cells for treatment of peripheral nerve injuries. Biochemical Society Transactions, 42(3), 697–701.
Fu, X. M., et al. (2019). The combination of adipose-derived schwann-like cells and acellular nerve allografts promotes sciatic nerve regeneration and repair through the JAK2/STAT3 signaling pathway in rats. Neuroscience, 422, 134–145.
Marconi, S., et al. (2012). Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Engineering Part A, 18(11–12), 1264–1272.
Schweizer, R., et al. (2020). Effect of Systemic adipose-derived stem cell therapy on functional nerve regeneration in a rodent model. Plastic Reconstruction Surgery Global Open, 8(7), e2953.
Lin, G., et al. (2011). Tracking intracavernously injected adipose-derived stem cells to bone marrow. International Journal of Impotence Research, 23(6), 268–275.
Rbia, N., et al. (2018). A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plastic Reconstruction Surgery, 142(2), 402–413.
Wang, D., Wang, S., & Shi, C. (2012). Update on cancer related issues of mesenchymal stem cell-based therapies. Current Stem Cell and Reserach Therapy, 7(5), 370–380.
Lee, A. S., et al. (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Natural Medicine, 19(8), 998–1004.
Wu, H., et al. (2018). Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury. Asian Journal of Andrology, 20(5), 442–447.
Burdick, J. A., Mauck, R. L., & Gerecht, S. (2016). To serve and protect: Hydrogels to improve stem cell-based therapies. Cell Stem Cell, 18(1), 13–15.
Sart, S., Ma, T., & Li, Y. (2014). Preconditioning stem cells for in vivo delivery. Bioresearch Open Access, 3(4), 137–149.
Hwang, D. W., et al. (2014). In vivo bioluminescence imaging for prolonged survival of transplanted human neural stem cells using 3D biocompatible scaffold in corticectomized rat model. PLoS ONE, 9(9), e105129.
Hyun, J. S., et al. (2013). Enhancing stem cell survival in vivo for tissue repair. Biotechnology Advance, 31(5), 736–743.
Masgutov, R., et al. (2018). Allogenic adipose derived stem cells transplantation improved sciatic nerve regeneration in rats: Autologous nerve graft model. Frontier Pharmacology, 9, 86.
Rbia, N., et al. (2019). In vivo survival of mesenchymal stromal cell-enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. The Journal of Hand Surgery, 44(6), 514.e1-514.e11.
Meyerrose, T. E., et al. (2007). In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells, 25(1), 220–227.
Erba, P., et al. (2010). Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. Journal of Plastic Reconstruction Aesthetics Surgery, 63(12), e811–e817.
Wolbank, S., et al. (2007). Labelling of human adipose-derived stem cells for non-invasive in vivo cell tracking. Cell and Tissue Banking, 8(3), 163–177.
Lequeux, C., et al. (2011). Adipose derived stem cells: Efficiency, toxicity, stability of BrdU labeling and effects on self-renewal and adipose differentiation. Molecular Cell Biochemistry, 351(1–2), 65–75.
Orbay, H., et al. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive and Aesthetic Surgery, 65(5), 657–664.
Liao, D., et al. (2010). Co-culture with Schwann cells is an effective way for adipose-derived stem cells neural transdifferentiation. Archies Medical Science, 6(2), 145–151.
Tomita, K., et al. (2013). Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience, 236, 55–65.
Faroni, A., et al. (2013). Differentiation of adipose-derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death Diseases, 4, e743.
Walsh, S. K., et al. (2012). Fate of stem cell transplants in peripheral nerves. Stem Cell Research, 8(2), 226–238.
Walsh, S., & Midha, R. (2009). Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurgical Focus, 26(2), E2.
Abbas, O. L., et al. (2016). Adipose-derived stem cells enhance axonal regeneration through cross-facial nerve grafting in a rat model of facial paralysis. Plastic and Reconstructive Surgery, 138(2), 387–396.
Guo, J., et al. (2017). Promoting potential of adipose derived stem cells on peripheral nerve regeneration. Molecular Medicine Reports, 16(5), 7297–7304.
Xu, Y., et al. (2008). Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Research, 1239, 49–55.
Wei, Y., et al. (2010). Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Proliferation, 43(6), 606–616.
Jiang, L., et al. (2008). Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. NeuroReport, 19(10), 1015–1019.
Kingham, P. J., et al. (2007). Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Experimental Neurology, 207(2), 267–274.
Xu, Y., et al. (2008). Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neuroscience, 9, 21.
Gao, S., et al. (2015). Different methods for inducing adipose-derived stem cells to differentiate into Schwann-like cells. Archives Medical Sciences, 11(4), 886–892.
Younesi, E., et al. (2015). Differentiation of adipose-derived stem cells into Schwann-like cells: Fetal bovine serum or human serum? Anatomy and Cell Biology, 48(3), 170–176.
Ladak, A., et al. (2011). Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Experimental Neurology, 228(2), 242–252.
Hou, S. Y., et al. (2006). Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience, 140(1), 101–110.
Tse, K. H., et al. (2015). Intrinsic mechanisms underlying the neurotrophic activity of adipose derived stem cells. Experimental Cell Research, 331(1), 142–151.
Piovesana, R., et al. (2019). M2 receptors activation modulates cell growth, migration and differentiation of rat Schwann-like adipose-derived stem cells. Cell Death Discovery, 5, 92.
Kingham, P. J., Mantovani, C., & Terenghi, G. (2009). Notch independent signalling mediates Schwann cell-like differentiation of adipose derived stem cells. Neuroscience Letter, 467(2), 164–168.
Faroni, A., et al. (2016). Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. European Journal of Neurosciences, 43(3), 417–430.
Mortimer, A. E., et al. (2017). Maintenance of a Schwann-like phenotype in differentiated adipose-derived stem cells requires the synergistic action of multiple growth factors. Stem Cells and International, 2017, 1479137.
Moosazadeh Moghaddam, M., et al. (2019). Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artificial Cells Nanomedicine and Biotechnology, 47(1), 1022–1035.
Kingham, P. J., et al. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Development, 23(7), 741–754.
Tomita, K., et al. (2012). Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. Journal of Neuroscience Research, 90(7), 1392–1402.
Scholz, T., et al. (2011). Neuronal differentiation of human adipose tissue–derived stem cells for peripheral nerve regeneration in vivostem cells for peripheral nerve regeneration. Archives of Surgery, 146(6), 666–674.
Gao, S., et al. (2014). Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds. Tissue Engineering Part A, 20(7–8), 1271–1284.
Watanabe, Y., et al. (2017). Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. Journal of Tissue Engineering and Regenerative Medicine, 11(2), 362–374.
Mathot, F., et al. (2020). Gene expression profiles of differentiated and undifferentiated adipose derived mesenchymal stem cells dynamically seeded onto a processed nerve allograft. Gene, 724, 144151.
Di Summa, P. G., et al. (2018). Adipose derived stem cells reduce fibrosis and promote nerve regeneration in rats. The Anatomical Record (Hoboken), 301(10), 1714–1721.
Sowa, Y., et al. (2016). Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plastic Reconstruction Surgery, 137(2), 318e–330e.
Zuk, P. (2013). Adipose-derived stem cells in tissue regeneration: A review. ISRN Stem Cells, 2013, 1–35.
Kalbermatten, D. F., et al. (2011). Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Research, 344(2), 251–260.
Wilkins, A., et al. (2009). Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Research, 3(1), 63–70.
Taghi, G. M., et al. (2012). Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biology International, 36(12), 1239–1249.
Osugi, M., et al. (2012). Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Engineering Part A, 18(13–14), 1479–1489.
Kaewkhaw, R., Scutt, A. M., & Haycock, J. W. (2011). Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia, 59(5), 734–749.
Engels, P. E., et al. (2013). Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology, 65(3), 437–445.
Mathias, T., et al. (2015). The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplation, 24(2), 203–211.
Mantovani, C., et al. (2012). Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Experimental Cell, 318(16), 2034–2048.
Yoshihiro, S., et al. (2012). Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: Influence of age and anatomic site of origin. Stem Cells and Development, 21(11), 1852–1862.
Kingham, J. P., et al. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells and Development, 23(7), 741–754.
Ochoa, J., et al. (1971). Nature of the nerve lesion caused by a pneumatic tourniquet. Nature, 233(5317), 265–266.
Masgutov, R. F., et al. (2016). Human adipose-derived stem cells stimulate neuroregeneration. Clinical Experimental Medicine, 16(3), 451–461.
Xie, S., et al. (2017). Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle, 16(9), 841–851.
Qing, L., et al. (2018). Exosomes and their MicroRNA cargo: New players in peripheral nerve regeneration. Neurorehabiliation Neural Repair, 32(9), 765–776.
Bucan, V., et al. (2019). Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Molecular Neurobiology, 56(3), 1812–1824.
Ching, R. C., Wiberg, M., & Kingham, P. J. (2018). Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cells and Research Theraphy, 9(1), 266.
Liu, C. Y., et al. (2019). Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neuroscience and Theraphy.
Farinazzo, A., et al. (2015). Murine adipose-derived mesenchymal stromal cell vesicles: In vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy, 17(5), 571–578.
Jacob, J. M., & Robbins, N. (1990). Differential effects of age on neuromuscular transmission in partially denervated mouse muscle. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 10(5), 1522.
Syu, W. Z., et al. (2019). Adipose-derived neural stem cells combined with acellular dermal matrix as a neural conduit enhances peripheral nerve repair. Cell Transplantation, 28(9–10), 1220–1230.
Kim, Y. M., et al. (2009). Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. The Laryngoscope, 119(5), 993–999.
Park, J. U., & Kwon, S. T. (2017). Potential of autologous adipose-derived stem cells to regenerate atrophied muscle in a rat model. Wound Repair Regeneration, 25(6), 944–955.
Rbia, N., et al. (2019). In vivo survival of mesenchymal stromal cell-enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. Journal of Hand Surgery American, 44(6), 514.
Funding
XJ was supported in part by Maryland Stem Cell Research Fund, USA (2018-MSCRFD-4271 and 2020-MSCRFD-5384) (both to XJ), NIH RO1 NS117102 (to XJ).
Author information
Authors and Affiliations
Contributions
LJ contributed with data acquisition and analysis, searched and reviewed the literature, drafted and revised the manuscript; XZ searched and reviewed the literature, and drafted the manuscript; TM reviewed the literature and revised the manuscript; XJ conceived the idea, designed and formulated the review theme, viewed the literature, data analysis, and revised and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict Interest
The authors declared no potential conflicts of interest. The founding sponsors had no role in the writing of this review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement: This concise review seeks to highlight the recent advances in augmenting nerve regeneration after peripheral nerve injury using adipose-derived stem cells with a focus on administration routes, cell dosages, cell fates, and underlying therapeutic mechanisms.
Rights and permissions
About this article
Cite this article
Jiang, L., Mee, T., Zhou, X. et al. Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells. Stem Cell Rev and Rep 18, 544–558 (2022). https://doi.org/10.1007/s12015-021-10236-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10236-5