Abstract
Hepatocellular carcinoma (HCC) remains a major global health problem with high incidence and mortality. Diagnosis of HCC at late stages and tumour heterogeneity in patients with different genetic profiles are known factors that complicate the disease treatment. HCC therapy becomes even more challenging in patients with drug resistance such as resistance to sorafenib, which is a common drug used in HCC patients. Sorafenib resistance can further aggravate HCC by regulating various oncogenic pathways such as autophagy and nuclear factor-kappa Beta (NF-ĸβ) signalling. Sirtuin 1 (SIRT1), is a nicotinamide adenosine dinucleotide (NAD)-dependent histone deacetylases that regulates various metabolic and oncogenic events such as cell survival, apoptosis, autophagy, tumourigenesis, metastasis and drug resistance in various cancers, but its role in HCC, particularly in sorafenib resistance is underexplored. In this study, we generated sorafenib-resistant HepG2 and Huh-7 liver cancer cell models to investigate the role of SIRT1 and its effect on autophagy and nuclear factor-kappa Beta (NF-ĸβ) signalling pathways. Western blot analysis showed increased SIRT1, altered autophagy pathway and activated NF-ĸβ signalling in sorafenib-resistant cells. SIRT1-silenced HCC cells demonstrated down-regulated autophagy in both parental and chemoresistant cells. This may occur through the deacetylation of key autophagy molecules such as FOXO3, beclin 1, ATGs and LC3 by SIRT1, highlighting the role of SIRT1 in autophagy induction. Silencing of SIRT1 also resulted in activated NF-ĸβ signalling. This is because SIRT1 failed to deacetylate p65 subunit of NF-κB, translocate the NF-κB from nucleus to cytoplasm, and suppress NF-κB activity due to the silencing. Hence, the NF-κB transcriptional activity was restored. These findings summarize the role of SIRT1 in autophagy/NF-ĸβ regulatory axis, with a similar trend observed in both parental and sorafenib-resistant cells. The present work promotes a better understanding of the role of SIRT1 in autophagy and NF-ĸβ signalling in HCC and sorafenib-resistant HCC. As some key proteins in these pathways are potential therapeutic targets, a better understanding of SIRT1/autophagy/NF-ĸβ axis could further improve the therapeutic strategies against HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the third leading cause of cancer-related death worldwide in 2020 [1], being more common in sub-Saharan Africa and Southeast Asia. Hepatocellular carcinoma (HCC) is the major type of liver cancer followed by cholangiocarcinoma. In total, 80% of HCC patients are diagnosed at advanced stage with a reported median survival of 6–8 months [2]. Sorafenib is the first-line targeted therapy for advanced HCC and has also shown excellent effects with recurrent tumours [3]. However, acquisition of chemoresistance remains the main challenge which renders the treatment ineffective [2].
Sirtuin 1 (encoded by SIRT1 gene), a NAD-dependent class III deacetylase, is mainly localized in the nucleus and is a key regulator for cell proliferation and apoptosis. Transcription factors such as p53, E2F1, FOXO, NF-ĸβ and c-Myc have been identified as the targets for SIRT1 [4], and these interactions give rise to cancer development and metastasis in various cancers [5,6,7]. In HCC, overexpression of SIRT1 could drive tumour cell survival and growth [8,9,10], and promote metastasis [11]. SIRT1 localizes predominantly in the nucleus where it promotes tumorigenesis, while it has been reported that cytoplasmic sirtuin 1 may have a tumour-suppressive role in HCC [12, 13]. SIRT1 is also known to regulate chemoresistance in various cancers such as ovarian, breast and gastric cancers [14,15,16], but the role of SIRT1 in HCC chemoresistance is underexplored.
Autophagy is a self-digestive defensive mechanism that degrades damaged cellular organelles and wastes through lysosomal degradation. Over the years, the dysregulation of autophagy has also been associated with various cancers including colorectal cancer (CRC) [17] and HCC [6]. Autophagy could either promote or suppress tumour growth in HCC and is regulated by sirtuin 1 and other pathways involving proto-oncogenes or tumour suppressors such as PI3K, AKT, mTOR and TP53 [6]. Interestingly, autophagy is also known to associate with drug resistance for HCC [18, 19]. However, the underlying mechanisms involving autophagic proteins and the related signalling pathways are not known.
Nuclear factor kappa B (NF-ĸβ) signalling is the main pathway in inflammation and other cellular respincluding cancer. SIRT1 can inhibit NF-ĸβ signalling directly or indirectly [20] while NF-ĸβ subunits such as p65 and p50 transcription factors could target downstream targets of SIRT1 and inhibit the function [21]. Many studies have also reported the crosstalks between NF-ĸβ signalling and autophagy in cancer where autophagy could either promote or inhibit tumour growth by regulating NF-ĸβ pathway [22].
In this study, we generated sorafenib-resistant cells using HepG2 and Huh-7 liver cancer cell lines and used these models to investigate the role of SIRT1 and its effect on autophagy and NF-ĸβ signalling pathways. We demonstrated the increased SIRT1 expression, altered autophagy pathway and activated NF-ĸβ signalling in sorafenib-resistant cells. Knockdown of SIRT1 inhibited autophagy in both parental and chemoresistant cells. In contrast, NF-ĸβ signalling was not affected by knockdown of SIRT1 in all cells. Our findings reveal the key role of SIRT1 in activating autophagy pathway in both parental and chemoresistant HCC cells. Interestingly, silencing of SIRT1 activated NF-ĸβ signalling, and this could also be led by the SIRT1-mediated downregulation of autophagy. This study promotes a better understanding of the role of SIRT1 in autophagy and NF-ĸβ signalling in HCC and sorafenib-resistant HCC, and this could improve the current therapeutic strategies against HCC and chemoresistant HCC.
Methods
Cell culture
HepG2 was purchased from AddexBio (#C0015002). Cells were maintained in Roswell Park Memorial Institute Medium (RPMI) supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/ streptomycin. Huh-7 was purchased from Abcam (#T112). Huh-7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin.
IC50 determination of sorafenib
Cells were seeded onto a 96-well plate with a density of 5000 cells per well for 24 h. Once adhered, cells were treated with sorafenib (#HY-10201, MedChemExpress) at various final concentrations (0 to 100 µM) for 72 h. Next, 10 µL of MTS reagent (#G3582, CellTiter 96 Aqueous One Solution, Promega) was added to each well and incubated at 37 °C for 4 h as previously described [23, 24]. Next, the absorbance was measured at 490 nm by a microplate reader (Tecan). Cell viability versus drug concentration graphs were plotted. The IC50 of sorafenib towards each cell type were obtained from an online IC50 calculator (AAT bioquest) as previously described [25, 26].
Generation of sorafenib-resistant cells
Chemoresistant cells were generated following a protocol described previously [27]. Cells were seeded in 10 cm dish with a density of 1.5 × 106 cells per dish. Once adhered, medium containing IC50 concentration of drugs were added and incubated for 72 h. Then, the drug-containing medium was replaced with fresh complete medium and incubated for another 72 h. The medium was then replaced again with medium containing IC50 concentration of drugs and incubated for 72 h for the second round. The medium was replaced as mentioned above. The medium was changed every 3–4 days for one to two weeks. The surviving cells from the first cycle (termed as HepG2-S1, Huh-7-S1) were pooled and replated onto 10 cm dish for the next treatment cycle using two times IC50 (2X IC50). The steps were repeated following a dose escalation of drug concentration until the surviving chemoresistant cells were generated. We managed to generate sorafenib-resistant HepG2 up to the forth cycle (HepG2-S4) and Huh-7 up to the fifth cycle (Huh-7-S5), respectively.
Clonogenic assay
Clonogenic assay was performed on a 6-well plate as previously described [26]. Cells ranging from a density of 100 to 100,000 cells per well were seeded onto the plate. After 24 h, cells in each plate were treated with five different concentrations (0, 6.25, 12.5, 25, 50 µM) of sorafenib. After 72 h of incubation in 37 °C, 5% CO2 incubator, medium containing sorafenib was removed and the cells were cultured in fresh complete medium for additional 7–10 days for visible colony formation. At the end of incubation, medium was removed and the cells were washed with 1 mL PBS. The cells colonies were fixed in 10% formalin for 15 min before they were stained with 0.25% w/v crystal violet (#548-62-9, Merck) in 95% ethanol. After air drying, the images of the plates were captured and colonies with more than 50 cells per well were quantified and counted using Image J software (NIH). Plating efficiency (PE) and surviving fractions (SF) were calculated following the equations below:
Sirtuin 1 knock-down by siRNA transfection
Lipofectamine 3000 (#L3000015, Invitrogen) was used to transfect the cells with FlexiTube siRNA (target sequence: AGCCATCGGAATGTTAAATTA, #1027417, Qiagen Biotechnology) to silence the sirtuin 1 expression in cells. Nonsilencing siRNA (AllStars Negative Control, #1027280, Qiagen Biotechnology) was used as a control siRNA in this experiment. Cells were seeded onto a 12-well plate at density of 2 × 105 cells/well a day before the transfection. Transfection was performed following the manufacturer’s instruction. The cells were monitored for any morphological changes or cell death under the microscope. After 48 h, cells were trypsinized and harvested for subsequent assay.
Western blot analysis
Cells were harvested and lysed in RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 5 mM EDTA) containing protease inhibitor cocktain (#78430, Thermo Scientific) and PMSF (#36978, Thermo Scientific). The protein concentration was determined by BCA (Bicinchoninic Acid) reagent using Pierce™ BCA Protein Assay Kit (#23225, Thermo Scientific) following the manufacturer’s instruction. Samples were loaded for SDS-PAGE analysis followed by immunoblot on PVDF membrane (#GE10600023, Merck). Primary antibodies: ABCB1(#13978) (1:1000), ABCC1(#72202) (1:1000), ABCG2(#42078 S) (1:1000), Beta-actin(#3700 S) (1:1000), p62/SQSTM1(#5114) (1:1000), LC3A(#4599 S) (1:1000), LC3B(#3868 S) (1:1000), Beclin-1(#4211) (1:1000), sirtuin 1 (#8469) (1:1000), RelA/p65(#4764) (1:1000), c-Rel(#4727) (1:1000) and p105/p50(#3035) (1:1000) purchased from Cell Signalling Technology were used followed by respective secondary antibodies: anti-mouse(#7076) (1:3000), anti-rabbit(#7074) (1:3000) from Cell Signalling Technology. Bands were visualized using electrochemiluminescence (ECL) buffers (#K-12045-D20, Advansta) and captured using ImageQuant™ LAS 500 (GE Healthcare). The densitometric analysis was performed using Image J software (NIH), normalized by beta-actin expression.
Statistical analysis
Independent experiments were performed three times and the data were expressed as the mean ± standard deviation for all triplicates within an individual experiment. Data were analyzed with one-way ANOVA with Tukey post-hoc test using Graphpad. p < 0.05 was considered statistically significant.
Results
Sensitivity of hepatocellular carcinoma cells to sorafenib
Concentration-dependent inhibition was observed in all sorafenib-treated cells (Fig. 1). Resistant cells showed less sensitivity to sorafenib compared to non-resistant or parental cells. In HepG2 cells, the IC50 values for the resistant (S4) and parental cells were 31.8 and 5.5 µM, respectively (Fig. 1a). In Huh-7 cells, the IC50 values for the resistant (S5) and parental cells were 50 and 7.7 µM, respectively (Fig. 1b). The IC50 values were approximately 6– and 7-fold higher in sorafenib-resistant HepG2 and Huh-7 cells, respectively, suggesting the successful establishment of chemoresistant cell lines. The resistant HepG2-S4 and Huh7-S5 cells could grow up to 4 and 5 repetitive cycles of sorafenib treatment, respectively but could not form surviving subclones in the further cycles.
Sensitivity of non-resistant and chemoresistant hepatocellular carcinoma (HCC) cells to sorafenib treatment. (a) HepG2, and (b) Huh-7. Inset table shows different concentrations of sorafenib used to repetitively treat HCC cells to generate different cycles of chemoresistant cells. *p < 0.05, **p < 0.01, ***p < 0.001
Clonogenic survival assay
To evaluate clone-forming capacity of resistant cells following sorafenib treatment, we then performed a clonogenic assay. As shown in Fig. 2, number of visible colonies reduced in both HepG2 and Huh-7 cells following the increase of sorafenib concentration. Parental HepG2 was more susceptible to sorafenib compared to the resistant cells. This difference is more apparent at concentration of 25 µM where only a few colonies are seen in parental cells while more colonies are seen in resistant counterparts (Fig. 2a). Similarly, an increased number of surviving colonies were seen in Huh7-S5 at 12.5 µM, but not in the parental cells (Fig. 2b). Figure 2c shows the survival fraction of the cells treated with sorafenib. Both HepG2 and Huh-7 cells showed sustained and higher survival at increasing concentrations of sorafenib.
Clonogenic assays of chemoresistant HCC cells and the survival fractions. Colony-forming assays of (a) HepG2; and (b) Huh-7 cells and the corresponding graphs showing the total number of colonies following different concentrations of sorafenib; (c) survival fraction calculations of HepG2 (left) and Huh-7 (right) cells. *p < 0.05, **p < 0.01, ***p < 0.001
Expression level of multidrug resistance (MDR) markers
Increased expression of ABC transporters such as ABCB1, ABCC1 and ABCG2 are often seen in drug-resistant cells, hence they are readily used as drug resistance markers in cancer cells. Figure 3a shows the western blot analysis of the markers while Fig. 3b shows the densitometry analysis of the bands. Elevated expression of ABCB1 and a slight increase of ABCG2 were observed in both resistant cells compared to the parental cells. ABCB1 drastically increased in HepG2-S2 and Huh7-S5 while only a low to modest increase was seen in HepG2-S4 and Huh7-S3 compared to the parental cells. Expression level of ABCC1 was also seen between Huh7-S3 and Huh7-S5, but surprisingly found to reduce in resistant HepG2 cells compared to the parental cells. Collectively, the elevated IC50 values, formation of resistant subclones, and increased expression of ABCC1 and ABCG2 transporters supported that the resistant HCC cells conferred resistance to sorafenib.
Expression level of chemoresistance proteins in chemoresistant HCC cells. a Western blot analysis using ABCB 1, ABCC 1, ABCG 2 and β-actin (endogenous control) antibodies in HepG2 (left) and Huh-7 (right) cells. b Densitometry analysis of ABCC 1, ABCC 1 and ABCG 2 expression in HepG2 (left) and Huh-7 (right) cells using ImageJ software (normalized with β-actin expression). P parental; *p < 0.05, **p < 0.01, ***p < 0.001
Changes of SIRT1, autophagy and NF-ĸβ in chemoresistant HCC cells
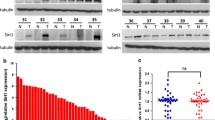
Compared to parental cells, high expression of SIRT1 was seen in chemoresistant HepG2 and Huh-7 cells, especially with higher expression levels in later cycles (Fig. 4a). In chemoresistant Huh-7 cells, reduced LC3A-I and LC3B-I expression, and increased LC3A-II and LC3B-II expression were observed, suggesting the conversion of LC3-I to LC3-II and reflecting the progression of autophagy. However, the activation of autophagy was not seen in chemoresistant HepG2 cells, suggesting that this upregulation could be cell-dependent (Fig. 4b).
Increased expression of Sirtuin 1, autophagy and NF-ĸβ signalling in chemoresistant cells. a Western blot analysis using sirtuin 1, autophagy, NF-ĸβ proteins, and β-actin antibodies in HepG2 (left) and Huh-7 (right) cells. b Densitometry analysis of autophagy (left) and NF-ĸβ (right) protein expression using ImageJ software (normalized with β-actin). P parental; *p < 0.05, **p < 0.01, ***p < 0.001
When NF-ĸβ proteins were assessed, increased expression of subunits such as RelA/p65 and cRel were seen in chemoresistant HepG2 and Huh-7 cells compared to the parental counterparts (Fig. 4b). This suggests the upregulation of NF-ĸβ signalling in the chemoresistant cells.
Effect of SIRT1 knock-down on autophagy and NF-ĸβ signalling
Decreased expression of SIRT1 was observed after siRNA silencing in all HCC cells (Figs. 5a, 6a). In parental HepG2 cells, increased LC3A-II expression was seen compared to the mock-transfected control, but not in LC3B-II expression. Decreased LC3A-I and LC3B-I expression were seen which suggested the LC3-I to LC3-II conversion. However, this was not seen in HepG2-S4 resistant cells which showed decreased LC3A-I and II expression and comparable level of LC3B-I and II (Fig. 5b). In addition, decreased p62 and beclin-1 expression were consistently seen in both parental and resistant HepG2 cells. Overall this shows the downregulation of autophagy following the silencing of SIRT1. In NF-ĸβ signalling, increased RelA/p65 and p50 expression but reduced cRel expression were seen in SIRT1-silenced HepG2 parental cells. In contrast, increased cRel expression was seen in HepG2-S4 resistant cells, but reduced expression of NF-ĸβ subunits, p50 and p105 were observed.
Effect of situin-1 silencing on autophagy and NF-ĸβ signalling in HepG2 cells. a Western blot analysis using sirtuin 1, autophagy, NF-ĸβ proteins, and β-actin antibodies in parental (left) and resistant (right) HepG2 cells. b Densitometry analysis of autophagy (left) and NF-ĸβ (right) protein expression using ImageJ software (normalized with β-actin). UT untreated, MT mock transfected, ST siRNA transfected, *p < 0.05, **p < 0.01, ***p < 0.001
Effect of situin-1 silencing on autophagy and NF-ĸβ signalling in Huh-7 cells. a Western blot analysis using sirtuin 1, autophagy, NF-ĸβ proteins, and β-actin antibodies in parental (left) and resistant (right) Huh-7 cells. b Densitometry analysis of autophagy (left) and NF-ĸβ (right) protein expression using ImageJ software (normalized with β-actin). UT untreated, MT mock transfected, ST siRNA transfected, *p < 0.05, **p < 0.01, ***p < 0.001
In parental Huh-7 cells, decreased expression of LC3A-II and LC3B-II, and increased expression of LC3A-I and LC3B-I were observed in the SIRT1-knocked down cells compared to the mock-transfected cells (Fig. 6b). Similar trend was also observed in Huh7-S5 resistant cells, suggesting that autophagy was not active after the knock-down of SIRT1. Decreased p62 and beclin-1 expression were also seen in both parental and resistant Huh-7 cells. In NF-ĸβ signalling, increased expression of RelA/p65 and cRel was observed in Huh-7 cells. Similarly, NF-ĸβ signalling was activated in Huh7-S5 resistant cells as shown by the increased expression of RelA/p65 and p50. Decreased expression of p105 was observed in both parental and resistant Huh7 cells.
Discussion
Increased IC50 values of sorafenib in HCC cells (5.5 and 7.7 µM in HepG2 and Huh-7 to 31.8 and 50 µM, respectively) confirmed the acquired sorafenib resistance. From a previous study, IC50 of HepG2 (6 µM) and Huh-7 cells (5 µM) was shifted to ±16 µM after long-term exposure to increasing sorafenib [28]. While another study reported the increased IC50 of sorafenib-resistant HepG2 and Huh-7 from approximately 5 and 7.5 µM, to 17.5 and 20 µM, respectively [29]. The variation of IC50 value after gaining sorafenib resistance could be due to the different methods used for generating resistant cells as well as different sources and passages of cells and sorafenib. Sorafenib resistance is known to involve ABC transporters, which efflux drugs from cancer cells and reduce the effectiveness of chemotherapy [30]. Consistent with Wang et al. [31], the sorafenib-resistant HCC cells showed higher expression of ABCB1 and ABCG2. However, only an increased level of ABCC1 was seen in resistant Huh-7, but not in HepG2 cells which is contradictory to a report by Chow et al. [32] which demonstrated increased ABCC1-3 expression in sorafenib-resistant HepG2 cells. This variation could be due to the gradual development of sorafenib resistance in the cells which affected the intracellular concentrations of ABC transporters, and hence resulting in the heterogeneous expression [33]. Furthermore, colony-forming assay also demonstrated the increased clonogenic capacity of surviving sorafenib-resistant cells compared to parental cells. These resistant cells were then utilized as models to investigate the expression level of SIRT1, autophagy, NF-ĸβ signalling, and their interactions.
Interaction of sirtuin 1 and autophagy has been previously studied in melanoma [34] and gastric cancer [35], but little is known in HCC. Our findings showed that sirtuin 1 expression was upregulated in sorafenib-resistant cells, and the activation of autophagy was cell-dependent. Activated autophagy was seen in Huh-7 cells but opposite effect was seen in HepG2 cells after they acquired sorafenib resistance. Autophagy has been reported to support cell survival in sorafenib-resistant HCC [36, 37]. Glucose deprivation can activate AMPK and ULKI complex by phosphorylation followed by PI3K complex which binds to beclin 1 to activate LC3 proteins to induce autophagy [35]. AMPK can also activate SIRT1 and induce autophagy through deacetylation of ATGs genes [35]. In another study, SIRT1 deacetylates FOXO3 which then reduce oxidative stress induction and inhibit cell apoptosis to promote cell survival [38]. Deacetylation of FOXO3 may also induce autophagy as previously reported [35, 39]. SIRT1 can also directly act on and deacetylate nuclear LC3 protein under starvation to induce autophagy [40]. Similarly, SIRT1 may induce autophagy through deacetylation of NF-ĸβ subunits, p65 and p50 to activate NF-ĸβ signalling pathway [35]. While much evidence highlight that SIRT1 most likely upregulates autophagy, it may also inhibit autophagy through p53 [35]. Deacetylation of p53 may suppress p53-dependent apoptosis, therefore leading to cell survival and promote chemoresistance [38]. This could be the reason why inhibited autophagy was observed in HepG2 sorafenib-resistant cells in our present study, which warrants future investigations.
Our findings demonstrate that NF-ĸβ signalling was also upregulated in HCC cells after acquiring sorafenib resistance. Sorafenib might have activated AMPK signal [19], leading to SIRT1 upregulation in a NAD + -dependent manner [35]. SIRT1 then deacetylates p65 and p50, thereby activating NF-ĸβ signalling pathway [35]. Both SIRT1 and NF-ĸβ signalling have been previously shown to upregulate ABC transporters, thereby reducing drug accumulation in cancer cells [41. 42]. Sorafenib could affect autophagy process by chemically modifying beclin 1 through ubiquitination and acetylation, thereby affecting the activity of beclin 1 [19]. Beclin 1 is upregulated by sorafenib which is then ubiquitinated by LATS1 to inhibit autophagy and promote sorafenib resistance [19]. Sorafenib may also affect the formation of LC3-II, resulting in autophagy inhibition and drug resistance [19]. On the other hand, activation of NF-ĸβ could induce autophagy through regulation of p62 and LC3-II.
To investigate the role of SIRT1 in autophagy regulation and NF-ĸβ signalling in HCC chemoresistance, SIRT1 in HCC cells was knocked down using siRNA, and the changes of autophagy and NF-ĸβ signalling were evaluated. Following the knock-down, autophagy was downregulated in both parental and sorafenib-resistant cells, while NF-ĸβ signalling was activated. This suggests the autophagy process is highly regulated by SIRT1. The acquired chemoresistance did not affect the effect of SIRT1 knockdown on both autophagy and NF-ĸβ signalling since similar trends were seen in both parental and sorafenib-resistant cells. Interestingly, activated NF-ĸβ signalling was observed after SIRT1 knock-down. This could be explained by the reduced expression of SIRT1 which functions to inactivate activity of NF-ĸβ by driving the translocation of NF-ĸβ complex from the nucleus back to cytoplasm [21]. As autophagy is reported to degrade NF-ĸβ signalling components and subsequently inactivate NF-ĸβ pathway [43], the activated NF-ĸβ signalling in this study may also due to the downregulated autophagy. However, it is more likely that other target genes such as TNF, RANKL and IL6 [44] interact and activate NF-ĸβ signalling in HCC as the result of SIRT1 knock-down. Future investigations are required to evaluate the involvement of other genes, proteins or pathways in SIRT1/autophagy/NF-ĸβ regulatory axis.
There are several limitations in this study. Since the role of SIRT1 in down-regulating autophagy pathway may be cell-dependent, it is logical to include more HCC cell lines or more clinically relevant models such as organoids from HCC patients or patient-derived xenograft (PDX) in future study. Ideally, normal hepatocytes should also be included to examine the role of SIRT1 in the related pathways in the non-cancer state. Due to the technical constrains, only limited key proteins of autophagy and NF-ĸβ pathways were selected and investigated in current study. In future work, more target proteins or genes such as ATGs and FOXO3 for autophagy, IKB and p65 for NF-ĸβ pathways or other target gene such as p53, can be included to provide a more comprehensive understanding of the role of SIRT1 in autophagy, NF-ĸβ and other related pathways.
Conclusion
We conclude that SIRT1 and NF-ĸβ signalling are activated in sorafenib-resistant cells. SIRT1 is found to primarily activate autophagy pathway in both parental and sorafenib-resistant cells. Reduced level of SIRT1 led to NF-ĸβ activation, but other intermediate proteins or pathways may have been involved which require future investigations.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
American Cancer Society (ACS). Cancer Statistics Center. http://cancerstatisticscenter.cancer.org. Accessed 24 June 2022.
Lohitesh, K., Chowdhury, R. & Mukherjee, S. (2018). Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: an insight. Cancer Cell International, 18(44), 44. https://doi.org/10.1186/s12935-018-0538-7.
Huang, A., Yang, X. R., Chung, W. Y., Dennison, A. R. & Zhou, J. (2020). Targeted therapy for hepatocellular carcinoma. Signal Transduction and Targeted Therapy, 5, 146. https://doi.org/10.1038/s41392-020-00264-x.
Mao, B., Hu, F., Cheng, J., Wang, P., Xu, M., Yuan, F., Meng, S., Wang, Y., Yuan, Z., & Bi, W. (2014). SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene, 33(11), 1468–1474. https://doi.org/10.1038/onc.2013.88.
Ayob, A. Z. & & Ramasamy, T. S. (2018). Cancer stem cells as key drivers of tumour progression. Journal of Biomedical Science, 25(1), 20. https://doi.org/10.1186/s12929-018-0426-4.
Wong, M. M., Chan, H. Y., Aziz, N. A., Ramasamy, T. S., Bong, J. J., Ch’ng, E. S., Armon, S., Peh, S. C. & Teow, S. Y. (2021). Interplay of autophagy and cancer stem cells in hepatocellular carcinoma. Molecular Biology Reports, 48(4), 3695–3717. https://doi.org/10.1007/s11033-021-06334-9.
Ong, A. L. C. & Ramasamy, T. S. (2018). Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Research Reviews, 43, 64–80. https://doi.org/10.1016/j.arr.2018.02.004.
Chen, J., Zhang, B., Wong, N., Lo, A. W., To, K. F., Chan, A. W., Ng, M. H., Ho, C. Y., Cheng, S. H., Lai, P. B., Yu, J., Ng, H. K., Ling, M. T., Huang, A. L., Cai, X. F. & Ko, B. C. (2011). Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Research, 71(12), 4138–4149. https://doi.org/10.1158/0008-5472.CAN-10-4274.
Jang, K. Y., Noh, S. J., Lehwald, N., Tao, G. Z., Park, H. S., Moon, W. S., Felsher, D. W., & Sylvester, K. G. (2012). SIRT1 and c-Myc promote liver tumor cell survival and predict poor survival of human hepatocellular carcinomas. Plos One, 7(9), e45119. https://doi.org/10.1371/journal.pone.0045119.
Molla, M. D., Dessie, G., Akalu, Y. & Ayelign, B. (2020). Hepatocellular expression of SIRT1 and its effect on hepatocellular carcinoma progression: A future therapeutic perspective. International Journal of Hepatology, 2020, 2374615. https://doi.org/10.1155/2020/2374615.
Hao, C., Zhu, P. X., Yang, X., Han, Z. P., Jiang, J. H., Zong, C., Zhang, X. G., Liu, W. T., Zhao, D. Q., Fan, T. T., Zhang, L., & Wei, X. L. (2014). Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma. BMC Cancer, 14, 978 https://doi.org/10.1186/1471-2407-14-978.
Farcas, M., Gavrea, A. A., Gulei, D., Ionescu, C., Irimie, A., Catana, C. S. & Berindan-Neagoe, I. (2019). SIRT1 in the development and treatment of hepatocellular carcinoma. Frontiers in Nutrition, 6, 148. https://doi.org/10.3389/fnut.2019.00148.
Song, S., Luo, M., Song, Y., Liu, T., Zhang, H. & Xie, Z. (2014). Prognostic role of SIRT1 in hepatocellular carcinoma. Journal of College of Physicians and Surgeons Pakistan, 24, 849–854.
An, Y., Wang, B., Wang, X., Dong, G., Jia, J. & Yang, Q. (2020). SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop. Cell Death and Disease, 11, 115. https://doi.org/10.1038/s41419-020-2308-4.
Mvunta, D. H., Miyamoto, T., Asaka, R., Yamada, Y., Ando, H., Higuchi, S., Ida, K., Kashima, H. & Shiozawa, T. (2017). SIRT1 regulates the chemoresistance and invasiveness of ovarian carcinoma cells. Translational Oncology, 10(4), 621–631. https://doi.org/10.1016/j.tranon.2017.05.
Wang, Y. Z., Zeng, J., Wu, W. P., Xie, S. D., Yu, H. T., Li, G. L., Zhu, T., Li, F. Y., Lu, J., Wang, G. Y., Xie, X. Y. & Zhang, J. (2019). Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Research, 21, 64. https://doi.org/10.1186/s13058-019-1150-z.
Awi, N. J., Yap, H. Y., Armon, S., Low, J. S. H., Peh, K. B., Peh, S. C., Lee, C. S. & & Teow, S. Y. (2021). Association between autophagy and KRAS mutation with clinicopathological variables in colorectal cancer patients. Malaysian Journal of Pathology, 43(2), 269–279.
Sheng, J., Qin, H., Zhang, K., Li, B. & Zhang, X. (2018). Targeting autophagy in chemotherapy-resistant of hepatocellular carcinoma. American Journal of Cancer Research, 8(3), 354–365.
Wu, Y., Zhang, J. & Li, Q. (2021). Autophagy, an accomplice or antagonist of drug resistance in HCC?. Cell Death and Disease, 12(3), 266. https://doi.org/10.1038/s41419-021-03553-7.
De Gregorio, E., Colell, A., Morales, A. & Marí, M. (2020). Relevance of SIRT1-NF-κB axis as therapeutic target to ameliorate inflammation in liver disease. International Journal of Molecular Sciences, 21(11), 3858. https://doi.org/10.3390/ijms21113858.
Kauppinen, A., Suuronen, T., Ojala, J., Kaarniranta, K., & Salminen, A. (2013). Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal, 25(10), 1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007.
Verzella, D., Pescatore, A., Capece, D., Vecchiotti, D., Ursini, M. V., Franzoso, G., Alesse, E. & Zazzeroni, F. (2020). Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death and Disease, 11(3), 210. https://doi.org/10.1038/s41419-020-2399-y.
Izadiyan, Z., Shameli, K., Teow, S. Y., Yusefi, M., Kia, P., Rasouli, E. & Tareq, M. A. (2021). Anticancer activity of 5-fluorouracil-loaded nanoemulsions containing Fe3O4/Au core-shell nanoparticles. Journal of Molecular Structure, 1245, 131075. https://doi.org/10.1016/j.molstruc.2021.131075.
Yusefi, M., Chan, H. Y., Teow, S. Y., Kia, P., Soon, M. L. K., Che Sidik, N. A., & Shameli, K. (2021). 5-fluorouracil encapsulated chitosan-cellulose fiber bionanocomposites: Synthesis, characterization and in vitro analysis towards colorectal cancer cells. Nanomaterials, 11(7), 1691. https://doi.org/10.3390/nano11071691.
Yusefi, M., Soon, M. L. K., Shameli, K., Teow, S. Y., Ali, R. R., Siew, K. K., Chan, H. Y., Wong, M. M. T., Lim, W. L. & Kuca, K. (2021). 5-Fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydrate Polymers, 273, 118523. https://doi.org/10.1016/j.carbpol.2021.118523.
Yusefi, M., Soon, M. L. K., Teow, S. Y., Monchouguy, E. I., Neerooa, B. N. H. M., Izadiyan, Z., Jahangirian, H., Rafiee-Moghaddam, R., Webster, T. J. & Shameli, K. (2022). Fabrication of cellulose nanocrystals as potential anticancer drug delivery systems for colorectal cancer treatment. International Journal of Biological Macromolecules, 199, 372–385. https://doi.org/10.1016/j.ijbiomac.2021.12.189.
Mohr, L., Carceles-Cordon, M., Woo, J., Cordon-Cardo, C., Domingo-Domenech, J. & Rodriguez-Bravo, V. (2017). Generation of prostate cancer cell models of resistance to the anti-mitotic agent docetaxel. Journal of Visualized Experiments, 127, 56327. https://doi.org/10.3791/56327.
Verslype, C., van Malenstein, H., Dekervel, J., Windmolders, P., Libbrecht, L., Eijsden, R., Nevens, F., van Pelt, J. (2012) Resistance development after long-term sorafenib exposure in hepatocellular cancer cell lines and risk of rebound growth and epithelial to mesenchymal transition. Journal of Clinical Oncology, 30(4), 216. https://doi.org/10.1200/jco.2012.30.4_suppl.216.
He, C., Dong, X., Zhai, B., Jiang, X., Dong, D., Li, B., Jiang, H., Xu, S., & Sun, X. (2015). MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget, 6(30), 28867–28881. https://doi.org/10.18632/oncotarget.4814.
Tang, W., Chen, Z., Zhang, W., Cheng, Y., Zhang, B., Wu, F., Wang, Q., Wang, S., Rong, D., Reiter, F. P., De Toni, E. N. & Wang, X. (2020). The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduction and Targeted Therapy, 5(1), 87. https://doi.org/10.1038/s41392-020-0187-x.
Wang, H., Qian, Z., Zhao, H., Zhang, X., Che, S., Zhang, H., Shang, H., Bao, J., Hao, C., Liu, J. & Li, Z. (2015). CSN5 silencing reverses sorafenib resistance of human hepatocellular carcinoma HepG2 cells. Molecular Medicine Reports, 12(3), 3902–3908. https://doi.org/10.3892/mmr.2015.3871.
Chow, A. K., Ng, L., Lam, C. S., Wong, S. K., Wan, T. M., Cheng, N. S., Yau, T. C., Poon, R. T., & Pang, R. W. (2013). The Enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PloS One, 8(11), e78675. https://doi.org/10.1371/journal.pone.0078675.
Estevinho, M. M., Fernandes, C., Silva, J. C., Gomes, A. C., Afecto, E., Correia, J., & Carvalho, J. (2022). Role of ATP-binding cassette transporters in sorafenib therapy for hepatocellular carcinoma: An overview. Curr Drug Targets, 23(1), 21–32. https://doi.org/10.2174/1389450122666210412125018.
Sun, T., Jiao, L., Wang, Y., & Ming, L. (2018). SIRT1 induces epithelial-mesenchymal transition by promoting autophagic degradation of E-cadherin in melanoma cells. Cell Death Dis, 9, 136. https://doi.org/10.1038/s41419-017-0167-4.
Qiu, G., Li, X., Che, X., Wei, C., He, S., Lu, J., Jia, Z., Pang, K., & Fan, L. (2015). SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment. FEBS Letters, 589(16), 2034–2042. https://doi.org/10.1016/j.febslet.2015.05.042.
Lu, S., Yao, Y., Xu, G. L., Zhou, C., Zhang, Y., Sun, J., Jiang, R. Q., Shao, Q. & Chen, Y. (2018). CD24 regulates sorafenib resistance via activating autophagy in hepatocellular carcinoma. Cell Death and Disease, 9, 646. https://doi.org/10.1038/s41419-018-0681-z.
Shimizu, S., Takehara, T., Hikita, H., Kodama, T., Tsunematsu, H., Miyagi, T., Hosui, A., Ishida, H., Tatsumi, T., Kanto, T., Hiramatsu, N., Fujita, N., Yoshimori, T. & Hayashi, N. (2012). Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. International Journal of Cancer, 131(3), 548–557. https://doi.org/10.1002/ijc.26374.
Olmos, Y., Brosens, J. J. & Lam, E. W. (2011). Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resistance Updates, 14(1), 35–44. https://doi.org/10.1016/j.drup.2010.12.001.
Kitada M., Ogura Y. & Koya D. (2016) Role of SIRT1 as a regulator of autophagy. In Hayat M.A. (Ed.), Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. (1st edn) 8:89−100. https://doi.org/10.1016/B978-0-12-802937-4.00003-X.
Huang, R., Xu, Y., Wan, W., Shou, X., Qian, J., You, Z., Liu, B., Chang, C., Zhou, T., Lippincott-Schwartz, J. & Liu, W. (2015). Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Molecular Cell, 57(3), 456–466. https://doi.org/10.1016/j.molcel.2014.12.013.
Cicchini, M., Karantza, V. & Xia, B. (2014). Molecular pathways: Autophagy in cancer—A matter of timing and context. Clinical Cancer Research, 21(3), 498–504. https://doi.org/10.1158/1078-0432.CCR-13-2438.
Velaei, K., Samadi, N., Soltani, S., Barazvan, B. & Soleimani Rad, J. (2017). NFκBP65 transcription factor modulates resistance to doxorubicin through ABC transporters in breast cancer. Journal of Breast Cancer, 24(4), 552–561. https://doi.org/10.1007/s12282-016-0738-8.
Trocoli, A. & Djavaheri-Mergny, M. (2011). The complex interplay between autophagy and NF-kB signalling pathways in cancer cells. American Journal of Cancer Research, 1(5), 629–649.
Xia, L., Tan, S., Zhou, Y., Lin, J., Wang, H., Oyang, L., Tian, Y., Liu, L., Su, M., Wang, H., Cao, D. & Liao, Q. (2018). Role of the NFkB-signaling pathway in cancer. OncoTargets and Therapy, 11, 2063–2073. https://doi.org/10.2147/OTT.S161109.
Author contributions
T.S.R., F.F.-L.C. and S.-Y.T. contributed to the study conception and design. Material preparation, data collection and analysis were performed by H.-Y.C. The first draft of the manuscript was written by H.-Y.C. and S.-Y.T. All authors revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Ministry of Higher Education Malaysia (FRGS/1/2019/SKK08/SYUC/02/1) and Sunway University Individual Research Grant Scheme 2022 [GRTIN-IGS-DMS(S)-04-2022]. We also thank Wenzhou-Kean University for partly supporting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chan, HY., Ramasamy, T.S., Chung, F.FL. et al. Role of sirtuin 1 (SIRT1) in regulation of autophagy and nuclear factor-kappa Beta (NF-ĸβ) pathways in sorafenib-resistant hepatocellular carcinoma (HCC). Cell Biochem Biophys 82, 959–968 (2024). https://doi.org/10.1007/s12013-024-01247-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-024-01247-3