Abstract
Hydrogen sulfide (H2S) is reported to be effective in the management of the myocardial ischemia–reperfusion (I/R) injury via PI3K/GSK3β pathway in normal rats. However, its efficacy against I/R in the presence of diabetic cardiomyopathy is relatively obscure. Thus, the present work aimed to find out H2S-mediated cardioprotection against I/R in diabetic cardiomyopathy and to evaluate its mode of action using Langendorff isolated heart perfusion system. The present work includes three groups of rat, viz. (i) normal, (ii) diabetes mellitus (DM: streptozotocin: 35 mg/kg; normal diet), and (iii) diabetes + high-fat diet (DCM) (streptozotocin: 35 mg/kg; high-fat diet). The effect of NaHS (an H2S donor; 20 µM) on cardiac function in isolated rat hearts demonstrates that H2S preconditioning (HIPC) significantly attenuated myocardial injury in both DM and DCM hearts, as evidenced by the (i) improvement in hemodynamics, which includes rate pressure product [(in mmHg × 103 × bpm) DM: 40 to 56; DCM: 21 to 58] and left ventricular developed pressure [(in mmHg) DM: 53 to 74; DCM: 28 to 74), (ii) reduction in infarct size (25% to 8%) and attenuated caspase activity, compared to their respective I/R controls. Also, the observed positive recovery of mitochondrial function during HIPC treatment reinforces the cardioprotection by HIPC in DCM heart against I/R injury. However, HIPC could not repair I/R-induced oxidative stress in DCM rat heart. Further, to study the H2S mode of action, the experimental rats were exposed to a PI3K inhibitor (Wortmannin) and GSK3β inhibitor (SB216763) before HIPC protocol, whose results suggest that unlike in normal and DM, HIPC mediates its cardioprotective effect independent of PI3K/GSK3β pathway. To conclude, HIPC ameliorates I/R injury in DCM rat via an alternative pathway other than existing PI3K pathway, which is required to be probed under disease conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary therapeutic strategy to treat the ischemic heart is the immediate reperfusion of the affected area, which paradoxically leads to further damage, in a process called myocardial ischemia–reperfusion injury (I/R) [1]. The significant mediators identified in the pathology of I/R injury are compromised mitochondrial function and reactive oxygen species (ROS)-mediated oxidative stress [2]. However, these mediators are common in the pathology of diabetes mellitus as well [3]. Altered metabolism, impaired micro-vasculature, dysfunction in the cardiac autonomic system, and depleted immune response are the major adverse effects found in hearts with diabetes mellitus [4]. These pathological or adaptive changes may modulate the structure and function of the myocardium by altering the events in cardioprotective signaling pathways in the absence of any pre-existing coronary artery diseases resulting in the development of diabetic cardiomyopathy [5]. The number of diabetic patients undergoing treatment for their ischemic heart kept climbing alarmingly [6], and there exist only a limited number of drugs to ameliorate reperfusion-induced injury in diabetic cardiomyopathy hearts where these hearts are resistant to many promising preclinical drugs and cardioprotective protocols like preconditioning and post-conditioning.
Even though very limited inroads are made in the management of I/R, the three endogenous gasotransmitters, namely nitric oxide (NO), H2S, and carbon monoxide (CO), were found to be key modulators of I/R [7]. A recent study in db/db mice had shown that H2S could render cardioprotection by activating RISK pathway [8]. Another group demonstrated the attenuation of I/R injury in db/db mice by H2S preconditioning via NrF2 signaling in an Erk-dependent manner [9]. The cardiac dysfunction in diabetic cardiomyopathy (occurs in patients with prolonged diabetes) is generally associated with increased Akt activation, insulin resistance, and hyperinsulinemia, which was not considered/addressed in the above-mentioned studies. Similarly, a recent study emphasizes the possible deficiency of endogenous H2S production and subsequent endoplasmic stress in diabetic cardiomyopathy that may be due to lipotoxicity [10]. Another important characteristic feature of a chronic diabetic condition that leads to diabetic cardiomyopathy was overlooked in the existing preclinical studies of H2S action in the diabetic animal. Hence, it is required to re-evaluate the efficacy of the ability of H2S to attenuate I/R in the heart with pre-existing comorbidities like diabetes (considering the fact that diabetic patients undergoing coronary interventions often present with prolonged DM complications), despite the conclusion from few studies that H2S could alleviate the development of diabetic cardiomyopathy [11]. Thus, the present study aims to investigate the efficacy of H2S as a preconditioning agent against I/R injury in diabetic (short-term effect of DM) and diabetic cardiomyopathic (chronic effect of DM) rat hearts and explore its probable mode of action.
Materials and Methods
All chemicals used are of analytical grade procured from Sigma-Aldrich (St. Louis, MO, USA) and HiMedia (Mumbai, India).
Animals
Male Wistar rats obtained from the central animal facility of SASTRA University, India, were housed in a departmental animal house in a controlled temperature (25 ± 2 °C), relative humidity (60 ± 5%) with 12-h light/dark cycle. Standard diet fed rats have induced diabetes (DM) by injecting streptozotocin (STZ: 35 mg/kg in 0.1 M cold citrate buffer, pH 4.5) (DM) and checked for blood glucose after 72 h to confirm hyperglycemia. To develop diabetic cardiomyopathy, rats were injected with STZ (35 mg/kg) and were fed a high-fat diet (HFD) throughout the study period. After STZ injection, DM rats were maintained in the normal diet for 10 days, whereas diabetes + high-fat diet rats were kept in the high-fat diet for 30 days.
Ethics Statement
The experimental protocol was carried out in accordance with the Indian National Science Academy guidelines for use and care of experimental animals in research and approved by the Institutional animal ethics committee (300/SASTRA/IAEC/RPP).
Perfusion Experimental Protocol
The male Wistar rats were anesthetized with sodium thiopentone (80 mg/kg, i.p.), 30 min after heparin treatment (500 IU/kg). Later, rat heart was rapidly excised and connected to the perfusion cannula via the aorta. Retrograde perfusion pressure was maintained at 70–80 mmHg with Krebs–Henseleit (KH) buffer, which was equilibrated with 95% O2 and 5% CO2 (pH 7.4, 37 °C). A polyethylene balloon filled with saline/distilled water was inserted into the left ventricle and adjusted the base-line end-diastolic pressure (EDP) ranging from 5 to 10 mmHg. After a stabilization period of 20 min, hearts were subjected to the perfusion protocol as described below. With the help of LabChart Pro software (Power lab, AD Instruments), heart rate, systolic pressure (LVSP), end-diastolic pressure (LVEDP) were computed and calculated. Rate pressure product was calculated as the product of systolic pressure and heart rate and expressed as mmHg × beats/min × 103.
Experimental Perfusion Groups
After excision of heart from the animal, it was mounted onto Langendorff perfusion apparatus that was stabilized by KH buffer for a minimum of 10 min to attain the normal cardiac hemodynamics. Accordingly, 10-min stabilization time was imparted in H2S conditioning group and for 25 min in the remaining groups. The rat hearts were randomly divided into following nine experimental groups with n = 6 in each group: (1) normal perfusion (Sham), (2) ischemia–reperfusion (I/R), (3) hydrogen sulfide preconditioning (HIPC), (3) diabetes heart with normal perfusion (DM-sham), (4) diabetes heart with ischemia–reperfusion (DM-I/R), (5) diabetes heart with hydrogen sulfide preconditioning (DM-HIPC), (6) diabetes + high-fat diet-treated heart (DCM) with normal perfusion (DCM-sham), (7) diabetes + high-fat diet-treated heart with ischemia–reperfusion (DCM-I/R), and (9) diabetes + high-fat diet-treated heart with hydrogen sulfide preconditioning (DCM-HIPC). At the end of the experiment, hearts were flash frozen, and stored at − 80 °C until use. Sodium hydrosulfide (NaHS—20 µM) was used as a donor of H2S.
Determination of Infarct Size
At the end of the experiment protocol, hearts were stained with 2,3,5-triphenyltetrazolium chloride (TTC) to determine the area of the infarcted regions, as described previously [12].
Estimation of Cardiac Injury Markers
Necrotic cell death was evaluated by analyzing the leakage of lactate dehydrogenase (LDH) and creatine kinase (CK) enzyme in the coronary efflux as per previously described methods [13].
Assay of Glucose-6-Phosphate Dehydrogenase (G6PdH) and Hexokinase
The rate-limiting enzyme of the pentose phosphate pathway and glycolysis were measured via the enzyme activity of glucose-6-phosphate dehydrogenase and hexokinase, respectively, as described earlier [14, 15].
Isolation of Mitochondria
The mitochondria from rat heart were isolated by differential centrifugation according to the method described elsewhere [16].
Mitochondrial Electron Transport Chain (ETC) Complex Activities
ETC enzyme activities of mitochondria were measured spectrophotometrically as per the protocol described previously [17].
Lipid Peroxidation and Antioxidant Enzymes
The concentration of thiobarbituric acid reactive substances (TBARS) in the myocardium was used to evaluate lipid peroxidation and estimated by the method of Fraga et al. [18]. Antioxidant enzymes like glutathione peroxidase (GPx), glutathione reductase, and catalase activities were measured by the method described elsewhere [19].
Caspase-3 Activity
Caspase-3 activity was assayed by using caspase substrate, Ac-DEVD-AMC (acetyl Asp-Glu-Val-Asp 7-amido-4-methyl coumarin), and the fluorescence for AMC was quantified using a multimode reader (Synergy H1, BioTek) with excitation at 385 nm and emission at 460 nm [20].
Measurement of H2S Metabolizing Enzymes
Cystathionine Beta Synthase (CBS), Cystathionine gamma-lyase (CSE), and rhodanase were estimated according to the procedure described by Stepien and Pieniazer [21] and Lee et al. [22], respectively.
Gene Expression Analysis
LV tissues (30 mg) were dissected, and total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, India). Briefly, tissues were homogenized using TRIzol, and phase separation was done using chloroform. RNA precipitation was carried out by isopropanol addition, and the RNA pellet was washed with 75% ethanol. RNA with the integrity of 1.8–2 (Nanodrop 2000, Thermo Fisher Scientific) was reverse-transcribed using a cDNA synthesis kit (Thermo Fisher Scientific, India). Quantitative gene expression was analyzed in duplicates using the ABI3000 (Applied Biosystems) using SYBR green-based quantitative polymerase chain reaction (qPCR) super mix (Thermo Fisher Scientific, India). Primer sequences for qPCR are listed in Table 3.
Histopathological Staining
At the end of the perfusion experiments, hearts were collected for histopathology, fixed in neutral buffered formaldehyde solution. Later, 5-µm-thick sections obtained from the paraffin-embedded block were placed on adhesive slides and stained with hematoxylin/eosin (H&E).
Statistical Analysis
Intergroup comparisons were performed by using a one-way analysis of the variance, followed by Student’s unpaired t test with Bonferroni’s correction for multiple comparisons using GraphPad Prism software (Version 6). Statistical significance was defined as a value of p < 0.05. Analyzed data are represented as mean with standard deviation (mean ± SD).
Results
Diabetic Cardiomyopathic Hearts Exhibit Marked Hypertrophy Along with Hyperglycemia and Hypoinsulinemia
The metabolic characteristics of the experimental animals were evaluated, and the results are given in Fig. 1. Animals injected with STZ showed significantly elevated serum glucose level, where STZ- and high-fat diet-treated animals (Fig. 1a) demonstrated a prominent increase. Insulin tolerance test conducted in STZ-administered rat suggests that DCM rats exhibited a significant resistance to insulin (Fig. 1b). HOMA-IR index as calculated from plasma insulin and blood glucose level confirms insulin insensitivity in DCM rats (Supplementary Table 1). Furthermore, we observed a substantial increase in heart-to-body weight ratio in DCM animal compared with the DM and normal control (Fig. 1c, d), thereby suggesting the cardiac hypertrophy, further confirmed with the higher myocardial expression of BNP and lower expression of ANP in DCM heart (Supplementary Table 1).
Plasma glucose (a) and insulin (b) levels in normal, DM, and DCM rats. Heart was weighed and plot as a graph (c). Cardiac hypertrophy was calculated as heart weight-to-body weight ratio (d). Data were represented as mean ± SD of 8 individual experiments. *p < 0.05 versus normal control. DM diabetes mellitus, DCM diabetes + high-fat diet treated
H2S Preconditioning Reversed Hemodynamic Indices and Attenuated Cardiac Injury in Normal and Diseased Rat Hearts
Reperfusing the ischemic heart from normal, DM, and diabetes + high-fat diet animals significantly (p < 0.05) reduced the cardiac performance as measured by a decrease in the rate pressure product (I/R: 66%, DM-I/R: 47%, DCM-I/R: 77%) and LVDP compared with the sham perfusion control (Table 1). Conditioning the diabetes + high-fat diet rat heart and DM heart with H2S before global ischemia recovered the RPP significantly (p < 0.05) by 64% and 29%, respectively.
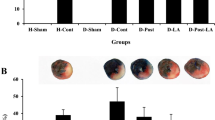
As shown in Fig. 2d, the area of infarction was prominent (indicated by pale/white color in the myocardial tissue) in diabetes + high-fat diet heart (36.4 ± 2.4) than DM (18 ± 0.8) and sham (24 ± 0.9) heart subjected to I/R protocol (Fig. 2b-I–b-III). Treating these hearts with HIPC provides tolerance for the diabetic heart to withstand I/R, as evident from the results shown in Fig. 2c-I–c-III. This result was well correlated with an elevated level of lactate dehydrogenase (LDH) and creatinine kinase (CK) enzymes in the coronary perfusate of reperfused heart (Fig. 2e, f) and the rat hearts treated with HIPC protocol where the attenuated levels of LDH and CK in both DM and diabetes + high-fat diet rat hearts were observed.
Cardiac injury analysis of normal, DM, and DCM rats. Image (a)-I, (b)-I, and (c)-I represents photograph of TTC-stained normal, DM, and DCM rat heart without I/R induction; image (a)-II, (b)-II, and (c)-II represents a photograph of TTC-stained normal, DM, and DCM rat heart subjected to ischemia–reperfusion; image (a)-III, (b)-III, and (c)-III represents a photograph of TTC-stained normal, DM, and DCM rat heart subjected to hydrogen sulfide preconditioning; graph (d) represents the quantified infarct size using ImageJ software; graph (e) and (f) represents leakage of lactate dehydrogenase and creatine kinase in the coronary perfusate respectively; and graph (g) represents the activity of an apoptotic marker—Caspase 3 enzyme. Data were represented as mean ± SD of 6 individual experiments. ap < 0.05 DCM-Sham, *p < 0.05 versus Sham, #p < 0.05 versus DM-Sham, $p < 0.05 versus DCM-Sham,. DM diabetes mellitus, DCM diabetes + high-fat diet treated, I/R ischemia/reperfusion, HIPC hydrogen sulfide preconditioning
Further evaluation of cardiac injury was done by measuring the apoptotic death mediator, namely caspase-3 activity. An elevated level of caspase-3 in normal, DM, and diabetes + high-fat diet rat hearts subjected to I/R was significantly (p < 0.05) reversed by H2S preconditioning in all experimental rat hearts, which again reinforces the above finding that HIPC showed positive protection against I/R-induced myocardial injury (Fig. 2g).
Furthermore, I/R-induced cardiac histo-architectural variation was analyzed using H&E staining that confirms the perturbed structural variations in diabetes + high-fat diet rat heart. Alteration in the myofibrillar arrangement and intercellular spaces in DM and diabetes + high-fat diet rat hearts was distinct and varied from their corresponding I/R-induced normal heart histological pattern (Fig. 3b, c vs. a). These ultrastructural changes were further confirmed with transmission electron microscopy (Fig. S1) and affirmed the I/R-associated changes in diabetes + high-fat diet heart (high degree of myocardial necrosis, fiber destruction) rather than a normal and DM heart (Fig. 3d–F). I/R-induced pathological changes in these experimental rat hearts were recovered significantly (p < 0.05) by HIPC, near to its respective sham control (Fig. 3h, i), but not to the level of the normal myocardial architecture (Fig. 3a).
Myocardial architecture analysis of normal, DM, and DCM rats using H&E staining. Image (a–c) represents the photograph of H&E-stained normal, DM, and DCM rat heart without I/R induction; Image (d–f) represents the photograph of H&E-stained normal, DM, and DCM rat heart subjected to I/R protocol; and Image (g–i) represents the photograph of H&E-stained normal, DM, and DCM rat heart subjected to hydrogen sulfide preconditioning. DM diabetes mellitus, DCM diabetes + high-fat diet treated, I/R ischemia/reperfusion, HIPC hydrogen sulfide preconditioning
Attenuation of I/R Induced Marked Mitochondrial Dysfunction by HIPC
Mitochondrial dysfunction is considered to be a critical characteristic feature of diabetes associated with cardiac abnormalities and I/R-induced injury. Even in the absence of I/R protocol, both DM and diabetes + high-fat diet rat hearts displayed a severe decline in the activities of mitochondrial ETC enzymes, with a higher degree of impairment in diabetes + high-fat diet rat heart than diabetic heart, compared with the normal (Fig. 4). Results from the present study showed that I/R-associated mitochondrial ETC enzymes dysfunction was effectively reversed by H2S preconditioning in the normal heart. However, similar extend of protection by HIPC was not found in DM and diabetes + high-fat diet rat heart (Fig. 4).
Mitochondrial OXPHOS analysis. Graphs represent complex I activity (a), complex II activity (b), complex III activity (c), and complex IV activity (d) in total mitochondria. Data were represented as mean ± SD of 6 individual experiments. *p < 0.05 versus Sham, #p < 0.05 versus DM-Sham, $p < 0.05 versus DCM-Sham. DM diabetes mellitus, DCM diabetes + high-fat diet treated, I/R ischemia/reperfusion, HIPC hydrogen sulfide preconditioning
Hydrogen Sulfide Preconditioning Improved I/R-Associated Declined Antioxidant Defense and Subsequent Elevated Oxidative Stress in the Normal Rat But Not in DM and Diabetes + High-Fat Diet Rats
Myocardial oxidative stress occurs due to the imbalance between antioxidant enzyme defenses and free radical release. This stress was evaluated by measuring the enzyme activities like superoxide dismutase (SOD), catalase, glutathione Peroxidase (GPx), glutathione reductase (GR), and concentration of non-enzymatic antioxidants like glutathione (GSH) along with the level of free radical-induced lipid peroxidation (Malondialdehyde or MDA level) and the results are shown in Fig. 5. Basal level oxidative stress was found to be higher in diabetic animals with or without cardiomyopathy. Reperfusion of ischemic heart induced a prominent elevation in oxidative stress in the normal heart (MDA: Sham—65%, DM—32%, DCM—72%), but the limited increase was observed in DM and diabetes + high-fat diet rat hearts (Fig. 5). Hearts subjected to HIPC protocol did not effectively ameliorate I/R-induced oxidative damage in I/R-challenged DM or diabetes + high-fat diet rat heart when compared with the normal heart (Fig. 5). Moreover, the down-regulated mRNA level of CuZnSOD in normal, DM, and diabetes + high-fat diet rat heart supports the above finding that DM/diabetes + high-fat diet heart experienced elevated oxidative stress. However, unlike I/R in normal heart, no prominent change was observed with the expression pattern in DM/diabetes + high-fat diet rat heart subjected to I/R (Fig. S2).
Myocardial levels of lipid peroxidation (a), reduced glutathione—GSH (b), and activities of glutathione peroxidase—GPx (c), Glutathione reductase—GR (d), catalase (e), and superoxide dismutase—SOD (f). Data were represented as mean ± SD of 6 individual experiments. *p < 0.05 versus Sham, $p < 0.05 versus DCM-Sham. DM diabetes mellitus, DCM diabetes + high-fat diet treated, I/R ischemia/reperfusion, HIPC hydrogen sulfide preconditioning
Reperfusion Mediated Altered Glucose Oxidation in DM, and Diabetes + High-Fat Diet Heart was Not Effectively Reversed by Hydrogen Sulfide Preconditioning
The rate-limiting enzymes of glycolysis and pentose phosphate pathway (glucose oxidative pathways which modulate the effective utilization of glucose) were determined using the enzyme activity of hexokinase and glucose-6-phosphate dehydrogenase, respectively (Table 2). Reperfusing the ischemic diabetes + high-fat diet rat heart affects both the glucose oxidative pathways (glycolysis—86% and PPP—50% decline), while in DM heart, only the pentose phosphate pathway was found to be impaired by 37%, but the glycolytic pathway was functional, and this could be responsible for the improved cardioprotection in DM than diabetes + high-fat diet group, compared with the normal. Moreover, conditioning the diseased heart with H2S before ischemia showed a less improvement (Table 2) in the carbohydrate metabolism in both DM and diabetes + high-fat diet rats.
Endogenous H2S-Metabolizing Enzyme Activities Were Declined in Normal Heart Subjected to I/R But Not in DM and Diabetes + High-Fat Diet Rat Heart
According to Table 3, both the DM and diabetes + high-fat diet rat myocardium exhibited reduced activities of CBS, CSE, and rhodanase suggesting that H2S metabolism was deteriorated severely in DM and diabetes + high-fat diet rat heart, compared to the normal. However, unlike in normal, I/R-induced decline in CBS, CSE enzymes were not observed in DM and diabetes + high-fat diet rat heart. Interestingly, H2S-degrading enzyme, rhodanase, was found to be significantly elevated in diabetes + high-fat diet group, but the activity was low in DM heart (Table 3) when subjected to I/R. Conditioning the DM heart with H2S before I/R did not improve the activity of CBS, CSE, or rhodanase effectively. On the other hand, HIPC protocol could effectively enhance the H2S-metabolizing enzymes in diabetes + high-fat diet heart.
Further evaluation of H2S-metabolizing enzymes at the molecular level was carried out by measuring the mRNA expression of CBS, CSE, and rhodanase. According to Table 3, the basal level expression of CBS was significantly declined in DM (% fold change: 0.12 vs. 1) and diabetes + high-fat diet rat heart (% fold change: 0.02 vs. 1), compared to the normal heart. On the other hand, CSE expression was upregulated in DM (% fold change: 6.72 vs. 1) than diabetes + high-fat diet (% fold change: 1.72 vs. 1) heart, compared to normal control. When these hearts were subjected to I/R, normal heart displayed lower expression of CBS, but both DM and diabetes + high-fat diet rat heart exhibited a higher expression level. However, the CSE expression was found to be increased in both normal and diabetes + high-fat diet group, but declined in DM heart on reperfusion protocol. Unlike normal heart, preconditioning the heart with H2S showed a significant decline in the expression level of CSE in both DM (1.38 vs. 6.51) and diabetes + high-fat diet (0.15 vs. 6.51), whereas the same was not observed in CBS expression (Table 3). The gene expression of H2S-catabolizing enzyme, rhodanase, was not significant in normal I/R, but show a distinct change in both DM (44% decline) and diabetes + high-fat diet (92% increase) during I/R, compared to their respective control. HIPC procedure improved the expression of rhodanase significantly in normal and diabetes + high-fat diet rat heart, but not in DM heart (Table 3), suggesting that the H2S metabolism is not complete in DM, compared to normal and diabetes + high-fat diet group.
Hydrogen Sulfide-Mediated Cardioprotection Against I/R Triggers Different Signaling Pathways in the Normal and Diseased Heart
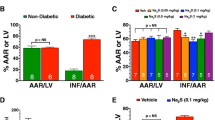
It is well established that H2S attenuated I/R injury in rat hearts without any comorbidities by activating signaling molecules of the PI3K pathway and by inhibiting GSK3β pathway. To check whether HIPC-mediated protection against I/R in diabetes heart is similar as observed in normal heart, we used inhibitors to arrest PI3K activation (using Wortmannin) and GSK3β inhibition (using SB216763). The cardiac physiological recovery data, as shown in Fig. 6, suggest that HIPC protection efficacy on diabetes + high-fat diet rat heart differs from normal and DM heart. Results suggest that the normal heart showed minimal recovery with PI3K inhibition along with I/R, whereas in diabetic and diabetes + high-fat diet rat heart, the recovery was moderate and least, respectively (Fig. 6a–c). However, diabetes + high-fat diet rat heart showed a maximum cardiac performance with H2S conditioning in the presence of PI3K inhibitor with less/no improvement in normal and DM heart (Fig. 6g–i) suggesting that H2S mediates its cardioprotection in diabetes + high-fat diet rat heart via an alternate pathway. Thus, we checked the I/R-associated cardiac hemodynamics in the presence of GSK3β inhibitor in all three experimental hearts (normal, DM, and DCM). As reported, the GSK3β inhibition as such provides improved recovery in normal and DM heart, but not in diabetes + high-fat diet rat heart (Fig. 6d–f). However, HIPC provided improved cardiac physiological recovery in diabetes + high-fat diet rat heart signifying the existence of an alternate signaling pathway which might be responsible for H2S-mediated cardioprotection in diabetes + high-fat diet rat heart.
Mechanism of action by H2S. Representative left ventricular (LV) pressure wave pattern of normal (a, d), DM (b, e), and DCM (c, f) rat heart subjected to I/R injury after treated with PI3K inhibitor (Wortmannin) and GSK3β inhibitor (SB612763), respectively; graphs from (g–i) represent LV pressure waveforms of normal, DM, and DCM rats subjected to HIPC after PI3K inhibitor treatment, and graphs from (j–l) represent LV pressure waveforms of normal, DM, and DCM rats subjected to HIPC after GSK3β inhibitor treatment. DM diabetes mellitus, DCM diabetes + high-fat diet treated, I/R ischemia/reperfusion, HIPC hydrogen sulfide preconditioning
Discussion
Accumulated early evidence from the literature and our previous study results suggested that hydrogen sulfide preconditioning renders protection against I/R injury in normal rat hearts and the underlying mechanisms linked to the preservation of mitochondria, particularly at the level of mitochondrial sub-sarcolemma and by attenuating both oxidative stress and apoptosis [23,24,25,26,27]. Though few investigators had demonstrated the protective effect of H2S in I/R-challenged diabetic rat hearts, its therapeutic potential against I/R in the diabetic heart that develops myopathy is largely unknown [8, 9, 24]. Thus, the present study was aimed to evaluate the efficacy of H2S in ameliorating I/R injury in diabetic rat with and without myopathy. The major findings are summarized as follows: HIPC ameliorated the damage from I/R in DM and DCM hearts by reducing the infarct size and improving the hemodynamics via attenuating the apoptosis. However, unlike the I/R-challenged normal heart, HIPC protocol was unable to reverse the I/R-induced oxidative stress, mitochondrial dysfunction, and endogenous H2S-metabolizing enzymes completely. Importantly, the underlying mechanism by which HIPC-mediated cardioprotection in diabetes + high-fat diet rat heart differs from the normal heart. Accordingly, the mode of action of HIPC protocol in normal heart depends on PI3k/GSK3β signaling axis, whereas cardioprotective events triggered by HIPC in diabetes + high-fat diet rat heart were independent of PI3k/GSK3β signaling axis.
Diabetes mellitus is known to increase myocardial lipid and glucose accumulation that leads to myocardial lipotoxicity, glucotoxicity, and insulin insensitivity which has a negative impact on myocardial contractility and ATP production [28, 29], and eventually increases the susceptibility of heart towards ischemia–reperfusion injury. The sensitivity of the heart towards I/R injury may vary with the ischemic duration, the extent of pathology, the degree of physiological adaptation, and stage of the disease. In the present study, we demonstrated that the diabetic heart with myopathy exhibited higher infarct size than a heart with or without diabetes. The significance of this finding is that the above observations were identified in an isolated rat heart where the influence of the neurohormonal axis and inflammatory response was absent or limited. It is well established that pro-inflammatory molecules can trigger the transition of adaptive to maladaptive tissue changes in the diabetic heart [30, 31]. Hence, the enhanced negative effect of I/R on diabetes + high-fat diet rat heart compared to DM or normal heart may be the direct impact of metabolic alterations in the diabetes + high-fat diet rat heart.
Contrary to a previous report that displayed abrogated protection by mechanical preconditioning protocol in diabetic heart [32], we demonstrated a beneficial effect (even though the protection was not the same as that of the normal heart) of HIPC in the diabetic heart with and without myopathy. Few investigators had reported that altering the IPC protocol time intervals can protect the diabetic heart from I/R pathology. HIPC is known to reduce reperfusion-induced injury via triggering multiple targets that involved PI3K activation and inhibition of GSK3β signaling pathway [33]. In a diabetic condition, the components of the PI3K/Akt signaling pathway were reported to be defective [34] without providing clarity in which stage of diabetes this defect occurs. In the present study, we evaluated HIPC mediated cardioprotection in the diabetic animal that develops and not develop cardiomyopathy. By using Wortmannin (PI3K inhibitor) and SB216763 (GSK3β inhibitor), we checked whether HIPC-mediated protection in I/R-challenged diabetic heart works via PI3k/GSK3β signaling axis. The hemodynamic data obtained from the study indicate that the protective mechanism of HIPC in normal and diabetes + high-fat diet rats were different (Fig. 6). Unlike the normal I/R-challenged heart conditioned with HIPC protocol in the presence of Wortmannin or SB216763, diabetes + high-fat diet rat heart exhibited cardiac contractile recovery with HIPC in the presence of above-said inhibitors. This observation underlines the existence of a distinct mode of action of HIPC in myocardium with different tissue-specific cues. Importantly, the similar mode of action in HIPC-mediated protection in both normal and DM heart subjected to I/R emphasizes the influential role of metabolic changes accrued during the pathological changes of the diabetic condition.
A shift from adaptive physiology to maladaptive hypertrophy widely persists in diabetic conditions, and many studies have shown that these changes will impair the contractile function of the myocardium that is regulated by intact functional cardiac mitochondria [35,36,37,38]. At the basal level, the spatial orientation of mitochondria was deteriorated in the diabetic heart and the alterations were severe in diabetes + high-fat diet rat (measured via TEM) (Fig. S1). Mitochondria is considered to be the converging target for the cardioprotective signaling pathway and the mitochondria-dependent signaling has a diverse pathological and physiological outcome. According to the present study, elevated mitochondrial oxidative stress may initiate the intrinsic pathway that triggers myocardial adaptation to I/R-associated stress. The beneficial effect of ROS may be restricted to its low level (picomolar to the nanomolar range) in mitochondria. Owing to the elevated ROS in the basal level of diabetic hearts, further elevation via I/R triggers pathological signaling rather than physiological adaptation. Earlier studies had suggested that the H2S-mediated improved antioxidant potential observed in I/R-challenged heart was attributed to its ability to increase the reduced glutathione content, thioredoxin level, and to augment Nrf2 gene expression [39], and this ability depends on cellular redox homeostasis [40]. Thus, altered oxidation–reduction state prevails in the myocardium of DM and DCM [41] may adversely affect the potential of H2S antioxidant capacity.
Szabo et al. had demonstrated that H2S could influence the activity of mitochondria in different mechanisms that include post-translational modification via sulfhydration, modulation of KATP channel, and modulation of redox antioxidant potential, and even alter the mitochondrial calcium handling [42]. Few studies have demonstrated that diabetic conditions can induce mitochondrial protein modification in heart and thus may adversely affect the H2S efficacy and that accounts for the minimal responsiveness of HIPC in DCM when compared with DM or normal rat hearts. Similarly, S-sulfhydration of ATP synthase subunit α (ATP5A1) by endogenous H2S regulates ATP synthesis. Thus, the level of endogenous H2S-generating enzymes like CBS, CSE, or rhodanase [43] may affect the endogenous H2S action on the myocardium against I/R. Few studies have shown that expression of CSE, the key enzyme that regulates the catalytic conversion of l-cysteine to H2S, was found to be low in rat fed on a high-fat diet [44]. Unlike in normal rat, hearts which were under the influence of DM exhibited low levels of endogenous H2S-producing enzymes that remain intact during I/R induction, even when exposed the heart to H2S (Table 3). Notably, the significant increase in the expression of H2S-synthesizing enzymes was not reflected in the enzymatic activities of CBS and CSE in both DM and diabetes + high-fat diet rat heart (Table 3), attributed to the altered endogenous H2S metabolic phase in the deteriorated mitochondria in the DM/DCM heart, when compared with the sham. Therefore, we assumed that H2S-metabolizing enzymes might not be the significant players in HIPC-associated protection in DM/DCM heart against I/R.
In conclusion, results from the present study demonstrated that HIPC protocol was found to be effective in ameliorating I/R injury in DCM rat heart. The underlying mechanism of protection works via reducing the infarct size, improving cardiac hemodynamics, and preserving mitochondrial function. Unlike I/R-challenged normal and DM heart, the protective effect of HIPC procedure did not depend on PI3k/GSK3β signaling axis.
References
Hausenloy, D. J., & Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. Journal of Clinical Investigation,123(1), 92.
Heusch, G., Boengler, K., & Schulz, R. (2010). Inhibition of mitochondrial permeability transition pore opening: The Holy Grail of cardioprotection. Basic Research in Cardiology,105(2), 151–154.
Grundy, S. M., Benjamin, I. J., Burke, G. L., Chait, A., Eckel, R. H., Howard, B. V., et al. (1999). Diabetes and cardiovascular disease: A statement for health professionals from the American Heart Association. Circulation,100(10), 1134–1146.
Vinik, A. I., Erbas, T., & Casellini, C. M. (2013). Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. Journal of Diabetes Investigation,4(1), 4–18.
Rubler, S., Dlugash, J., Yuceoglu, Y. Z., Kumral, T., Branwood, A. W., & Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology,30(6), 595–602.
Kannel, W. B., & McGee, D. L. (1979). Diabetes and cardiovascular risk factors: The Framingham study. Circulation,59(1), 8–13.
Andreadou, I., Iliodromitis, E. K., Rassaf, T., Schulz, R., Papapetropoulos, A., & Ferdinandy, P. (2015). The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. British Journal of Pharmacology,172(6), 1587–1606.
Lambert, J. P., Nicholson, C. K., Amin, H., Amin, S., & Calvert, J. W. (2014). Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med. Gas Res.,4, 20.
Peake, B. F., Nicholson, C. K., Lambert, J. P., Hood, R. L., Amin, H., Amin, S., et al. (2013). Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. American Journal of Physiology-Heart and Circulatory Physiology,304(9), H1215–H1224.
Guo, R., Wu, Z., Jiang, J., Liu, C., Wu, B., Li, X., et al. (2017). New mechanism of lipotoxicity in diabetic cardiomyopathy: Deficiency of Endogenous H2S Production and ER stress. Mechanisms of Ageing and Development,162, 46–52.
Zhou, X., An, G., & Lu, X. (2015). Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clinical Science,128(5), 325–335.
Mensah, K., Mocanu, M. M., & Yellon, D. M. (2005). Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: A potential role for phosphatase and tensin homolog deleted on chromosome ten. Journal of the American College of Cardiology,45(8), 1287–1291.
Kurian, G. A., Sachu, P., & Thomas, V. (2005). Effect of aqueous extract of Desmodium Gangeticum root in the severity of isoproterenol induced Myocardial infarcted rats. Journal of Ethnopharmacology,97(3), 457–461.
Noltmann, E. A., Gubler, C. J., & Kuby, S. A. (1961). Glucose 6-phosphate dehydrogenase (Zwischenferment) I. Isolation of the crystalline enzyme from yeast. Journal of Biologiocal Chemistry,236(5), 1225–1230.
Bergmeyer, H. U., Grassl, M., & Walter, H. E. (1983). Reagents for enzymatic analysis. Methods of Enzymatic Analysis,2, 185–223.
Gostimskaya, I., & Galkin, A. (2010). Preparation of highly coupled rat heart mitochondria. Journal of Visualized Experiments,43, 2202.
Frazier, A. E., & Thorburn, D. R. (2012). Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods in Molecular Biology,837, 49–62.
Fraga, C. G., Leibovitz, B. E., & Tappel, A. L. (1988). Lipid peroxidation measured as thiobarbituric acid reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radical Biology and Medicine,4, 155–161.
Ansari, S. B., & Kurian, G. A. (2016). Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chemico-Biological Interactions,252, 28–35.
Denton, D., Mills, K., & Kumar, S. (2008). Methods and protocols for studying cell death in Drosophila. Methods in Enzymology,446, 17–37.
Stepien, P. P., & Pieniazek, N. J. (1973). The use of the l-serine sulfhydrylase assay for the estimation of cystathionine b-synthase. Analytical Biochemistry,54, 294–299.
Lee, C. H., Hwang, J. H., Lee, Y. S., & Cho, K. S. (1995). Purification and characterization of mouse liver rhodanase. Journal of Biochemistry and Molecular Biology,28, 170–176.
Elrod, J. W., Calvert, J. W., Morrison, J., Doeller, J. E., Kraus, D. W., Tao, L., et al. (2007). Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America,104(39), 5560–15565.
Karwi, Q. G., Bornbaum, J., Boengler, K., Torregrossa, R., Whiteman, M., Wood, M. E., et al. (2017). AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. British Journal of Pharmacology,174, 287–301.
Chatzianastasiou, A., Bibli, S. I., Andreadou, I., Efentakis, P., Kaludercic, N., Wood, M. E., et al. (2016). Cardioprotection by H2S donors: nitric oxide-dependent and -independent mechanisms. Journal of Pharmacology and Experimental Therapeutics,106, 432–442.
Sivarajah, A., Collino, M., Yasin, M., Benetti, E., Gallicchio, M., Mazzon, E., et al. (2009). Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock,31, 267–274.
Yao, L. L., Huang, X. W., Wang, Y. G., Cao, Y. X., Zhang, C. C., & Zhu, Y. C. (2010). Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. American Journal of Physiology-Heart and Circulatory Physiology,298, H1310–H1319.
Buchanan, J., Mazumder, P. K., Hu, P., Chakrabarti, G., Roberts, M. W., Yun, U. J., et al. (2005). Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology,146, 5341–5349.
Ouwens, D. M., Boer, C., Fodor, M., De Galan, P., Heine, R. J., Maassen, J. A., et al. (2005). Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia,48, 1229–1237.
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E., & Blaxall, B. C. (2016). Cardiac fibrosis: the fibroblast awakens. Circulation Research,118(6), 1021–1040.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circulation Research,110, 159–173.
Yang, Z., Tian, Y., Liu, Y., Hennessy, S., Kron, I. L., & French, B. A. (2013). Acute hyperglycemia abolishes ischemic preconditioning by inhibiting Akt phosphorylation: Normalizing blood glucose before ischemia restores ischemic preconditioning. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2013/329183.
Jiang, H., Xiao, J., Kang, B., Zhu, X., Xin, N., & Wang, Z. (2016). PI3K/SGK1/GSK3β signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Experimental Cell Research,345(2), 134–140.
Heydrick, S. J., Jullien, D., Gautier, N., Tanti, J. F., Giorgetti, S., Van Obberghen, E., et al. (1993). Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. Journal of Clinical Investigation,91(4), 1358.
Boudina, S., Sena, S., O’Neill, B. T., Tathireddy, P., Young, M. E., & Abel, E. D. (2005). Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation,112, 2686–2695.
Bugger, H., Boudina, S., Hu, X. X., Tuinei, J., Zaha, V. G., Theobald, H. A., et al. (2008). Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes,57, 2924–2932.
Duncan, J. G., Fong, J. L., Medeiros, D. M., Finck, B. N., & Kelly, D. P. (2007). Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation,115, 909–917.
Ansari, M., Gopalakrishnan, S., & Kurian, G. A. (2019). Streptozotocin-induced type II diabetic rat administered with nonobesogenic high-fat diet is highly susceptible to myocardial ischemia–reperfusion injury: An insight into the function of mitochondria. Journal of Cellular Physiology,234(4), 4104–4114.
Tian, D., Dong, J., Jin, S., Teng, X., & Wu, Y. (2017). Endogenous hydrogen sulfide-mediated MAPK inhibition preserves endothelial function through TXNIP signaling. Free Radical Biology and Medicine,110, 291–299.
Xie, Z. Z., Liu, Y., & Bian, J. S. (2016). Hydrogen sulfide and cellular redox homeostasis. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2016/6043038.
Mahalakshmi, A., & Kurian, G. A. (2018). Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radical Biology and Medicine,118, 35–43.
Szabo, C., Ransy, C., Módis, K., Andriamihaja, M., Murghes, B., Coletta, C., et al. (2014). Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British Journal of Pharmacology,171, 2099–2122.
Fu, M., Zhang, W., Wu, L., Yang, G., Li, H., & Wang, R. (2012). Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proceedings of the National Academy of Sciences United States of America,109(8), 2943–2948.
Bravo, E., Palleschi, S., Aspichueta, P., Buqué, X., Rossi, B., Cano, A., et al. (2011). High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids in Health and Disease,10(1), 60.
Acknowledgements
The authors sincerely thank the Department of Science and Technology (DST/INSPIRE Fellowship/2013/326). The authors also place on record their thanks to Kausthubh R. for his editing of this manuscript.
Author information
Authors and Affiliations
Contributions
Dr. Gino A Kurian has contributed to the design and implementation of the research, the interpretation of the results, and writing of the manuscript. Ms. Mahalakshmi has processed the experimental data, performed analysis, drafted the manuscript, designed figures and tables, and compiled the literature sources.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Handling Editor: Filomain Nguemo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ansari, M., Kurian, G.A. Mechanism of Hydrogen Sulfide Preconditioning-Associated Protection Against Ischemia–Reperfusion Injury Differs in Diabetic Heart That Develops Myopathy. Cardiovasc Toxicol 20, 155–167 (2020). https://doi.org/10.1007/s12012-019-09542-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09542-9