Abstract
Our previous study demonstrated that hydrogen sulfide post-conditioning (HPOC) renders cardioprotection against ischemia-reperfusion (I/R) injury in normal rat by preserving mitochondria. But its efficacy in ameliorating I/R in the diabetic heart with (DCM) or without cardiomyopathy (DM) is unclear and is the focus of the present study. Normal (N), diabetes mellitus (streptozotocin, 35 mg/kg; normal diet), and DCM (streptozotocin, 35 mg/kg; high-fat diet) rats were subjected to I/R (30 min global ischemia followed by 60 min reperfusion) in presence and absence of HPOC using ex vivo Langendorff perfusion system. At the end of heart perfusion, subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) fractions from the tissue were isolated and measured for the ATP production, electron transport chain (ETC) enzyme activity, and membrane potential. The prominent I/R-associated injury in DCM rat was not subsequently attenuated by HPOC protocol unlike in the normal or diabetic rat heart (latter rat heart showed moderate protection) (HPOC recovery on infarct size: N 75% vs. DM 63% vs. DCM 48%). The baseline ATP content and subsequent ATP-producing capacity in DCM rat heart were low as compared with those in normal or DM rat heart, especially in SSM. HPOC protocol reversed the I/R-induced low mitochondrial ATP content and low ATP-producing capacity (both in non-energized and energized with glutamate/malate) significantly in normal and DM hearts, but not in DCM heart. Moreover in DCM, decreased activity of mitochondrial electron chain enzymes (complexes I, II, III, and IV) in SSM (26%, 88%, 57%, and 17%) and IFM (76%, 89%, 60%, and 13%) from sham control was maintained even after the conditioning of heart with hydrogen sulfide donor. Results altogether suggest that significantly higher levels of perturbing mitochondria in DCM rat heart underline the deteriorated cardiac recovery by HPOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic cardiomyopathy (DCM) has been defined as ventricular dysfunction that occurs independently of coronary artery disease and hypertension carrying a substantial risk for the subsequent development of heart failure (Rubler et al. 1972). It is characterized with hyperglycemia, lipid accumulation, increased oxidative stress, impaired calcium handling, mitochondrial dysfunction, renin–angiotensin system activation, myocardial inflammation, and apoptosis (Miki et al. 2013; Voulgari et al. 2010; van de Weijer et al. 2011; Duncan 2011). The existence of DCM as a distinct clinical condition from diabetes was evident from the Framingham Heart Study in 1991, which also reported an increased prevalence of heart failure in diabetic patients (Galderisi et al. 1991). Apparently, diabetic patients exhibit high sensitivity towards myocardial ischemia-reperfusion injury that leads to heart failure (Algeria et al. 2007; Marso et al. 2007). DCM, one of the key clinical conditions displayed by the myocardium of chronic diabetes mellitus patients, is associated with heart failure, but its contributory role in the ischemia-reperfusion (I/R) pathological outcome is not known.
Importantly, the prevalence of DCM kept on increasing in parallel with the rise in diabetes mellitus patients, demanding an effective therapeutic management option. Better glycemic control is considered to be an ideal approach, but often impractical in patients undergoing revascularization procedure that induce unavoidable I/R injury in the process. One of the emerging treatment modalities is to target mitochondria as this is the key player in the pathology of I/R injury. In this direction, many investigators have shown that endogenous gasotransmitters (nitric oxide, hydrogen sulfide, and carbon monoxide) are potential molecules in the management of I/R injury. It is widely known that these gasotransmitters are involved in multiple physiological functions. Moreover, the cardioprotective signaling triggered by these molecules is thought to converge on mitochondria. But our previous study has demonstrated perturbed mitochondrial function in DCM rat heart (Mahalakshmi and Kurian 2018).
Among the different gasotransmitters, H2S has been identified as a promising molecule that can render protection against I/R injury and DCM. Accordingly, an experiment in db/db mice showed that H2S preconditioning could attenuate the I/R injury by activating NRF2 (nuclear factor erythroid 2–related factor 2) signaling pathway, without explaining its anti I/R effect in diabetic cardiomyopathy animal (Peake et al. 2013). Another study in adult male C57BL/6J mice has demonstrated that H2S can regulate FoxO1 (forkhead box protein O1) in cardiac cells, thereby inhibit the progression of DCM. The physiological functions of H2S are mediated by a different molecular target and are regulated by systemic hormone and neuroendocrine secretions. To understand further, few investigators have studied the direct impact of the molecule on cardiac tissue (Calvert et al. 2009; Lambert et al. 2014; Peake et al. 2013), by using isolated rat heart experimental model that paved the way to identify H2S-linked cardioprotective signaling pathway. Thus, it is noteworthy to consider the direct impact of H2S in the outcome of I/R injury by using isolated rat heart model in DCM heart that exhibits structural and functional alterations.
Many investigators have reported the presence of well-defined mitochondrial subpopulations (interfibrillar (IFM) and subsarcolemmal (SSM)) in the heart that possesses distinct biochemical, morphological, and functional characteristics. Kurian and his co-worker (Kurian et al. 2012) had shown that both IFM and SSM appeared to be differently influenced by I/R injury. Later, the same group had demonstrated a clear difference in H2S action on IFM and SSM from I/R-challenged rat heart (Banu et al. 2016). Apparently, some investigators have pointed out the significant functional deficiency of SSM in the diabetic heart (Croston et al. 2014). An array of pathological disturbances in H2S metabolism was found in ischemia reperfused heart in an acute and chronic diabetic animal. Thus, more studies at the level of mitochondrial subpopulation are required to understand the underlying mechanism of H2S action in the diabetic heart that developed myopathy, as evidence from the literature suggesting mitochondria as the key player in H2S-mediated cardioprotection. The present study aimed to investigate the efficacy of H2S as a post-conditioning agent against I/R injury in DCM heart, which is being investigated for the first time.
Methods
Animals
Male Wistar rats from central animal facility, SASTRA University, India, were housed in cages and given free access to tap water and standard rat chow (Altromin no. 1324, Altromin, Lage, Germany) or high-fat diet, maintained in a room with a 12:12-h light/dark cycle at a temperature of around 25 °C. The high-fat diet was prepared by adding 40% fat (saturated fat 50%, monounsaturated fat 42%) to a standard rat chow diet. All procedures for the handling of the animals during the investigations were reviewed and approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA Approval No. 300/SASTRA/IAEC/RPP), India.
Preparation of experimental animals
Rats were grouped into three groups, namely (i) normal, (ii) diabetes mellitus (DM), and (iii) diabetic cardiomyopathy (DCM). In the diabetes group, rats (7–8 weeks) were subjected to streptozotocin (STZ) injection (i.p. 35 mg/kg b.wt.) and maintained at normal diet throughout the study. In the DCM group, rats (2–3 weeks) were fed with a high-fat diet, and once they reached the age of 8 weeks (comparable to the DM group), STZ was injected (i.p. 35 mg/kg b.wt.) and further maintained in high-fat diet (HFD) for 4 weeks.
Isolated rat heart perfusion
The rats were anesthetized by an intraperitoneal injection of sodium thiopental (80 mg/kg) 30 min after heparin treatment (500 IU/kg, i.p.). Later, rat chest was opened, and the hearts were excised immediately and perfused with KH buffer containing (in mM) NaCl 118.5, KCl 5.8, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, glucose 11, and NaHCO3 25, via Langendorff perfusion system. The perfusion buffer was equilibrated with carbogen gas (95% O2 and 5% CO2 at 37 °C (pH 7.4)). A latex balloon filled with saline/distilled water coupled with pressure transducer was inserted into the left ventricular cavity, and the baseline end-diastolic pressure was adjusted to 5–10 mmHg.
Hemodynamic parameters like heart rate, left ventricular end-diastolic pressure (LVEDP), rate pressure product (RPP), and left ventricular developed pressure (LVDP) were computed and calculated using Lab chart Pro software (AD Instruments Inc., Sydney, Australia). LVDP was calculated as the difference of left ventricular systolic and diastolic pressure, and RPP was calculated as the product of left ventricular systolic pressure and heart rate.
Perfusion experimental groups
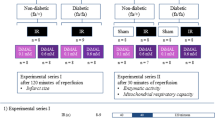
The rat hearts were randomly divided into the following nine experimental perfusion groups: (1) sham (n = 6): normal rat hearts were perfused for 120 min with KH buffer; (2) I/R (n = 6): normal rat hearts were subjected to 30 min global (no-flow) ischemia and reperfused with KH buffer for 60 min; (3) HPOC (n = 6): normal rat hearts were subjected to sodium hydrosulfide (NaHS) perfusion for 15 min after ischemia followed by 60-min reperfusion; (3) DM-sham (n = 6): diabetic rat hearts were perfused for 120 min with KH buffer; (4) DM-I/R (n = 6): diabetic rat hearts were subjected to 30 min global (no-flow) ischemia and reperfused with KH buffer for 60 min; (5) DM-HPOC (n = 6): diabetic rat hearts were subjected to NaHS perfusion for 15 min after ischemia followed by 60-min reperfusion; (6) DCM-sham (n = 6): diabetic cardiomyopathy rat hearts were perfused for 120 min with KH buffer; (7) DCM-I/R (n = 6): diabetic cardiomyopathy rat hearts were subjected to 30 min global (no-flow) ischemia and reperfused with KH buffer for 60 min; (9) DCM-HPOC (n = 6): diabetic cardiomyopathy rat hearts were subjected to NaHS perfusion for 15 min after ischemia followed by 60-min reperfusion. Sodium hydrosulfide (NaHS 20 μM) was used as a donor of H2S.
At the end of the perfusion, hearts were (i) fixed in neutral buffered formaldehyde solution and embedded in paraffin for histological analysis and (ii) flash-frozen and stored at − 80 °C for further biochemical analysis. From the paraffin-embedded tissue block, 5-μm-thick sections were made and placed on adhesive slides followed by staining with hematoxylin and eosin (H&E).
Determination of infarct size
At the end of the perfusion, hearts were stained with 2,3,5-triphenyltetrazoliumchloride (TTC) to determine infarcted regions. Briefly, the transverse heart tissue sections were incubated with 1.5% TTC for 30 min at 37 °C with shaking at every 5 min. The viable myocardium was stained with brick red due to the formation of a precipitate that resulted from a reaction of TTC with dehydrogenase enzymes. The loss of these enzymes from the infarcted myocardium prevents the formation of the precipitate, and the infarcted area within the region at risk remains pale yellow. The tissue samples were then fixed in 4% formalin solution for at least 24 h. Then, the sections were photographed using an Olympus microscope, and the infarcted area was calculated using ImageJ software.
Mitochondrial analysis
According to the method described by Palmer et al. (1977), rat cardiac mitochondria subpopulations, viz., subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria, were isolated. Mitochondrial ATP content was determined using ATP lite (Perkin Elmer) according to the manufacturer’s instructions. ETC enzyme activities in IFM and SSM were measured spectrophotometrically as per the protocol described previously (Frazier and Thorburn 2012). The mitochondrial swelling and mitochondrial membrane potential were estimated as described elsewhere (Chinopoulos et al. 2003; Baracca et al. 2003). The level of glutathione (GSH) and activities of antioxidant enzymes were measured as per the protocol described elsewhere (Ansari and Kurian 2016).
RT-PCR analysis
Total cellular RNA from the sampled tissues was extracted using TRIzol® reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. RNA concentrations and purity were determined spectrophotometrically (Nanodrop 2000, Thermo Scientific, USA). First-strand complementary DNA (cDNA) was synthesized using the cDNA Reverse Transcription Kit (Thermo Scientific, USA) in accordance with the manufacturer’s guidelines and stored at − 20 °C until use. End-point RT-PCR was used for the detection of β-actin, ANP (atrial natriuretic peptide), and BNP (B-type natriuretic peptide) genes. Briefly, 1 μL of first-strand cDNA (100 ng), PCR buffer (1 ×), dNTP mix (0.2 mM), specific primers (0.4 μM), and AmpliTaq DNA polymerase (0.025 U/μL) were used according to the manufacturer’s instructions. The program for the detection is as follows: 30 cycles of 15 s of melting at 95 °C, 20 s of annealing at respective temperature, and 20 s of extension at 72 °C using Applied Biosystems 3000. Primer sequences for β-actin are as follows: TGACGATATCGCTGCGCTC (forward), CAGTTGGTGACAATGCCGTG (reverse); ANP: AGAGAGTGAGCCGAGACAGC (forward), TGTTGGACACCGCACTGTAT (reverse); BNP: GACTCCGGCTTCTGACTCTG (forward), ACTGTGGCAAGTTTGTGCTG (reverse).
Statistical analysis
Data are reported as mean ± SD unless stated otherwise. Comparisons between groups and treatments were made using two-way analysis of variance (ANOVA) followed by a pairwise post hoc test like Bonferroni test where appropriate. Calculations were performed using GraphPad Prism (Graph-Pad Software, CA, USA). P < 0.05 was considered statistically significant.
Results
Characteristics of rats
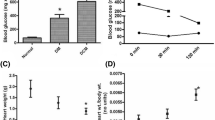
In the present study, STZ injection was combined with high-fat diet to induce cardiomyopathy in Wistar rat that led to high plasma glucose level (33 ± 7 mM) with increased insulin resistance (HOMA-IR index; 9.65 ± 0.48). STZ-injected animal fed with standard diet exhibited the plasma glucose level as 20 ± 3 mM and HOMA index as 7.89 ± 0.39, compared with normal control (Supplementary Table 1). At the end of 12 weeks, diabetic animal fed with high-fat diet exhibited decreased plasma insulin (6.42 ± 0.19 vs 12.56 ± 0.37) level, elevated expression of BNP and reduced expression of ANP, increased heart weight/body weight ratio (6 ± 0.9), and high collagen content (4.24 ± 0.08), compared with the normal animal, indicating the presence of cardiac hypertrophy and fibrosis in DCM animals. Unlike DCM animals, diabetic animal fed with standard diet did not show any significant change in hypertrophic and fibrosis parameters as mentioned above (Table S1).
Hydrogen sulfide post-conditioning improved cardiac hemodynamic function in I/R-challenged diabetes heart with and without myopathy
The hemodynamic changes of different experimental groups are shown in Supplementary Fig. 1. The cardiac performance of isolated perfused DCM rat heart was low (rate pressure product, 13 ± 0.6) with a higher LVEDP, indicating ventricular hypertrophy (Fig. S1), as compared with that of DM (RPP = 24 ± 1.2) and normal rat hearts (RPP = 35 ± 1.7). Upon reperfusion, the hemodynamic indices were further declined significantly in all groups and post-conditioning the respective groups with H2S improved the physiological parameters significantly in both normal and DM rat hearts, but the moderate improvement was observed with DCM rat heart (Fig. S1).
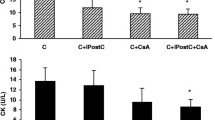
The cardiac injury was evaluated via TTC staining, caspase-3 enzyme activity, and perfusate cardiac marker levels, and the results are shown in Table 1 and in Supplementary Fig. 1 and Supplementary Fig. 2. Measured myocardial infarct size was 24 ± 0.7%, 18 ± 1.8%, and 37 ± 2.4% in normal I/R, DM-I/R, and DCM-I/R rat hearts, respectively, when compared with the sham control rats (4 ± 0.2) (Fig. S1 (d)), and was supported by elevated caspase activity in the tissue and LDH and CK activities in coronary perfusate. Conditioning these hearts with H2S significantly reduced the infarct size in I/R-challenged normal and DM hearts, whereas in DCM, only a minimal improvement was observed (Fig. S1 (d) and Fig. S2).
Effect of hydrogen sulfide post-conditioning on mitochondrial function
Electron transport chain enzyme activities
The mitochondrial functional activity was measured by evaluating the electron transport chain (ETC) enzyme activities and estimating the ATP levels. The baseline measurement of ETC enzymes suggests low activity in both DM and DCM rat hearts with prominent impact on SSM fraction, compared with the normal rat heart. When diabetic heart fed with high-fat diet and standard diet has undergone I/R, all the ETC complex activities were declined in both IFM and SSM (Fig. 1), compared with the respective sham control and normal control rat hearts. HPOC treatment improved the mitochondrial complex activities (both in IFM and SSM) significantly (P < 0.05) in normal heart challenged to I/R. However, the same trend was not observed with diabetic heart with and without myopathy. In DCM-HPOC, except complex II, the rest of the complex activities improved compared with DCM-I/R and the effect was prominent in SSM. But in DM-HPOC, most of the ETC enzyme complexes were not significantly recovered in both IFM and SSM, compared with those in DCM-I/R group (Fig. 1).
Mitochondrial ETC enzymes of normal, DM, and DCM rat myocardium subjected to I/R injury and HPOC protocol. a Complex I activity. b Complex II activity. c Complex III activity. d Complex IV activity. Complex I activity was expressed as μmol NADH oxidized min−1 mg−1 protein; Complex II activity was expressed in μmol DCPIP reduced min−1 mg−1 protein; Complex III activity was expressed in μmol cytochrome C reduced min−1 mg−1 protein; Complex IV activity was expressed in μmol cytochrome C oxidized min−1 mg−1 protein; Data were represented as mean ± SD of 6 individual experiments. *p < 0.05 sham vs. DM-sham vs. DCM-sham; #p < 0.05 I/R vs. respective sham control; $p < 0.05 HPOC vs. respective I/R control. DM: diabetes mellitus; DCM: diabetic cardiomyopathy; I/R: ischemia-reperfusion
Mitochondrial ATP level
The ATP concentration in the rat heart isolated mitochondria from all experimental groups under non-energized and energized conditions by utilizing GM (glutamate/malate) and succinate as substrate were measured and the results are shown in Fig. 2. Baseline ATP level under non-energized condition was found to be different among the experimental groups, where DM heart exhibited similar ATP concentration as that of normal, while DCM heart showed declined ATP level. This difference was more prominent with IFM. However, in the energized condition, the ATP concentration in the DCM heart was improved. The decreased I/R-associated ATP concentration was increased by HPOC procedure in all energized conditions (Fig. 2). Recovery of ATP concentration was conspicuous in SSM fraction of DM heart and IFM fraction of DCM heart (Fig. 2).
ATP content of normal, DM, and DCM rat myocardium subjected to I/R injury and HPOC protocol. Panels a–c represent measured ATP content using ATPlite luminescence kit, in non-energized, glutamate/malate energized, and succinate energized medium. Data were represented as mean ± SD of 6 individual experiments. *p < 0.05 sham vs. DM-sham vs. DCM-sham; #p < 0.05 I/R vs. respective sham control; $p < 0.05 HPOC vs. respective I/R control. DM: diabetes mellitus; DCM: diabetic cardiomyopathy; I/R: ischemia-reperfusion
Mitochondrial membrane potential and swelling
Mitochondrial membrane potential and swelling in isolated mitochondria were measured in non-energized and energized medium (GM and succinate) conditions, and the results are shown in Fig. 3. Similar to the level of ATP, the baseline behavior of membrane potential and swelling was altered in isolated mitochondria from DCM rat heart, compared with other groups. Unlike the mitochondria from normal and DM hearts, HPOC protocol did not arrest the change in membrane potential and swelling behavior (Fig. 3).
Mitochondrial morphological analysis. Graphs a–c represent mitochondrial membrane potential measured in non-energized, glutamate/malate, and succinate energized conditions, respectively; graphs d–f represent mitochondrial swelling analysis data measured in non-energized, glutamate/malate, and succinate energized conditions, respectively. *p < 0.05 sham vs. DM-sham vs. DCM-sham; #p < 0.05 I/R vs. respective sham control; $p < 0.05 HPOC vs. respective I/R control. DM: diabetes mellitus; DCM: diabetic cardiomyopathy; I/R: ischemia-reperfusion
Effect of HPOC on reperfusion-induced oxidative stress
Figure 4 shows the activities of antioxidant enzymes and lipid peroxidation level in the mitochondria (SSM and IFM) from different experimental groups. Unlike normal cardiac mitochondria (both IFM and SSM), I/R induced elevated lipid peroxidation, and subsequent low antioxidant enzymes were not prominent in diabetic rat heart (including DCM heart). Hence, the HPOC-mediated attenuation of lipid peroxidation and subsequent improvement in antioxidant enzymes were observed only in normal rat heart.
Oxidative stress analysis in cardiac mitochondria subpopulation. a Levels of lipid peroxidation. b Level of reduced glutathione (GSH). c Activity of glutathione peroxidase. d Activity of glutathione reductase. e Activity of catalase. f Activity of superoxide dismutase in cardiac mitochondria subpopulation. Data were represented as mean ± SD of 6 individual experiments. *p < 0.05 sham vs. DM-sham vs. DCM-sham; #p < 0.05 I/R vs. respective sham control; $p < 0.05 HPOC vs. respective I/R control. DM: diabetes mellitus; DCM: diabetic cardiomyopathy; I/R: ischemia-reperfusion
Discussion
Numerous studies have revealed that both exogenous and endogenous H2S could exert cardioprotection in normal and diabetic hearts and the underlying mechanism is well-established using different animal models (Elrod et al. 2007; Chatzianastasiou et al. 2016; Calvert et al. 2009; Bibli et al. 2015). But no study exists in the literature that distinguishes the protective effect of H2S against ischemia-reperfusion injury in the diabetic heart that develops myopathy (basal diabetic pathology varies in the presence of myopathy) and the one that is without it.
In our present approach, we demonstrated that the cardioprotective effect of H2S post-conditioning towards I/R was inferior in the diabetic rat heart with myopathy.
Declined mitochondrial function and subsequent elevation of I/R injury in DCM rat heart compared with those in the normal and DM rat hearts might have adversely affected the HPOC efficiency to cardioprotection. These mitochondrial perturbations are a part of the progression of diabetes leading to myopathy. Among the two mitochondrial subpopulations, HPOC protected IFM better than SSM in the DCM heart probably due to the deteriorated basal SSM activity. However, upon isolating the IFM and SSM from DCM rat heart and studying their ATP-producing capacity in vitro, we found that there was no difference in the functional capacity of mitochondrial subpopulations suggesting that the distinct response of HPOC in DCM rat heart may be due to spatial location of mitochondria in the myocardium.
Evidence from the literature showed that H2S imparts cardioprotection against I/R injury by enabling the activation of cardioprotective signaling pathways that converge towards the mitochondria and utilize the organelle as their target site (Banu et al. 2016; Murphy 2004). In the present study, we found perturbed myocardial architecture (at the basal level of the tissue itself) and dysfunctional mitochondria in DCM rat heart that might have contributed to the abrogated cardioprotection by HPOC upon I/R challenge. In support to these findings, we observed that DCM heart experienced higher oxidative stress at its basal level and imparted negative influence to the protective effect of HPOC protocol, despite of lower I/R-associated elevation of oxidative stress (Fig. 4) in the tissue. For a better understanding, the schematic representation of the HPOC effect on normal, DM, and DCM hearts is depicted in Fig. 5.
A schematic representation that describes the molecular events in post-conditioning-mediated cardioprotection, when the hearts from normal, DM, and DCM rats were subjected to I/R and how POC modulates the I/R-mediated changes. (A) describes the I/R-associated cardiac injury (measured via TTC), declined physiological recovery (measured via RPP), increased oxidative stress, and defective mitochondrial function even at its subpopulation (SSM and IFM) level in normal rat heart. POC followed by I/R significantly improved the abovementioned changes. (B) and (C) describe the I/R-mediated changes in DM and DCM hearts. The molecular events described for normal myocardium on subjected to I/R are similar in DM heart and DCM heart as well. Post-conditioning was found to be cardioprotective for DM heart, as evident by inhibiting declined mitochondrial function (both SSM and IFM), and reducing infarct size, but not effective in attenuating oxidative stress. This protective effect is lost in diabetic cardiomyopathy (DCM) condition, where it fails to protect the mitochondria, thereby the infarct size and oxidative stress
Contrary to the previous LAD ligation model in rat, where two different outputs for the effect of neurohormonal axis, the present study was carried out in an isolated rat heart model. The advantage of the latter model was the absence of neurohormonal effect that enables us to measure the direct impact of H2S action on the heart. Few investigators pointed out the influential role of hormones for the pleiotropic effect of H2S in different organ systems (Hine et al. 2017) that may contribute to the cumulative impact of H2S on heart that resulted different outcomes in I/R-challenged diabetic animals. However, the impact of diabetes-mediated structural modification alone and its impact on H2S action cannot be clearly studied in the LAD model, and thus, we utilized isolated rat heart model in the present study to evaluate the efficacy of H2S-mediated cardioprotection.
According to the present study data, I/R-associated oxidative stress or mitochondrial dysfunction was low in the DCM heart (where cardiac cell showed structural alterations), but the basal biochemical changes associated with the previously mentioned factors were relatively high when compared with normal and DM rat hearts. Apparently, at the basal level, SSM experienced more oxidative stress (higher TBARS [thiobarbituric acid reactive substances] and reduced GSH level; Fig. 4) in the DCM rat heart, which is in agreement with the previous study done by Dabkowski and his co-workers (Dabkowski et al. 2010) in type II diabetic heart. It has been reported that diabetes mellitus condition is associated with elevated ROS (reactive oxygen species) release from mitochondria (Du et al. 2000) and SSM being large intricate system mainly involved in metabolism is considered to be the key target and source of free radicals. IFM, primarily present between the myofibrils that provide ATP to the contractile unit, displayed prominent mitochondrial dysfunction in DCM rat heart at its basal level. Our results are in agreement with others observing increased I/R-associated oxidative stress and mitochondrial damage in DCM heart, which differs at the level of mitochondrial subpopulations. Apparently, IFM showed significant mitochondrial damage, and SSM exhibited higher oxidative stress. But, Skulachev (2001) identified that SSM provides proton to IFM through mitochondrial filaments for ATP generation to maintain the contractility even at low concentration of oxygen. In the present study, the inability of the HPOC procedure to reverse the mitochondrial dysfunction may be because of the deteriorated function of IFM and SSM.
Diabetes is associated with a low level of H2S by downregulating CSE (cystathionine γ-lyase) gene expression. The protective effect of H2S supplementation has been shown to mitigate many diseases that include cardiomyopathy, nephropathy, retinopathy, and endothelial dysfunctions (Ye et al. 2018; Zhou et al. 2014; Si et al. 2013). Hence, by principle, H2S administration to I/R-challenged diabetic rat may be beneficial (Banu et al. 2016). But contrary to our expectation, HPOC did not work well in DCM rat heart, and our data indicated the need of sufficient healthy mitochondria for H2S action (Banu et al. 2016), which was found to be low in DCM rat heart.
Conclusion
Impaired mitochondria in diabetic heart participate in the deleterious cascade of events provoked by ischemia-reperfusion leading to myocardial cell death. The prevailed cellular energetics in terms of reducing equivalent in diabetic heart and its change in the biochemical environment during the development of myopathy will distinctly modulate the activity of spatially located different mitochondria. Intact mitochondria in the myofibrils in the diabetic heart deteriorated when the DM heart developed myopathy. HPOC effectively improved the cardiac mitochondria function in the diabetic heart without myopathy, but not in the DCM heart.
References
Algeria JR, Miller TD, Gibbons RJ, Yi QL, Yusuf S (2007) Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 154:743–750
Ansari SB, Kurian GA (2016) Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem Biol Interact 252:28–35
Banu SA, Ravindran S, Kurian GA (2016) Hydrogen sulfide post-conditioning preserves interfibrillar mitochondria of rat heart during ischemia reperfusion injury. Cell Stress Chaperones 21(4):571–582
Baracca A, Sgarbi G, Solaini G, Lenaz G (2003) Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta Bioenerg 1606(1–3):137–146
Bibli SI, Andreadou I, Chatzianastasiou A, Tzimas C, Sanoudou D, Kranias E, Brouckaert P, Coletta C, Szabo C, Kremastinos DT, Iliodromitis EK (2015) Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc Res 106:432–442
Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ (2009) Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105:365–374
Chatzianastasiou A, Bibli SI, Andreadou I, Efentakis P, Kaludercic N, Wood ME, Whiteman M, Di Lisa F, Daiber A, Manolopoulos VG, Szabó C (2016) Cardioprotection by H2S donors: nitric oxide-dependent and -independent mechanisms. J Pharmacol Exp Ther 106:432–442
Chinopoulos C, Starkov AA, Fiskum G (2003) Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem 278:27382–27389
Croston TL, Thapa D, Holden AA, Tveter KJ, Lewis SE, Shepherd DL, Nichols CE, Long DM, Olfert IM, Jagannathan R, Hollander JM (2014) Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 307(1):H54–H65
Dabkowski ER, Baseler WA, Williamson CL, Powell M, Ra-zunguzwa TT, Frisbee JC, Hollander JM (2010) Mito-chondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol 299:H529–H540
Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97:12222–12226
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta Mol Cell Res 1813(7):1351–1359
Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H (2007) Hydrogen sulfide attenuates myocardial ischemia–reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104:15560–15565
Frazier AE, Thorburn DR (2012) Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods Mol Biol 837:49–62
Galderisi M, Anderson KM, Wilson PW, Levy D (1991) Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 68:85–89
Hine C, Kim HJ, Zhu Y, Harputlugil E, Longchamp A, Matos MS, Ramadoss P, Bauerle K, Brace L, Asara JM, Ozaki CK (2017) Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metabol 25(6):1320–1333
Kurian GA, Berenshtein E, Kakhlon O, Chevion M (2012) Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. Biochem Biophys Res Commun 428(3):376–382
Lambert JP, Nicholson CK, Amin H, Amin S, Calvert JW (2014) Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res 4:20–31
Mahalakshmi A, Kurian GA (2018) Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radic Biol Med 118:35–43
Marso SP, Miller T, Rutherford BD, Gibbons RJ, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Mehran R, Krucoff MW, Lansky AJ (2007) Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD trial). Am J Cardiol 100:206–210
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18(2):149–166
Murphy E (2004) Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res 94(1):7–16
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252(23):8731–8739
Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW (2013) Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol 304(9):H1215–H1224
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J (2013) Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol 169(3):619–631
Skulachev VP (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci 26:23–29
van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P (2011) Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res 92(1):10–18
Voulgari C, Papadogiannis D, Tentolouris N (2010) Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag 6:883
Ye P, Gu Y, Zhu YR, Chao YL, Kong XQ, Luo J, Ren XM, Zuo GF, Zhang DM, Chen SL (2018) Exogenous hydrogen sulfide attenuates the development of diabetic cardiomyopathy via the FoxO1 pathway. J Cell Physiol 233(12):9786–9798
Zhou X, Feng Y, Zhan Z, Chen J (2014) Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem 289(42):28827–28834
Acknowledgments
The authors sincerely thank the Department of Science and Technology for providing INSPIRE fellowship (DST/INSPIRE Fellowship/2013/326).
Author information
Authors and Affiliations
Contributions
Dr. Gino A Kurian has designed the work and helped in data interpretation and writing the manuscript. Ms. Mahalakshmi A has executed the experiments, analyzed the data, interpreted the results, wrote the manuscript, and compiled the literature sources.
Corresponding author
Ethics declarations
All procedures for the handling of the animals during the investigations were reviewed and approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA Approval No. 300/SASTRA/IAEC/RPP), India.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of significance
Hydrogen sulfide post-conditioning failed to recover cardiac mitochondrial function in diabetic cardiomyopathy rat heart from ischemia-reperfusion resulted injury.
Electronic supplementary material
ESM 1
(DOCX 1462 kb)
Rights and permissions
About this article
Cite this article
A, M., Kurian, G.A. Mitochondrial dysfunction plays a key role in the abrogation of cardioprotection by sodium hydrosulfide post-conditioning in diabetic cardiomyopathy rat heart. Naunyn-Schmiedeberg's Arch Pharmacol 393, 339–348 (2020). https://doi.org/10.1007/s00210-019-01733-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01733-z