Abstract
Heavy metal exposure is associated with cardiovascular diseases such as myocardial infarction (MI). Vascular dysfunction is related to both the causes and the consequences of MI. We investigated whether chronic exposure to low doses of mercury chloride (HgCl2) worsens MI-induced endothelial dysfunction 7 days after MI. Male Wistar rats were divided into four groups: Control (vehicle), HgCl2 (4 weeks of exposure), surgically induced MI and combined HgCl2-MI. Morphological and hemodynamic measurements were used to characterize the MI model 7 days after the insult. Vascular reactivity was evaluated in aortic rings. Chronic HgCl2 exposure did not cause more heart injury than MI alone in terms of the morphological or hemodynamic parameters. Vascular reactivity increased in all groups, but the combination of HgCl2-MI increased the vasorelaxation induced by ACh compared with the HgCl2 and MI groups. Results showed reduced endothelial nitric oxide synthase (eNOS) protein expression in the MI group; increased iNOS activity in the HgCl2-MI group, although without enough magnitude to reverse the reduction in NO bioavailability; and increased phenylephrine response in the HgCl2-MI group due to an increase in ROS production, notably via xanthine oxidase (XO). Results suggest that the combination of 1 month pre-exposure of HgCl2 before MI changed the endothelial generation of oxidative stress induced by mercury exposure from NADPH oxidase pathway to XO (xanthine oxidase)-dependent ROS production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury is an environmental pollutant representing a high risk to public health. Human exposure usually occurs by the consumption of mercury-contaminated fish and the inhalation of mercury vapor during professional exposure [1,2,3,4,5]. Mercury exposure produces adverse effects on the cardiovascular system [1, 4,5,6], driving endothelial dysfunction by increasing ROS production [7, 8]. Similar pathological effects also occur in clinical conditions as hypertension, atherosclerosis, diabetes and MI [9]; therefore, mercury exposure is considered an important risk factor for the development of cardiovascular diseases [7, 10,11,12,13].
Oxidative stress at the endothelial level as an effect of mercury exposure is already reported for both conductance and resistance vessels [7, 14, 15]. In addition, oxidative stress is known as an efficient mechanism to produce oxidized low-density lipoprotein and consequently atherosclerosis [16]. Indeed, there is a close link between mercury and cardiovascular diseases, such as carotid atherosclerosis, myocardial infarction, coronary heart disease and hypertension [5, 14, 17]. Advanced glycation end products are generated, and subsequent participation of inflammatory cells maintains vascular injury [18].
Myocardial infarction (MI) is the leading cause of death worldwide and is associated with high mortality [19]. The consequences of MI can be catastrophic, such as sudden death soon after the ischemic event, or chronic, such as the development of heart failure (HF) [20,21,22].
At the vascular level, impaired NO bioavailability is associated with overproduction of vascular reactive oxygen species (ROS), generation of peroxynitrite and an inadequate antioxidant reserve [23, 24].
Potential sources of vascular ROS production include NADPH oxidase, xanthine oxidase and uncoupled NO synthases [25]. Mercury exposure and MI injury increase oxidative stress in the vessels mainly by the activation of NADPH oxidase, COX-2 and the renin–angiotensin system [8, 15, 26, 27]. Additionally, the activity of vascular xanthine oxidase is increased by > 200% in patients with chronic HF after myocardial damage and is inversely associated with endothelium-dependent vasodilatation [28,29,30,31]. Regarding vascular effects, results are controversial. It has been reported that heart failure in rats enhanced basal NO release in the aorta, and in rat caudal arteries iNOS inhibition prevented the augment of phenylephrine reactivity [32, 33]. However, no studies to date have investigated whether chronic exposure to low doses of mercury worsens the adverse effects that might result from MI in conductance vessels.
Although MI may have occurred in individuals who were first exposed to HgCl2, the underlying mechanisms that relate the consequences of MI and low doses of HgCl2 are still unknown. In addition, the fact that the endothelium is affected even by low concentrations of heavy metals highlights the important need to better understand the mechanisms by which these metals promote development of cardiovascular diseases in association with MI. Thus, the aim of this study was to investigate the consequences of MI in combination with chronic exposure to HgCl2 and the underlying mechanisms that affect vascular reactivity in these rats.
Materials and Methods
Animals
Studies were performed on male Wistar rats (2 months, 170–200 g). All experiments were conducted in compliance with the biomedical research guidelines stated by the Brazilian Societies of Experimental Biology and were approved by the local ethics committee of the Federal University of Espírito Santo (084/2011 CEUA-UFES). All rats had access to water and rodent chow ad libitum.
Induction of Myocardial Infarction and Experimental Groups
Rats were divided into four experimental groups, as previously reported [34]: non-infarcted animals with saline exposure (vehicle–saline solution, i.m.) as a control (Control), animals that underwent myocardial infarction with saline exposure (MI), non-infarcted animals with HgCl2 exposure and animals that underwent myocardial infarction and were treated with HgCl2 (HgCl2-MI). We performed the 30-day HgCl2 treatment as reported by Wiggers et al. [35]. As previously described, this HgCl2 treatment (1st dose 4.6 μg/kg i.m., subsequent doses 0.07 μg/kg/day i.m. to cover daily loss) led to HgCl2 blood levels of approximately 8 ng/mL [14, 35]. In the present study, this dose was considered a low dose of HgCl2 instead of a toxic dose because a blood mercury concentration of 8 ng/mL is similar to the levels observed in exposed humans [36, 37].
MI was induced as previously reported [20]. Briefly, animals were anaesthetized with ketamine (50 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). The thorax was opened via a thoracotomy in the fourth intercostal space, and the heart was quickly exposed. With a 6.0 suture string, the left anterior descending coronary artery was permanently occluded at approximately 2 mm from its origin. The heart was returned, and the thorax was closed. Mechanical respiratory support was used for animals that did not spontaneously resume respiratory movement. In the non-infarcted control groups of animals, a sham surgery was performed following the same protocol described above but without coronary occlusion.
Hemodynamic Measurements and Scar Area
Seven days after surgery, the animals were anaesthetized with ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) for hemodynamic evaluation, as previously described [20]. Briefly, the right common carotid artery was dissected and separated from the connective tissue. A fluid-filled polyethylene catheter (P50) was inserted in the carotid artery. Systolic (SBP) and diastolic (DBP) blood pressure and heart rate (HR) were measured after stabilization. The catheter was then advanced into the left ventricle, and the systolic (LVSP) and end-diastolic (LVEDP) pressures and the maximum rates of pressure rise and fall (± dP/dt) were obtained.
To determine the infarct size, the scar tissue was carefully separated from the non-infarcted myocardium, and the area of the scar tissue was measured. Infarct area is presented as the infarct area relative to the total left ventricular area [20, 21].
Vascular Reactivity Responses
Immediately after the hemodynamic measurements, the thoracic aorta was dissected, and the segments were mounted in an isolated tissue chamber containing Krebs–Henseleit buffer (in mmol L−1: NaCl 118, KCl 4.7, NaHCO3 23, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, glucose 11 and EDTA 0.01) that had been gassed with 95% O2 and 5% CO2 (pH 7.4). The aortas were then maintained at a resting tension of 1 g at 37 °C. The isometric tension was recorded using an isometric force transducer (TSD125C BIOPAC Systems, Inc., Santa Barbara, CA, USA) connected to an acquisition system (MP100 BIOPAC Systems, Inc., Santa Barbara, CA, USA).
After a 45-min equilibration period, all aortic rings were initially exposed twice to 75 mM KCl. The first exposure checks their functional integrity, and the second exposure assesses the maximal tension developed. Then, endothelial integrity was tested with acetylcholine (ACh, 10 µM) in segments that were previously contracted with phenylephrine (10 µM). After a washout period (30 min), increasing phenylephrine concentrations (10−10–3.10−4 M) were applied. A concentration–response curve for this contractile agonist was obtained, and tension was measured once a plateau was attained.
The role of endothelial-derived vasoactive factors on the phenylephrine-elicited contractile response was investigated. The effects of the following drugs were evaluated: (1) the nonspecific NOS inhibitor N-nitro-l-arginine methyl ester (L-NAME, 100 μM), (2) the specific iNOS inhibitor 1400 W (1 µM), (3) an enzyme scavenger of ROS (superoxide dismutase, 150 U mL−1), (4) an NADPH oxidase inhibitor (apocynin, 0.3 mM) and (5) a xanthine oxidase inhibitor (allopurinol, 100 µM). These drugs were added 30 min before the generation of the phenylephrine concentration–response curves. Importantly, to guarantee the quality of the experiments, only one drug was used for each aortic ring.
Additionally, the influence of the endothelium on the response to phenylephrine in aortic rings from all groups was investigated after the mechanical removal of the endothelium by rubbing the lumen with a needle. The absence of the endothelium was confirmed by the inability of 10 µM ACh to produce relaxation.
To evaluate relaxation dependent and independent of the endothelium, after the 45-min equilibration period, two different aortic rings were contracted with phenylephrine (1 µM) until they reached a plateau (approximately 15 min), and the concentration–response curves for ACh (10−10 – 3 * 10−4 M) or sodium nitroprusside (SNP, 10−10 – 3 * 10−4 M) were obtained.
Western Blot Analysis
In another set of animals, the eNOS, iNOS, Mn-SOD and Cu/Zn-SOD protein expression levels were analyzed in homogenates from the aortic segments used for the reactivity experiments, as previously described [14].
Proteins from the homogenized arteries were separated by 7.5–12% SDS-PAGE. The proteins were transferred to nitrocellulose membranes that were then incubated with mouse monoclonal antibodies against endothelial nitric oxide synthase (eNOS, 1:250; Transduction Laboratories™, UK), inducible nitric oxide synthase (iNOS, 1:200; BD Transduction Laboratories™, UK), Cu–Zn superoxide dismutase (Cu/Zn-SOD, 1:1000, Sigma Aldrich, Germany) or Mn superoxide dismutase (Mn-SOD, 1:500, Sigma Aldrich, Germany). After being washed, the membranes were incubated with an anti-mouse or anti-rabbit (1:5000, StressGen, Victoria, Canada) immunoglobulin antibody conjugated to horseradish peroxidase. After a thorough wash, the immunocomplexes were detected with an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL, Amersham International, Little Chalfont, UK) and film (Hyperfilm ECL International). The signals on the immunoblot were quantified with the National Institutes of Health ImageJ V1.56 computer program. The same membrane was used to examine β-actin expression using a mouse monoclonal antibody (1:5000, Sigma, USA).
Data Analyses and Statistics
Results are expressed as the mean ± SEM. Contractile responses are presented as the percentages of the maximal response induced by 75 mM KCl. For each concentration–response curve, the maximal contraction (R max) induced by depolarization to KCl of vascular smooth muscle and the agonist concentration that produced 50% of the maximal response (log EC50) were calculated using nonlinear regression analysis with GraphPad Prism™ (version 5.0, GraphPad Software, USA). Agonist sensitivities are expressed as pD2 (−log EC50). To compare the effect of drugs on the response of the aortic ring to phenylephrine, some results are expressed as the differences of the area under the concentration–response curves (dAUC) in the control and experimental situations. AUCs were calculated from individual concentration–response curve plots; the differences are expressed as the percentages of the control AUC.
For protein expression, the results are expressed as the ratio between signals on the immunoblot corresponding to the protein of interest and β-actin. The results are expressed as the mean ± SEM for the number of rats indicated; differences were analyzed using Student’s t test or one-way ANOVA, followed by Tukey’s test. p < 0.05 was considered statistically significant.
Results
General Characteristics
General characteristics of Control, HgCl2, MI and HgCl2-MI groups are shown in Table 1. Body weight (BW) gain was observed in all groups between the 1st and 3rd weeks. At the 4th week, the BW of the Control and HgCl2 rats continued to increase, while BW loss was evident in the animals that underwent MI surgery (p < 0.05, MI and HgCl2-MI). Additionally, the relative weight of the right ventricle (RV/BW) and relative weight of the lungs (LW/BW) increased in both MI groups compared with the Control and HgCl2 groups (p < 0.05). These results are a necessary condition to characterize the presence of heart failure [20]. The same scar size was observed in the MI and HgCl2-MI groups (p > 0.05) (Table 1).
Hemodynamic Measurements
Systolic and diastolic blood pressures, left ventricular systolic pressure (LVSP) and positive (+ dP/dt) and negative (− dP/dt) rates of pressure development in the left ventricle (LV) were reduced in the MI and HgCl2-MI groups compared with the Control and HgCl2 groups. Moreover, the left ventricular end-diastolic pressures (LVEDPs) of the MI and HgCl2-MI groups were significantly higher than those of the Control and HgCl2 groups (p < 0.05) (Table 1). In this context, hemodynamic function was impaired in both groups that underwent MI. However, chronic exposure to HgCl2 did not add other changes to the impairments caused by infarction.
Response to Vascular Reactivity
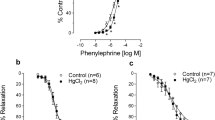
Given that both HgCl2 chronic exposure and MI affect vascular reactivity were investigated by endothelial and smooth muscle function, and endothelial-dependent and endothelial-independent vasodilatations, we examined the effect of HgCl2 plus myocardial infarction on vascular contraction and relaxation. Chronic HgCl2 exposure, acute MI and the combined effect of HgCl2 and MI increased the maximal response (R max) induced by phenylephrine in aortic rings compared with the Control group (Fig. 1a, Table 2). These similar results suggest that the combination of HgCl2 and MI did not enhance the increased contractile response to phenylephrine observed in the HgCl2 and MI groups.
Concentration–response curves of phenylephrine (a), acetylcholine (b) and sodium nitroprusside (c) in rat aortic rings from the Control, HgCl2, MI and HgCl2-MI groups. The results are reported as the mean ± SEM, with the number of animals used in parentheses. * p < 0.05 versus Control; & p < 0,05 versus HgCl2-MI one-way ANOVA followed by Tukey’s post hoc test
Exposure to acetylcholine and SNP produced concentration-dependent relaxation in the aortic rings of all groups. Chronic HgCl2 exposure and acute MI equally reduced the vascular response to acetylcholine. However, the combined effect of chronic HgCl2 exposure and MI (HgCl2-MI group) normalized the reduction of Rmax observed in HgCl2 and MI groups (Fig. 1b, Table 2). In addition, endothelium-independent vasodilatation, which was analyzed by SNP administration, was similar in all groups (Fig. 1c, Table 2).
Effects of Chronic HgCl2 Exposure Plus MI on Endothelial Function in Vascular Responses
To evaluate the influence of the endothelium, we mechanically removed it. Reactivity to phenylephrine was increased in all groups, but this response was smaller in the HgCl2, MI and HgCl2-MI groups than in the Control group (Fig. 2a–d). This difference is clearly observable when the dAUCs are compared with those of the Control group (Control: 115.4 ± 12.0, HgCl2: 80.5 ± 5.3, MI: 29.5 ± 4.4, HgCl2-MI: 49.3 ± 5.6, p < 0.05) (Fig. 2e).
Effects of endothelium removal (E−) on the concentration–response curves of phenylephrine in rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test. The inset graph shows differences in the area under the concentration–response curve in endothelium-denuded and intact segments of the four experimental groups (dAUC, e). * p < 0.05 versus Control, & p < 0.05 versus HgCl2, $ p < 0.05 versus MI, # p < 0.05 versus MI + HgCl2, one-way ANOVA followed by Tukey’s post hoc test
To investigate whether the reduced endothelial modulation of the vascular response to phenylephrine depended on NO, we used the NOS inhibitor L-NAME (100 µM). This drug promoted an increase in the response to phenylephrine in all groups evaluated (Fig. 3a–d), but this effect was smaller in the aortic rings from the HgCl2, MI and HgCl2-MI groups than in those from the Control group, as shown by the dAUC values (Control: 116.8 ± 12.8, HgCl2: 61.1 ± 7.6, MI: 28.9 ± 3.2, HgCl2-MI: 29.9 ± 2.3, p < 0.05) (Fig. 3e).
Effects of L-NAME on the concentration–response curves of phenylephrine in rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test. The inset graph shows differences in the area under the concentration–response curve of the four experimental groups (dAUC, e) in the presence and absence of L-NAME. Densitometry analyses of western blots for endothelial nitric oxide synthase (eNOS) (f). * p < 0.05 versus Control, & p < 0.05 versus HgCl2, $ p < 0.05 versus MI, # p < 0.05 versus MI + HgCl2, one-way ANOVA followed by Tukey’s post hoc tests
These results suggest that chronic HgCl2 exposure, acute MI and the combined effect of both conditions induce endothelial dysfunction in conductance arteries, thereby reducing endothelial NO modulation. Because alterations in the level of eNOS can explain this endothelial dysfunction, the expression of this protein was measured. However, we observed that eNOS protein expression was reduced only in the aorta of the MI group compared with the other groups (Fig. 3f).
Since eNOS was reduced only in the MI group, we performed experiments to investigate whether iNOS was involved in endothelial NO modulation. However, in both the HgCl2 and HgCl2-MI groups, iNOS expression was similar to the control. We then used the specific iNOS inhibitor 1400 W (1 µM) in vitro. Only the phenylephrine concentration–response curve of the HgCl2-MI group shifted to the left after drug administration (Fig. 4a–d), suggesting an increase in iNOS activity. These results suggest that only the infarcted animals exposed chronically to HgCl2 underwent MI-stimulated NO production via iNOS, even though the iNOS protein expression levels were similar among all groups evaluated (Fig. 4e). Therefore, our results suggest reduced NO bioavailability in the HgCl2, MI and HgCl2-MI groups. However, only in the MI group could this reduction be explained by a reduction in eNOS expression.
Effect of 1400 W on the concentration–response curves of phenylephrine in rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test. Densitometry analyses of western blots for inducible nitric oxide synthase (iNOS) (e)
The Role Of Free Radicals in the Effects of Chronic HgCl2 Exposure Plus MI in the Aorta
To explain the reduction in NO bioavailability, we investigated whether the endothelial changes observed in the aortic rings from the HgCl2, MI and HgCl2-MI groups were related to changes in O2•− production. The effects of the superoxide anion scavenger SOD (150 U/mL), the NADPH oxidase inhibitor apocynin (0.3 mM) and the xanthine oxidase inhibitor allopurinol (100 µM) on the vasoactive responses were examined. SOD reduced the contractile responses to phenylephrine in the HgCl2, MI and HgCl2-MI groups but not in the Control group (Fig. 5a–d). Indeed, the dAUC values of the HgCl2, MI and HgCl2-MI groups showed that the reactivity reduction produced by SOD treatment was similar (Control: 22.3 ± 2.5, HgCl2: 46.3 ± 8.6, MI: 43.9 ± 3.9, HgCl2-MI: 59.6 ± 7.4, p < 0.05) (Fig. 5e). These findings suggest that the HgCl2 and HgCl2-MI groups produced more ROS than the control group. To investigate the levels of antioxidant proteins, two important isoforms of SOD were evaluated. Western blot analysis revealed decreased protein expression levels of mitochondrial Mn-SOD in the aortas from the MI group compared with those from the Control group, but the cytosolic Cu/Zn-SOD expression level was unaltered among all groups evaluated (Fig. 5f, g).
Effect of SOD on the concentration–response curves of phenylephrine in rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test. The inset graph shows differences in the area under the concentration–response curve of the four experimental groups (dAUC, e) in the presence and absence of SOD. Densitometry analyses of western blots for superoxide dismutase Mn and superoxide dismutase Cu/Zn (Mn-SOD and Cu/Zn-SOD) (f). * p < 0.05 versus Control, one-way ANOVA followed by Tukey’s post hoc tests
The two major pathways that produce vascular oxidative stress are the NADPH oxidase and xanthine oxidase pathways [38]. To elucidate which signaling pathway was responsible for the increase in ROS production, we first examined NADPH oxidase by incubating the aortic rings with apocynin. Apocynin promoted a decrease in the response to phenylephrine in arteries from all groups (Fig. 6a–d). For the HgCl2 and MI groups, the increase in O2•− production was related to NADPH oxidase pathway, but a similar effect was not observed in the Control and HgCl2-MI groups, as indicated by the dAUC values (Control: 22.7 ± 5.0, HgCl2: 54.5 ± 6.5, MI: 44.9 ± 5.2, HgCl2-MI: 19.8 ± 5.9, p < 0.05) (Fig. 6e).
Effect of apocynin on the concentration–response curves for phenylephrine of rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test. The inset graph shows differences in the area under the concentration–response curve of the four experimental groups (dAUC, e) in the presence and absence of apocynin. * p < 0.05 versus Control and # versus HgCl2-MI, one-way ANOVA followed by Tukey’s post hoc tests
Previous reports suggested that xanthine oxidase is another pathway for ROS endothelial production [25]. To investigate the involvement of xanthine oxidase in vascular ROS production, we blocked this pathway with allopurinol. Only the HgCl2-MI group showed a leftward shift in the concentration–response curve to phenylephrine in the presence of the xanthine oxidase inhibitor (Fig. 7a–d). These results bring a novel finding that the ROS production found in animals that underwent chronic HgCl2 exposure plus MI was different from that of other groups due to an increase in xanthine oxidase activity.
Effect of allopurinol on the concentration–response curves of phenylephrine in rat aortic rings from the Control (a), HgCl2 (b), MI (c) and HgCl2-MI (d) groups. The results are reported as the mean ± SEM, with the number of animals in parentheses. § p < 0.05 compared with the corresponding control for each group, as determined by Student’s t test
Discussion
The present study demonstrates for the first time that chronic exposure to low doses of HgCl2 in combination with acute MI causes endothelial dysfunction in isolated rat aortic rings due to reduced NO bioavailability associated with an increase in ROS production via xanthine oxidase. These changes are characterized by the following: (a) increased phenylephrine response in the HgCl2-MI group, most likely related to an increase in ROS production, notably via xanthine oxidase; (b) eNOS protein expression reduction in the MI group; and (c) increased activity of iNOS in the HgCl2-MI, although without enough magnitude to reverse the reduction of NO bioavailability and the increased vascular reactivity.
It is important to emphasize that an increased reactivity of vascular smooth muscle of large arteries, reducing aortic compliance, adds further pressure overload to the left ventricle [39]. Considering that MI and Hg exposure increase ROS production and vascular reactivity [14, 15, 35], they might enhance the risk of developing heart failure.
Evidence from epidemiologic studies suggests that people with high levels of mercury in their urine, hair or toenails have an increased risk of cardiovascular diseases [12, 13]. Indeed, an association between the amount of mercury in hair and the risk of MI was reported in humans [17]. Other studies show a direct effect of acute or chronic mercury exposure on endothelial function, which is characterized by reduced NO bioavailability and increased ROS production [14, 35, 40].
To characterize the presence of HF in the animals that underwent MI injury (the MI and HgCl2-MI groups), we examined several relevant factors. The two groups with MI presented impaired morphological and hemodynamic parameters, increased relative weight of the lungs, and increased RV- and LVEDP, which are consistent with our previous findings [20]. However, chronic HgCl2 exposure plus MI did not cause alterations in the morphological or hemodynamic parameters beyond the changes already caused by infarction. Such behavior was expected because previous reports showed that both arterial blood and ventricular pressures were not affected by the HgCl2 treatment we used here [35, 41]. In the current study, we observed that chronic HgCl2 exposure plus MI promoted an increase in the aortic vascular reactivity to phenylephrine, which was similar to the effects of MI injury or HgCl2 exposure alone. These alterations seem to be explained by reduced endothelial modulation of the contractile response induced by phenylephrine. We previously reported that chronic HgCl2 exposure increases vascular reactivity to phenylephrine, most likely as a result of reduced NO bioavailability in the rat aorta and coronary vessels [14, 35, 40]. This reduced endothelial modulation produced by mercury exposure was explained mainly by increased NADPH oxidase activity [14], but without involvement of xanthine oxidase. In contrast, in the HgCl2-MI group, the reduced participation of NO in vessel tone modulation observed with L-NAME incubation was not accompanied by a reduction in eNOS expression, suggesting that another mechanism was involved.
Vascular NADPH oxidase in the aortic wall is a major source for ROS generation [42]. At present, it is accepted that this oxidase activity impairs endothelial function in both HF [43, 44] and chronic HgCl2 exposure [14, 35]. Thus, to verify whether NADPH oxidase mediates the ROS source, we used a non-selective NADPH oxidase inhibitor, apocynin. Apocynin treatment promoted a decrease in the vascular contraction response to phenylephrine in the aortic rings of the HgCl2 and MI groups, but the HgCl2-MI response was similar to what was found in Control animals suggesting that the oxidative stress produced by NADPH oxidase was reduced as shown by the dAUC reduction (Fig. 6e). However, as shown in Fig. 1a, the increased reactivity to phenylephrine was similar in the treated groups. This finding suggested that another source of ROS might play a role.
Another import source of vascular ROS is xanthine oxidase [28, 38]. To investigate this pathway, we blocked xanthine oxidase with allopurinol [38]. The aortic rings from rats in the chronic HgCl2 exposure and acute MI groups showed no changes in vascular reactivity to phenylephrine after allopurinol administration. However, after allopurinol administration, the group receiving both acute MI and chronic HgCl2 exposure showed a reduction in its vascular reactivity to phenylephrine. These results suggest that xanthine oxidase might be another major source of ROS in the HgCl2-MI group. A previous report showed an increase in xanthine oxidase activity after chronic MI, and treatment with allopurinol reduced the incidence of apoptosis in non-ischemic areas [45]. In addition, previous reports show activation of xanthine oxidase after reperfusion injury and myocardial damage [29,30,31]. Indeed, for the first time, we report that after acute MI injury associated with low doses of HgCl2, the activation of ROS production via the xanthine oxidase pathway modulates endothelial dysfunction. Results suggest that myocardial infarction changes the endothelial generation of oxidative stress induced by mercury exposure, to vascular XO-dependent ROS production.
It is important to emphasize that even with enhanced iNOS activity, which was present only in the HgCl2-MI group, increased NO production was not enough to counterbalance the reduction in NO bioavailability.
Interestingly, our results show reduced ACh-induced vasodilatation in the HgCl2 and MI groups. However, in the animals that underwent HgCl2 chronic exposure and MI injury (HgCl2-MI), the ACh relaxation dose–response curve was preserved. We argue that this finding could be related to an increase in iNOS activity as previously reported after MI [33]. We then incubated the aortic rings with the pharmacological iNOS inhibitor 1400 W. Aortic rings that underwent chronic HgCl2 exposure or were isolated from rats that underwent acute MI individually showed no changes in the vascular reactivity to phenylephrine. However, after iNOS blockade by 1400-W administration, the vascular response of animals that underwent chronic HgCl2 plus MI injury indicated that the NO production was associated with iNOS even though the iNOS protein levels showed no alterations. Thus, the NO production from iNOS might be a response to restore endothelium-dependent relaxation. However, despite this result, MI injury associated with chronic HgCl2 exposure caused vascular dysfunction, as characterized by increase in phenylephrine reactivity and reduced NO bioavailability.
The development of endothelial dysfunction is marked by the reduced endogenous production of antioxidant agents [23, 46]. Indeed, another important mechanism that counterbalances NO production is ROS generation [47]. Animals that underwent MI showed reduced Mn-SOD protein expression levels, which is consistent with the endothelial dysfunction developed in this group of animals. In contrast, the animals that underwent MI and chronic HgCl2 exposure did not exhibit altered Mn-SOD protein expression levels. However, even the apparently counterregulatory effect of iNOS associated with normal Mn-SOD protein expression, which maintains endothelium-mediated vasodilatation, was insufficient to prevent endothelial dysfunction caused by the overproduction of ROS observed in the HgCl2-MI group. The ROS excess reacts with NO, disrupting physiological signaling and leading to production of toxic and reactive molecules, such as peroxide nitrite [47]. To verify our hypothesis, we used a superoxide scavenger enzyme, SOD. SOD reduced the maximal response to phenylephrine in the HgCl2-MI group, suggesting that the combination of chronic HgCl2 exposure and MI also maintains oxidative stress. Our findings also lead us to speculate that the association between HgCl2 and MI might support the transition from an acute to a chronic phase of myocardial infarction by xanthine oxidase activation.
In conclusion, the present study demonstrates that the combination of chronic HgCl2 exposure plus MI injury also presented endothelial vascular dysfunction. Such dysfunction is mediated by the vascular xanthine oxidase pathway, different to what occurs with mercury exposure and HF by themselves, which activate NADPH oxidase, COX2 and RAS. These findings help to explain the reduced participation of NO in vascular reactivity due to: (a) increased phenylephrine response in the HgCl2-MI group, most likely related to an increase in ROS production, notably via xanthine oxidase; (b) increased activity of iNOS only in the HgCl2-MI although without enough magnitude to reverse the reduction of NO bioavailability; (c) eNOS protein expression reduction in the MI group. These findings suggest that the pre-exposure of HgCl2 before MI turns the endothelial generation of oxidative stress by mercury exposure from NADPH oxidase pathway to XO-dependent ROS production.
References
Clarkson, T. W., Magos, L., & Myers, G. J. (2003). The toxicology of mercury—current exposures and clinical manifestations. The New England Journal of Medicine, 349, 1731–1737.
Langworth, S., Sällsten, G., Barregard, L., Cynkier, I., Lind, M. L., & Söderman, E. (1997). Exposure to mercury vapour and impact on health in the dental profession in Sweden. Journal of Dental Research, 76, 1397–1404.
McKelvey, W., Gwynn, R. C., Jeffery, N., Kass, D., Thorpe, L. E., Garg, R. K., et al. (2007). A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environmental Health Perspectives, 115, 1435–1441.
Salonen, J. T., Seppänen, K., Nyyssönen, K., Korpela, H., Kauhanen, J., Kantola, M., et al. (1995). Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation, 91, 645–655.
Virtanen, J. K., Voutilainen, S., Rissanen, T. H., Mursu, J., Tuomainen, T. P., Korhonen, M. J., et al. (2005). Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 228–233.
Choi, A. L., Weihe, P., Budtz-Jørgensen, E., Jørgensen, P. J., Salonen, J. T., Tuomainen, T. P., et al. (2009). Methylmercury exposure and adverse cardiovascular effects in faroese whaling men. Environmental Health Perspectives, 117(3), 367–372.
Vassallo, D. V., Simões, M. R., Furieri, L. B., Fioresi, M., Fiorim, J., Almeida, E. A., et al. (2011). Toxic effects of mercury, lead and gadolinium on vascular reactivity. Brazilian Journal of Medical and Biological Research, 44, 939–946.
Lemos, N. B., Angeli, J. K., Faria, T. de O., Ribeiro Junior, R. F., Vassallo, D. V., Padilha, A. S., et al. (2012). Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS ONE, 7(11), e49005.
Rajendran, P., Rengarajan, T., Thangavel, J., Nishigaki, Y., Sakthisekaran, D., Sethi, G., et al. (2013). The vascular endothelium and human diseases. International Journal of Biological Sciences, 9, 1057–1069.
Guallar, E., Sanz-Gallardo, M. I., van’t Veer, P., Bode, O., Aro, A., Gómez-Aracena, J., et al. (2002). Mercury, fish oils, and the risk of myocardial infarction. The New England Journal of Medicine, 347, 1747–1754.
Houston, M. C. (2007). The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Alternative Therapies in Health and Medicine, 13, S128–S133.
Houston, M. C. (2011). Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. Journal of Clinical Hypertension (Greenwich), 13, 621–627.
Park, S. K., Lee, S., Basu, N., & Franzblau, A. (2013). Associations of blood and urinary mercury with hypertension in U.S. adults: The NHANES 2003–2006. Environmental Research, 123, 25–32.
Furieri, L. B., Galán, M., Avendaño, M. S., García-Redondo, A. B., Aguado, A., Martínez, S., et al. (2011). Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. British Journal of Pharmacology, 162, 1819–1831.
Peçanha, F. M., Wiggers, G. A., Briones, A. M., Perez-Giron, J. V., Miguel, M., Garcia-Redondo, A. B., et al. (2010). The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to mercury concentration. Journal of Physiology and Pharmacology, l61(1), 29–36.
Kishimoto, T., Oguri, T., & Tada, M. (1995). Effect of methylmercury (CH3HgCl) injury on nitric oxide synthase (NOS) activity in cultured human umbilical vascular endothelial cells. Toxicology, 103, 1–7.
Salonen, J. T., Seppanen, K., Lakka, T. A., Salonen, R., & Kaplan, G. A. (2000). Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis, 148, 265–273.
Harja, E., Bu, D. X., Hudson, B. I., Chang, J. S., Shen, X., Hallam, K., et al. (2008). Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE −/− mice. The Journal of Clinical Investigation, 118, 183–194.
Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J. D., Borden, W. B., et al. (2013). Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation, 127, e6–e245.
Faria, T. de O., Baldo, M. P., Simões, M. R., Pereira, R. B., Mill, J. G., Vassallo, D. V., et al. (2011). Body weight loss after myocardial infarction in rats as a marker of early heart failure development. Archives of Medical Research, 42, 274–280.
Mill, J. G., Stefanon, I., dos Santos, L., & Baldo, M. P. (2011). Remodeling in the ischemic heart: the stepwise progression for heart failure. Brazilian Journal of Medical and Biological Research, 44, 890–898.
Stefanon, I., Valero-Muñoz, M., Fernandes, A. A., Ribeiro, R. F., Jr., Rodríguez, C., Miana, M., et al. (2013). Left and right ventricle late remodeling following myocardial infarction in rats. PLoS ONE, 8, e64986.
Bauersachs, J., & Widder, J. D. (2008). Endothelial dysfunction in heart failure. Pharmacological Reports, 60, 119–126.
Förstermann, U. (2008). Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature Clinical Practice Cardiovascular Medicine, 5, 338–349.
Weseler, A. R., & Bast, A. (2010). Oxidative stress and vascular function: implications for pharmacologic treatment. Current Hypertension Reports, 12, 154–161.
Abbate, A., Santini, D., Biondi-Zoccai, G. G. L., Scarpa, S., Vasaturo, F., Liuzzo, G., et al. (2004). Cyclo-oxygenase-2 (COX-2) expression at the site of recent myocardial infarction: friend or foe? Heart, 90(4), 440–443.
Looi, Y. H., Grieve, D. J., Siva, A., Walker, S. J., Anilkumar, N., Cave, A. C., et al. (2008). Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension, 51(2), 319–325.
Landmesser, U., Spiekermann, S., Dikalov, S., Tatge, H., Wilke, R., et al. (2002). Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation, 106, 3073–3078.
Engberding, N., Spiekermann, S., Schaefer, A., Heineke, A., Wiencke, A., Müller, M., et al. (2004). Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug. Circulation, 110, 2175–2179.
Zweier, J. L., & Talukder, M. A. (2006). The role of oxidants and free radicals in 32 reperfusion injury. Cardiovascular Research, 70(2), 181–190.
Sagor, M. A., Tabassum, N., Potol, M. A., & Alam, M. A. (2015). Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxidative Medicine and Cellular Longevity, 2015, 478039.
da Cunha, V., Stefanon, I., & Mill, J. G. (2004). The role of nitric oxide in mediating cardiovascular alterations accompanying heart failure in rats. Canadian Journal of Physiology and Pharmacology, 82, 372–379.
Sartório, C. L., Pinto, V. D., Cutini, G. J., Vassallo, D. V., & Stefanon, I. (2005). Effects of inducible nitric oxide syinthase inhibition on the rat tail vascular bed reactivity three days after myocardial infarction. Journal of Cardiovascular Pharmacology, 45, 321–326.
Faria, T. de O., Costa, G. P., Almenara, C. C., Angeli, J. K., Vassallo, D. V., Stefanon, I., et al. (2014). Chronic exposure to low doses of HgCl2 avoids calcium handling impairment in the right ventricle after myocardial infarction in rats. PLoS ONE, 9(4), e95639.
Wiggers, G. A., Peçanha, F. M., Briones, A. M., Pérez-Girón, J. V., Miguel, M., Vassallo, D. V., et al. (2008). Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. American Journal of Physiology Heart and Circulatory Physiology, 295, H1033–H1043.
Gupta, M., Bansal, J. K., & Khanna, C. M. (1996). Blood mercury in workers exposed to the preparation of mercury cadmium telluride layers on cadmium telluride base. Industrial Health, 34, 421–425.
Chen, C., Qu, L., Li, B., Xing, L., Jia, G., Wang, T., et al. (2005). Increased oxidative DNA damage, as assessed by urinary 8-hydroxy-2-deoxyguanosine concentrations, and serum redox status in persons exposed to mercury. Clinical Chemistry, 51, 759–767.
George, J., & Struthers, A. D. (2009). Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vascular Health and Risk Management, 5, 265–272.
O’Rourke, M. F., & Pauca, A. L. (2004). Augmentation of the aortic and central arterial pressure waveform. Blood Pressure Monitoring, 9, 179–185.
Rizzetti, D. A., Torres, J. G., Escobar, A. G., Peçanha, F. M., Santos, F. W., Puntel, R. L., et al. (2013). Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS ONE, 8, e55806.
Furieri, L. B., Fioresi, M., Junior, R. F., Bartolomé, M. V., Fernandes, A. A., Cachofeiro, V., et al. (2011). Exposure to low mercury concentration in vivo impairs myocardial contractile function. Toxicology and Applied Pharmacology, 255(2), 193–199. doi:10.1016/j.taap.2011.06.015.
Schramm, A., Matusik, P., Osmenda, G., & Guzik, T. J. (2012). Targeting NADPH oxidases in vascular pharmacology. Vascular Pharmacology, 56, 216–231.
Hare, J. M., & Stamler, J. S. (2005). NO/Redox disequilibrium in the failing heart and cardiovascular system. The Journal of Clinical Investigation, 115, 509–517.
Ahn, B., Beharry, A. W., Frye, G. S., Judge, A. R., & Ferreira, L. F. (2015). NAD(P)H oxidase subunit p47phox knockout prevents diaphragm contractile dysfunction in heart failures. American Journal of Physiology. Lung Cellular and Molecular Physiology, 309, L497–L505.
Xiao, J., She, Q., Wang, Y., Luo, K., Yin, Y., Hu, R., et al. (2009). Effect of allopurinol on cardiomyocyte apoptopsis in rats after myocardial infarction. European Journal of Heart Failure, 11, 20–27.
Cai, H., & Harrison, D. G. (2000). Endothelial dysfunction in cardiovascular disease: The role of oxidative stress. Circulation Research, 87, 840–844.
Förstermann, U. (2010). Nitric oxide and oxidative stress in vascular disease. Pflügers Archv, 459, 923–939.
Acknowledgements
This study was supported by Grants from No 54685435/2011-FAPES (Fundação de Amparo à Pesquisa do Espírito Santo), No 48511935/2009 PRONEX—FAPES/CNPq (FAPES/Conselho Nacional de Desenvolvimento Cientifico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faria, T.d., Simões, M.R., Vassallo, D.V. et al. Xanthine Oxidase Activation Modulates the Endothelial (Vascular) Dysfunction Related to HgCl2 Exposure Plus Myocardial Infarction in Rats. Cardiovasc Toxicol 18, 161–174 (2018). https://doi.org/10.1007/s12012-017-9427-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9427-x