Abstract
Selenium (Se) is an essential trace element for human health and plays an important role in the development and maintenance of central nervous system functions. Se deficiency has been associated with cognitive decline and increased oxidative stress. The increase in oxidative stress is one of the hypotheses for the emergence and worsening of neurodegenerative diseases, such as Alzheimer’s disease (AD). To investigate the neuroprotective effects of organic Se compounds in human neuroblastoma cells (SH-SY5Y) differentiated into cholinergic neurons-like. The SH-SY5Y cells were differentiated into cholinergic neuron-like with retinoic acid (RA) and brain-derived neurotrophic factor (BDNF). AD was mimicked exposing the cells to okadaic acid (OA) and beta-amyloid protein (Aβ). The neuroprotective effect of organic Se compounds, selenomethionine (SeMet) and Ebselen, was evaluated through cell viability tests, acetylcholinesterase and antioxidant enzyme activities, and detection of reactive oxygen species (ROS). None of the SeMet concentrations tested protected against the toxic effect of OA + Aβ. On the other hand, previous exposure to 0.1 and 1 µM Ebselen protected cells from the toxic effect of OA + Aβ. Cell differentiation induced by RA and BDNF exposure was effective, showing characteristics of neuronal cells, and pointing to a promising model of AD. Ebselen showed a protective effect, but more studies are needed to identify the mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), described by Alois Alzheimer in 1907, is the most prevalent cause of dementia in the elderly population [1]. AD is characterized by severe and progressive loss of memory, with a progressive and sequential decline in cognition [2, 3]. Depression, apathy, and hallucinations are symptoms primarily observed in the early stages of AD [4]. On the other hand, in the advanced stages of AD, the subjects have a decline in motor function and a significant loss of language, ability to perform tasks, and name people and objects [5, 6]. Psychiatric and behavioral changes, such as agitation, irritability, delusions, and hallucinations are present in up to 75% of AD cases [2, 7].
In AD, neuronal loss occurs due to the deposition of amyloid β (Aβ) in extracellular senile plaques and tau hyperphosphorylation in intracellular neurofibrillary tangles contributing to neurodegeneration [8]. Current AD treatments, for example, cholinesterase inhibitors, e.g., donepezil, rivastigmine, and galantamine, are useful in delaying the AD progression but may cause side effects (e.g., nausea, vomiting, anxiety, agitation, tremors, drowsiness, and seizures) [9]. Treating dementia before the onset of clinical symptoms is as important as developing therapeutics for post-diagnostic treatment.
Trace elements are essential for normal health, suggesting that diet and lifestyle can reduce the risk of pathologies. Some trace elements have shown potential neuroprotective effects [10, 11]. Selenium (Se) is a trace element with antioxidant properties due to the selenol group present in selenoproteins [12,13,14,15,16]. It has been observed that the levels of Se in the brain tend to decrease during aging and are related to cognitive decline [17]. In vitro and in vivo studies indicate that Se is important for reducing oxidative stress and other harmful agents in AD [8]. Sufficient Se intake and adequate body Se levels may be related to decreased AD risk [2, 18]. Recently, our group [19] demonstrated, through a systematic review and meta-analysis, that Se supplementation improves the Se levels in plasma, erythrocytes, and cerebrospinal fluid, as well as improves the glutathione peroxidases activity, an important antioxidant selenoprotein, in AD and mild cognitive impairment (MCI) individuals.
Se can be found in organic (e.g., selenomethionine (SeMet), methyl selenocysteine, selenocysteine, and selenocystine) and inorganic (e.g., selenate and selenite) chemical forms [13]. Research on natural and synthetic organic Se compounds is increasing. Organic Se compounds have been targeted due to their greater bioavailability of Se and lower toxicity compared to inorganic Se molecules. SeMet and Ebselen are examples of natural and synthetic organic Se compounds, respectively, that demonstrate great pharmacological potential [13,14,15,16, 20, 21]. Studies conducted with animal models of AD have demonstrated that SeMet treatment reduces tau hyperphosphorylation [22] and Ebselen reverses memory impairment [23].
The difficulty of in vitro models that resemble the biochemical and molecular AD findings makes the search for new treatments arduous. In this context, in vitro models with cholinergic neuron-like cells are becoming important tools to evaluate the potential of new drugs. The SH-SY5Y human neuroblastoma cells are derived from metastatic neuroblastoma and are in immature stages of development [24, 25]. Medeiros et al. [26] demonstrated that this cell line can be differentiated into choline-producing cells using retinoic acid (RA) and brain-derived neurotrophic factor (BDNF). In the same study, after cholinergic differentiation, to mimic AD, neurotoxins such as okadaic acid (OA) and Aβ were used resulting in decreased neurite densities and cell viability. Thus, to contribute to research on the effects of Se on AD, this in vitro study investigated the neuroprotective effects of organic Se in human neuroblastoma cells (SH-SY5Y) differentiated into cholinergic neuron-like cells, analyzing cell viability and biochemical parameters.
Materials and Methods
SH-SY5Y Cell Line
SH-SY5Y (neuroblastoma) was acquired at the cell bank of Rio de Janeiro, Brazil. SH-SY5Y is a neuroblastic-type cell [24].
Cell Cultivation
SH-SY5Y cells were cultured with culture medium Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM-F12) (Sigma-Aldrich®, USA), supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich®, USA), and 1% penicillin/streptomycin (Sigma-Aldrich®, USA). Cells were kept in a 75 cm² culture flask at 37 ºC in an atmosphere with 5% CO2 until a minimum confluence of 80%. The medium was replaced 2–3 days a week.
SH-SY5Y Cholinergic Differentiation
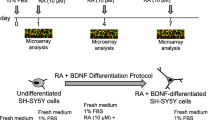
The cholinergic differentiation was performed according to Medeiros et al. [26] and Lopes et al. [27]. Briefly, SH-SY5Y cells were seeded at 6 or 48-well plates at a density of 5 × 104 or 1 × 105 cells per well, depending on the analysis performed, in a culture medium with DMEM-F12 supplemented with 10% of FBS and 1% penicillin/streptomycin. After 24 h, neuronal differentiation was induced by reducing FBS to 1% and adding 10 µM RA (Santa Cruz Biotechnology, USA). On the fourth day of differentiation, 50 ng/mL of BDNF was added. The treatment was replaced every three days for seven days (Fig. 1).
Immunocytochemistry
For immunofluorescence analysis, cells were seeded in 48-well plates at a density of 5 × 104 and cholinergic differentiation was performed. Subsequently, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature and then washed three times with phosphate buffer saline (PBS). After fixation, the cells were permeabilized with a PBS solution containing 3% Triton X-100 and 10% FBS for 5 min at room temperature and washed three times with PBS. The plates were incubated for 24 h (overnight) at 4 ºC with the primary antibody Anti-β Tubulin III (Sigma-Aldrich®, USA); after that, the cells were washed three times with PBS and incubated at room temperature with the corresponding secondary antibody conjugated to FITC (anti-mouse) (Sigma-Aldrich®, USA). To stamp the cell nucleus, Hoechst 33,342 (2’-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5’-bi-1 H-benzimidazole trihydrochloride trihydrate) (Sigma-Aldrich®, USA) was used [28]. The control was performed using undifferentiated SH-SY5Y cells. The reading was performed using an inverted fluorescence microscope and the images were obtained using the InCell Analyzer 2000. Reading analyses were performed using Fiji (ImageJ).
Neuroprotector Effects of Se
Selection of Concentration
To determine the toxicity of the Aβ1-42 protein fragment (Sigma-Aldrich®, USA) and OA (Sigma-Aldrich®, USA), a concentration curve of the compounds was performed (Aβ1-42: 0, 0.05, 0.1, 0.15, and 0.2 µM; OA: 0, 0.5, 1, 1.5, and 2 µM). On the other hand, to select the concentrations with pharmacological potential, SeMet (Cayman Chemical Company, USA) and Ebselen (Sigma-Aldrich®, USA) were tested at 0, 0.1, 0.3, 1, 3, 10, and 30 µM. The SH-SY5Y cells were seeded at 48-well plates at a density of 1 × 105 and, after seven days of differentiation, the cholinergic neuron-like cells were exposed to the compounds for 24 h. After, the cytotoxicity assay was performed as described in the cytotoxicity assay topic.
Selenium Pre-Treatment and OA + Aβ Exposure
After differentiation, the cholinergic neuron-like cells were pre-treated with SeMet or Ebselen for 24 h. After, the cells were exposed to OA + Aβ for 24 h. After that, the cytotoxicity and biochemical analyses were performed (Fig. 2).
Cytotoxicity Assay
After the exposure of cholinergic neuron-like cells to compounds as described selection of concentration and selenium pre-treatment and OA + Aβ exposure topics, the culture medium was replaced by 1 mg/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT; Invitrogen®, USA) solution, prepared in complete culture medium. After 3 h of incubation, at 37 ºC, all MTT solution was removed and 200 µL of dimethylsulfoxide (DMSO; Êxodo Cientifica, Br) was added. The absorbance was read spectrophotometrically at 595 nm [27]. Results were expressed as the percentage of control.
Biochemical Analyses
Sample Preparation
To perform the biochemical analysis, cholinergic neuron-like cells exposed to compounds as described in the selenium pre-treatment and OA + Aβ exposure topic were previously prepared. All the culture medium was collected, wells were washed two times with PBS, and cells were harvested by trypsin. To inactivate the action of trypsin, DMEM-F12 supplemented with 10% FBS and 1% penicillin/streptomycin was added. Samples were centrifuged at 400 g for 5 min, the supernatant was discarded, and the pellet was washed two times with PBS. The samples were resuspended in PBS, and the aliquots were stored at -80 ºC for thirty minutes before the biochemical analyses.
Acetylcholinesterase
Acetylcholinesterase (AChE) activity was measured by the method described by Ellman et al. [29]. In a 96-well plate, 25 µL of the sample (prepared as described in the sample preparation topic), 0.75 mM 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB; Sigma-Aldrich®, USA), and 10 mM acetylthiocholine (Sigma-Aldrich®, USA) were added. The absorbance was measured spectrophotometrically at 405 nm once a minute for 5 min. The activity was expressed as nmol ACh/min/mg of protein.
Glutathione S-Transferase
The activity of the enzyme glutathione S-transferase (GST) was evaluated by the method described by Keen et al. [30]. Briefly, 20 µL of the sample (prepared as described in the sample preparation topic) and 180 µL reaction solution (3.0 mM 1-chloro-2,4-dinitrobenzene (CDNB; Sigma-Aldrich®, USA), 3.0 mM reduced glutathione (GSH; Sigma-Aldrich®, USA), and 0.1 M phosphate buffer, pH 6.5) were added in a 96-well plate. Absorbance was measured spectrophotometrically at 340 nm once a minute for 4 min. The activity was expressed as nmol/min/mg of protein.
Superoxide Dismutase
The analysis of superoxide dismutase (SOD) activity was performed according to the method proposed by Gao et al. [31]. Briefly, 40 µL of the sample (prepared as described in the sample preparation topic), 1 M Tris (Sigma-Aldrich®, USA), 5 mM ethylenediaminetetraacetic acid (EDTA; VWR Life Science®, USA) buffer, pH 8.0, and 15 mM pyrogallic acid (Labsynth®, Br) were added in a 96-well plate. After 30 min of incubation at room temperature, 1 N hydrochloric acid (Sigma-Aldrich®, USA) was added to stop the reaction. The absorbance was measured spectrophotometrically at 440 nm. The results were expressed as U of SOD/mg of protein.
Glutathione Peroxidase
Glutathione peroxidase (GPX) activity was performed using the method of Paglia and Valentine [32]. In a 96-well plate, 10 µL of the sample (prepared as described in the sample preparation topic) and 130 µL of reaction solution 1 (0.1 M PBS, pH 7.0, 3.08 mM sodium azide (Labsynth®, Br), 0.31 mM NADPH (Sigma-Aldrich®, USA), 3.08 mM GSH, and 1.54 U/mL glutathione reductase (Sigma-Aldrich®, USA)) were added into the wells. After 2 min at room temperature, 60 µL of reaction solution 2 (5 mM hydrogen peroxide (Sigma-Aldrich®, USA) and 0.1 M sodium phosphate buffer, pH 7.0), were added to the wells. The plate was incubated at room temperature for 5 min. The absorbance was measured spectrophotometrically at 340 nm once a minute for 5 min. The GPX activity was expressed in nmol/min/mg protein.
Protein Quantification
Total protein was quantified as described by Bradford [33]. Briefly, 10 µL of the sample (prepared as described in the sample preparation topic) and 250 µL of Bradford reagent (Thermo Scientific®, USA) were pipetted into the 96-well plate. A standard curve of bovine serum albumin (BSA; Sigma-Aldrich®, USA) was prepared (0-500 mg BSA/mL). The absorbance was measured spectrophotometrically at 595 nm. Results are expressed in mg protein/mL.
Reactive Oxygen Species
After the exposure of cholinergic neuron-like cells to compounds as described in the selenium pre-treatment and OA + Aβ exposure topic, the cell medium was collected, the wells were washed twice with PBS, and the cells were harvested by trypsin. Samples were centrifuged at 400 g for 5 min, the supernatants were discarded, and the pellets were washed two times with PBS. After that, the pellets were resuspended with a PBS solution containing 10 µM 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich®, USA) and incubated for 15 min in the dark at room temperature (Adapted from Tetz et al. [34]). Reading was performed by flow cytometry (FACS Canto II- Becton Dickinson) and analyzed by Flowing software version 2.5.0. The reading was performed in the EP channel and results are expressed as median fluorescence intensity (MFI).
Statistical Analysis
For all tests, at least, three independent experiments were carried out. The data was statistically analyzed using the Prisma Graphpad software, version 6.0, using the Kruskal-Wallis test followed by Dunn’s test or Mann-Whitney U test; and presented as median ± interquartile interval. Results were considered statistically different when p < 0.05.
Results
SH-SY5Y Cholinergic Neuron-Like Differentiation
After microscopic analysis (400x magnification), it was observed that the treatment with RA and BDNF for seven days induced neural characteristics in SH-SY5Y cells (Fig. 3A and B). It was possible to observe the cell body with a pyramidal form and the extension of the neurites (Fig. 3B).
The immunocytochemistry showed that the Anti-β Tubulin III (represented by the green color) markers were greater in SH-SY5Y differentiated to cholinergic neuron-like cells when compared to undifferentiated SH-SY5Y cells (Fig. 4A and B). Moreover, the Mann-Whitney U test indicated that cholinergic neuron-like cells had a statistically significant increase in the AChE activity (U = 0; p = 0.014). In fact, the AChE activity was 3.6 times high in cholinergic neuron-like cells when compared to undifferentiated SH-SY5Y cells (Fig. 4C).
β Tubulin III immunocytochemistry and acetylcholinesterase activity. (A) undifferentiated SH-SY5Y cells (x100) and (B) SH-SY5Y differentiated to cholinergic neuron-like (x100); blue: Hoechst (DAPI; nucleus) and green: fluorochrome FTIC (β Tubulin III). (C) Acetylcholinesterase activity of SH-SY5Y undifferentiated cells and SH-SY5Y cells differentiated to cholinergic neuron-like. The results were analyzed by the Mann-Whitney U test and presented as a median ± interquartile interval (n = 3). * Means statistically different from undifferentiated cells (SH-SY5Y).
Cell Viability: Selection of Concentrations
OA + Aβ Concentration
After cell differentiation, a concentration curve was performed to analyze the concentration of OA + Aβ that made at least more than 20% of the cells unviable. The Kruskal-Wallis test revealed an effect of OA + Aβ on cholinergic neuron-like cells viability (H(4) = 7.205; p = 0.0382). In fact, cholinergic neuron-like cells exposed to 2 µM OA and 0.2 µM Aβ had a statistically significant decrease in their viability (~ 46%) when compared to the control cells (non-exposed cells) (Fig. 5), therefore, these concentrations were chosen to the AD mimetic experiments.
SeMet and Ebselen Concentration
The Kruskal-Wallis test showed the absence of SeMet effects on the cholinergic neuron-like cell viability (Fig. 6A). On the other hand, the Kruskal-Wallis test revealed the effect of Ebselen (H(6) = 16.98; p = 0.0045) exposure on the viability of cholinergic neuron-like cells (Fig. 6B). Ebselen (10 and 30 µM) exposure caused a statistically significant reduction (~ 50%) in the viability of cholinergic neuron-like cells. Even though the concentration of 3 µM did not cause a statistically significant reduction in cell viability, a decrease of approximately 50% was observed. Thus, all the SeMet concentrations and 0.1, 0.3, and 1 µM Ebselen were used in the neuroprotection tests, as they did not alter the cholinergic neuron-like cells viability.
Cytotoxicity analysis of cholinergic neuron-like cells exposed for 24 h to SeMet (A) and Ebselen (B). The results were analyzed by the Kruskal-Wallis test followed by Dunn’s post-test and presented as median ± interquartile interval (n = 4). * Means statistically different from control (unexposed cells; dashed line)
Cell Viability: Neuroprotection
The analysis of the neuroprotective effect of SeMet and Ebselen against the toxic effects of OA + Aβ is depicted in Fig. 7A and B. The Kruskal-Wallis test showed the absence of effects of the SeMet pre-treatment in the viability of cholinergic neuron-like cells challenged with OA + Aβ (Fig. 7A). On the other hand, the Kruskal-Wallis test revealed an effect of Ebselen pre-treatment (H(4) = 8.890; p = 0.0144) on the viability of cholinergic neuron-like cells challenged with OA + Aβ (Fig. 7B). In fact, previous exposure to 0.1 and 1 µM Ebselen, protected cells from the toxic effect of OA + Aβ, increasing the cell viability by approximately 40% compared to cells that were exposed to OA + Aβ. Due to the absence of SeMet protective effects, the biochemical tests were performed only with Ebselen.
SeMet and Ebselen neuroprotection. Cholinergic neuron-like cells were treated for 24 h with SeMet (A) or Ebselen (B) and challenged with OA + Aβ. The results were analyzed by the Kruskal-Wallis test followed by Dunn’s post-test and presented as median ± interquartile interval (n = 4). *Means statistically different from cells challenged with OA + Aβ. The dashed line represents the viability of non-exposed cells
Biochemical Analysis
Acetylcholinesterase
The AChE assay was performed on the cholinergic neuron-like cells pre-treated with Ebselen and challenged with OA + Aβ (Fig. 8A). The Kruskal-Wallis test demonstrated the effect of the treatment (H(4) = 11.05; p = 0.0114) on the AChE activity of cholinergic neuron-like cells. In fact, cholinergic neuron-like cells exposed to OA + Aβ had a statistically significant increase in the AChE activity (~ 48.5%) compared to the non-exposed cells. The pre-treatment with Ebselen had no protective effect.
Enzymatic activity of cholinergic neuron-like cells treated for 24 h to Ebselen and challenged with OA + Aβ. (A) AChE, (B) SOD, (C) GST, and (D) GPX activity. The results were analyzed by the Kruskal-Wallis test followed by Dunn’s post-test and presented as the median ± interquartile interval. (n = 6 AChE/ n = 3 SOD, GST, and GPX). *Means statistically different from control cells (non-exposed cells)
Superoxide Dismutase
The SOD activity was performed on the cholinergic neuron-like cells pre-treated with Ebselen and challenged with OA + Aβ (Fig. 8B). The Kruskal-Wallis test demonstrated the effect of the treatment (H(4) = 8.318; p = 0.0055) on the SOD activity of cholinergic neuron-like cells. Only the cholinergic neuron-like cells pre-treated with 0.1 µM Ebselen and challenged with OA + AB had a statistically significant increase in the SOD activity (~ 180%) when compared to the control (non-exposed cells).
Glutathione S-Transferase
The GST activity was performed on the cholinergic neuron-like cells pre-treated with Ebselen and challenged with OA + Aβ (Fig. 8C). The Kruskal-Wallis test demonstrated no effect of the treatment on this enzyme activity.
Glutathione Peroxidase Activity
The GPX activity was performed on the cholinergic neuron-like cells pre-treated to Ebselen and challenged with OA + Aβ (Fig. 8C). The Kruskal-Wallis test demonstrated no effect of the treatment on this enzyme activity.
Reactive Oxygen Species
The reactive oxygen species (ROS) was measured on the cholinergic neuron-like cells pre-treated to Ebselen and challenged with OA + Aβ (Fig. 9). The Kruskal-Wallis test demonstrated no effect of the treatment on ROS production. Although not significant, OA + Aβ increased reactive species (~ 57.51%) when compared to non-exposed cells, and when the cells were pre-treated with Ebselen there was a decrease in these ROS levels (~ 13.69%) compared to OA + Aβ challenged cells.
Discussion
Several studies have been carried out searching in vitro models to study neurodegenerative diseases, including AD [25,26,27]. SH-SY5Y neuroblastoma cell line has been used as an in vitro model to study neurodegenerative diseases, this cell can differentiate into dopamine, choline, and adrenergic neuron-like cells [25]. Based on previous studies of Medeiros et al. [26] and Jämsä et al. [35], we sought to standardize SH-SY5Y cell differentiation with RA and BDNF to cholinergic neuron-like cells. RA has been used to induce cell differentiation impeding cell cycle progression, increasing expression of cyclin-dependent kinase inhibitors, p21 and p27Kip1, and anti-apoptotic Bcl-2 and Bcl-xL proteins, and enhancing PI3K/AKT activity, which plays a role in neurite development and differentiation [36]. Conversely, BDNF is an important regulator of the expression of the axonal marker, tau, and dendritic microtubule-associated protein-2 (MAP2), as well as is involved in the activation of TrkB and in the stimulation of signaling pathways for the establishment and conservation of neural polarization [37]. Pretreatment with RA points to a better response of BDNF [38]. In our study, to prove cell differentiation through RA and BDNF, we demonstrated the presence of β Tubulin III, which is generally expressed in neurons and is involved in neurogenesis [39], as well as an increase in the AChE activity, which is highly expressed in cholinergic neurons [1].
The mixture OA + Aβ has been used in models to mimic AD symptoms, i.e., the biochemical and molecular alterations. Medeiros et al. [26] showed in an in vitro model of AD disease using OA + Aβ an increase in the phosphorylated tau and decrease of neurite density. Kamat and Nath [40] suggested that OA may alter several biochemical and molecular events, causing tau hyperphosphorylation, cholinergic alterations, cell death, synapse dysfunction, and neuroinflammation. Aβ exposure causes severe cellular stress, indicated by lysosomal and mitochondrial deficits [41]. In animal models, OA leads to Aβ deposition and neuronal degeneration, synaptic loss, and memory impairments, characteristic of AD progression [40,41,42,43]. Furthermore, Kamat and Nath [40] suggest that OA leads to Aβ deposition, with subsequent neuronal degeneration, synaptic loss, and memory impairment, which are marks of AD progression.
Se has been shown to have beneficial effects in neurodegenerative diseases due to its antioxidant properties linked to the activity of selenoproteins [2]. SeMet attenuates tau hyperphosphorylation and Aβ deposition, reduces glial activation, attenuates neuroinflammation, decreases neuronal death, and reverses synaptic deficit in vitro and in vivo [44, 45]. Conversely, Ebselen has anti-inflammatory and antioxidant properties [45, 46]. Studies have shown that this compound reduces hippocampal oxidation, and improves apoptosis, cell proliferation, and memory in animal AD models [47].
However, there are few studies that analyze the in vitro effects of organic Se compounds in AD. In your study, we observed the absence of SeMet effects on cell viability at relatively high concentrations (up to 100 µM), this may be due to its essential role in cellular metabolism, which has been demonstrated by other studies [48,49,50,51,52]. In fact, Costa et al. [53] demonstrated that the half maximal inhibitory concentration (IC50) of SeMet in a non-tumor breast cell line (MCF-10 A), was approximately 441.76 µM, on the other hand, the IC50 of Ebselen in the same cell line was approximately 82.07 µM. The cellular metabolism of SeMet and Ebselen may explain the differences regarding cell toxicity. SeMet is metabolized to selenide and is used in the selenoproteins turnover, and only in the situation of selenoproteins saturation, the selenide will be metabolized to the MeSe• (methylselenyl radical), which is toxic [2]. Meanwhile, Ebselen interacts with intracellular thiols, and a concentration higher than 10 µM can deplete important thiol-containing molecules, such as GSH, and lead to cell death [21]. Indeed, Shi et al. [54] observed that Ebselen exposure induced GSH depletion and cell death in C6 glioma cells.
Although some AD animal models demonstrated that SeMet treatment ameliorates AD symptoms [55, 56], in our study, the SeMet pre-treatment did not have a protective effect on cell viability, this could be due to the concentrations tested. Based on our results, only the Ebselen effects were explored in the biochemical analysis. Xie et al. [57] demonstrated that Ebselen exposure reduced Aβ levels in AD neurons and mouse brains, showing a significant improvement in animals’ spatial learning and memory. Recently, Li et al. [58] pointed out that probably Ebselen’s therapeutic effect is related to the regulation of mitochondrial function in AD animal models.
AChE catalyzes the hydrolysis of the neurotransmitter acetylcholine and is present in synapses to hydrolyze acetylcholine and stop the propagation of nerve impulses [59]. Patients with AD have biochemical changes, such as deterioration of cholinergic neurons in the brain with a reduction in acetylcholine synthesis, for this, the inhibition of AChE activity is one of the therapeutic strategies to increase cholinergic levels in the brain [1]. In our study, the exposure to OA + Aβ increased the cholinergic neuron-like AChE activity. Farzi et al. [60] observed that rats exposed to Aβ had an increase in cerebral AChE activity. In humans, Campanari et al. [61] observed in postmortem AD brains that AChE levels were increased in the zone of senile plaques and neurons with neurofibrillary tangles. Seems that Aβ stimulates AChE synthesis [62], and this increase may be related to oxidative stress [63]. In fact, Melo et al. [63] demonstrated that the enhancement of AChE activity, in retinal cells, induced by Aβ was mediated by oxidative stress. Although, in our study, Ebselen did not show a protective effect in the AChE activity increase induced by OA + Aβ exposure, Martini et al. [64] demonstrated that Ebselen inhibited the purified (Electrophorus electricus) and cerebral (Wistar rats) AChE activity in vitro (IC50 ~ 29 µM).
SOD is an enzyme that is part of the antioxidant defense system and converts the superoxide radical to hydrogen peroxide. In our analysis, it was possible to observe that SOD activity increased in cells pre-treated with 0.1 µM Ebselen pointing to an increase in the cell antioxidant responses. Aras et al. [65] showed that rats treated with Ebselen had an increase in cerebral SOD activity and an improvement in the symptoms of ischemia/reperfusion injury. The increase of antioxidant enzymes in AD patients is important, once Marcus et al. [66] observed that these patients had high levels of oxidative stress markers and reduced activity of antioxidant enzymes, such as SOD.
GST protects cells against oxidative stress, this enzyme acts in the detoxification process of xenobiotics by catalyzing the conjugation reaction between a GSH molecule and a given xenobiotic [67]. There is an association between GSH depletion and AD, with a significant decrease in GST activity in the brain and the cerebrospinal fluid [68]. However, in our study, GST activity inhibition was not observed. Similarly, Ianiski et al. [69] and Wilhelm et al. [70] observed no alteration in cerebral GST activity of rodents exposed to Aβ and Ebselen, respectively.
The GPX is an intracellular antioxidant enzyme that reduces hydrogen peroxide to water [71]. Li et al. [58], in a study in Wild-type murine neuroblastoma Neuro‐2 A (N2A) cells, and Unsal et al. [72], in a rodent AD model, observed that Ebselen exposure increased the GPX activity. Again, the increase in antioxidant enzymes induced by Ebselen is pivotal in the context of AD disease, for instance, Garlet et al. [73] observed that GPX activity in the blood of patients with AD was decreased when compared to the control group.
An important factor in the neurodegeneration process is the formation of ROS. In our study, OA + Aβ caused an increase in ROS levels, and Ebselen has shown to have an effect by reducing these levels (not significant). Martorell et al. [74] carried out a study on Caenorhabditis elegans which showed that Aβ exposure increased the ROS levels. Similarly, Ianiski et al. [69] observed that mice exposed to Aβ presented an increase in cerebral ROS levels. In agreement with our findings, Xie et al. [57] observed that Ebselen treatment significantly decreased ROS levels in N2a-SW cells.
Conclusion
Cholinergic differentiation induced by RA and BDNF was effective, showing characteristics of neuronal cells of the cholinergic type, and the proposed model to mimic AD reduced cell viability. Ebselen showed a protective effect, pointing to an increase in cell viability and a tendency to increase the activity of antioxidant enzymes, but more studies need to be carried out to identify Ebselen’s mechanism of action and whether a longer treatment time would have a better effect.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Sharma K (2019) Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol Med Rep 20:1479–1487. https://doi.org/10.3892/mmr.2019.10374
Oliveira CS, Piccoli BC, Nogara PA, Pereira ME, Carvalho KAT, Skalny AV, Tinkov AA, Aschner M, Rocha JBT (2021) Selenium neuroprotection in neurodegenerative disorders. In Handbook of Neurotoxicity, 2nd ed.; Kostrzewa RM, Ed.; Springer: Cham, Switzerland, 2021; Volume 1, p. 35. https://doi.org/10.1007/978-3-031-15080-7_238
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol 16:508–518. https://doi.org/10.2174/1570159X15666170720095240
Buchman AS, Bennett DA (2011) Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother 11:665–676. https://doi.org/10.1586/ern.11.57
Kueper JK, Lizotte DJ, Montero-Odasso M, Speechley M, Alzheimer’s Disease Neuroimaging Initiative (2020) Cognition and motor function: the gait and cognition pooled index. PLoS ONE 15(9):e0238690. https://doi.org/10.1371/journal.pone.0238690
Koenig AM, Arnold SE, Streim JE (2016) Agitation and irritability in Alzheimer’s Disease: evidenced-based treatments and the black-box warning. Curr Psychiatry Rep 18:3. https://doi.org/10.1007/s11920-015-0640-7
Dominiak A, Wilkaniec A, Wroczyński P, Adamczyk A (2016) Selenium in the therapy of neurological diseases. Where is it Going? Curr Neuropharmacol 14:282–299. https://doi.org/10.2174/1570159x14666151223100011
Pedrini S, Gupta VB, Hone E, Doecke J, O’Bryant S, James I, Bush AI, Rowe CC, Villemagne VL, Ames D, Masters CL, Martins RN, AIBL Research Group (2017) A blood-based biomarker panel indicates IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in an AD cohort. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-14020-9
Cardoso BR, Cominetti C, Cozzolino SM (2013) Importance and management of micronutrient deficiencies in patients with Alzheimer’s disease. Clin Interv Aging 8:531–542. https://doi.org/10.2147/CIA.S27983
Mehri A (2020) Trace elements in human nutrition (II) - an update. Int J Prev Med 11:2. https://doi.org/10.4103/ijpvm.IJPVM_48_19
Oliveira CS, Piccoli BC, Aschner M, Rocha JBT (2017) Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In Neurotoxicity of Metals; Aschner M, Costa L, Eds.; Springer: Cham, Switzerland, Volume 18, pp. 53–83. https://doi.org/10.1007/978-3-319-60189-2_4
Rocha JBT, Piccoli BC, Oliveira CS (2017) Biological and chemical interest in selenium: a brief historical account. Arkivoc 457–491. https://doi.org/10.24820/ark.5550190.p009.784
Hassan W, Oliveira CS, Noreen H, Kamdem JP, Nogueira CW, Rocha JBT (2016) Organoselenium compounds as potential neuroprotective therapeutic agents. Curr Org Chem 20:218–231
Nogara PA, Oliveira CS, Rocha JBT (2020) Chemistry and pharmacology of synthetic organoselenium compounds. Organoselenium Chem., De Gruyter, Berlin, 305–346. https://doi.org/10.1515/9783110625110-008
Rocha JB, Oliveira CS, Nogara PA (2017) Toxicology and anticancer activity of synthetic organoselenium compounds. Organoselenium Compounds in Biology and Medicine. Synthesis, Biological and Therapeutic Treatments, pp 342–376
Cardoso BR, Bandeira VS, Jacob-Filho W, Cozzolino SMF (2014) Selenium status in elderly: relation to cognitive decline. J Trace Elem Med Biol 28:422–426. https://doi.org/10.1016/j.jtemb.2014.08.009
Cardoso BR, Apolinário D, Bandeira VS, Busse AL, Magaldi RM, Jacob-Filho W, Cozzolino SMF (2016) Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: a randomized controlled pilot trial. Eur J Nutr 55:107–116. https://doi.org/10.1007/s00394-014-0829-2
Pereira ME, Souza JV, Galiciolli MEA, Sare F, Vieira GS, Kruk IL, Oliveira CS (2022) Effects of selenium supplementation in patients with mild cognitive impairment or Alzheimer’s Disease: a systematic review and meta-analysis. Nutrients 14:3205. https://doi.org/10.3390/nu14153205
Santi C, Scimmi C, Sancineto L (2021) Ebselen and Analogues: pharmacological properties and synthetic strategies for their preparation. Molecules 26:4230. https://doi.org/10.3390/molecules26144230
Nogara PA, Oliveira CS, Pereira ME, Bortoli M, Orian L, Aschner M, Rocha JBT (2022) Therapeutic applications of low-molecular-weight thiols and selenocompounds. In: Redox Chemistry and Biology of Thiols. Alvarez B, Comini MA, Salinas G, Trujillo M (Eds), Academic Press, Chap. 27, pp. 643–677. https://doi.org/10.1016/B978-0-323-90219-9.00005-4
Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, Hoffmann PR, Ni JZ, Song GL (2017) Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci 37:2449–2462. https://doi.org/10.1523/JNEUROSCI.3229-16.2017
Martini F, Rosa SG, Klann IP, Fulco B, Carvalho FB, Rahmeier FL, Fernandes MC, Nogueira CW (2019) A multifunctional compound Ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J Psychiatr Res 109:107–117. https://doi.org/10.1016/j.jpsychires.2018.11.021
Cogo SC, Nascimento TGFC, Pinhatti FAB, Junior NF, Rodrigues BS, Cavalli LR, Elifio-Esposito S (2020) An overview of neuroblastoma cell lineage phenotypes and in vitro models. Exp Biol Med 245:1637–1647. https://doi.org/10.1177/1535370220949237
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2
Medeiros LM, De Bastiani MA, Rico EP, Schonhofen P, Pfaffenseller B, Wollenhaupt-Aguiar B, Grun L, Barbé-Tuana F, Zimmer ER, Castro M, Parsons RB, Klamt F (2019) Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s Disease studies. Mol Neurobiol 56:7355–7367. https://doi.org/10.1007/s12035-019-1605-3
Lopes FM, Schröder R, da Frota ML Jr, Zanotto-Filho A, Müller CB, Pires AS, Meurer RT, Colpo GD, Gelain DP, Kapczinski F, Moreira JC, Fernandes M, Klamt F (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94. https://doi.org/10.1016/j.brainres.2010.03.102
Trzaska KA, Rameshwar P (2011) Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells. Methods Mol Biol 698:295–303. https://doi.org/10.1007/978-1-60761-999-4_22
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251:6183–6188
Gao R, Yuan Z, Zhao Z, Gao X (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelect Bioenerg 45:41–45. https://doi.org/10.1016/S0302-4598(98)00072-5
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R (2013) Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods 67:56–60. https://doi.org/10.1016/j.vascn.2013.01.195
Jämsä A, Hasslund K, Cowburn RF, Bäckström A, Vasänge M (2004) The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun 319:993–1000. https://doi.org/10.1016/j.bbrc.2004.05.075
Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp 108:53193. https://doi.org/10.3791/53193
Hromadkova L, Bezdekova D, Pala J, Schedin-Weiss S, Tjernberg LO, Hoschl C, Ovsepian SV (2020) Brain-derived neurotrophic factor (BDNF) promotes molecular polarization and differentiation of immature neuroblastoma cells into definitive neurons. Biochim Biophys Acta Mol Cell Res 1867:118737. https://doi.org/10.1016/j.bbamcr.2020.118737
Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimer’s Dis 20:1069–1082. https://doi.org/10.3233/JAD-2010-091363
Duly A, Kao F, Teo WS, Kavallaris M (2022) βIII-tubulin gene regulation in health and disease. Front Cell Dev Biol 10:851542. https://doi.org/10.3389/fcell.2022.851542
Kamat PK, Nath C (2015) Okadaic acid: a tool to study regulatory mechanisms for neurodegeneration and regeneration in Alzheimer’s disease. Neural Regen Res 10:365–367. https://doi.org/10.4103/1673-5374.153679
Rostami J, Mothes T, Kolahdouzan M, Eriksson O, Moslem M, Bergström J, Ingelsson M, O’Callaghan P, Healy LM, Falk A, Erlandsson A (2021) Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation 18:124. https://doi.org/10.1186/s12974-021-02158-3
Kamat PK, Tota S, Rai S, Shukla R, Ali S, Najmi AK, Nath C (2012) Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats. Eur J Pharmacol 690:90–98. https://doi.org/10.1016/j.ejphar.2012.06.006
Kaushal A, Wani WY, Bal A, Gill KD, Kaur J (2019) Okadaic acid and hypoxia-induced dementia model of Alzheimer’s type in rats. Neurotox Res 35:621–634. https://doi.org/10.1007/s12640-019-0005-9
Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, Hoffmann PR, Ni JZ, Song GL (2017) Selenomethionine mitigates Cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s Disease Mouse Model. J Neurosci 37(9):2449–2462. https://doi.org/10.1523/JNEUROSCI.3229-16.2017
Li X, Shi Q, Xu H, Xiong Y, Wang C, Le L, Lian J, Wu G, Peng F, Liu Q, Du X (2022) Ebselen Interferes with Alzheimer’s Disease by Regulating Mitochondrial Function. Antioxidants (Basel) 11(7):1350. Published 2022 Jul 11. https://doi.org/10.3390/antiox11071350
Nogara PA, Pereira ME, Orian L, Oliveira CS, Rocha JBT (2023) The Long Story of Ebselen: From About One Century of its Synthesis to Clinical Trials. In: Vito Lippolis; Claudio Santi; Eder J. Lenardão; Antonio L. Braga. (Org.). The Long Story of Ebselen: From About One Century of its Synthesis to Clinical Trials. 1ed. p. 567–591
Klann IP, Martini F, Rosa SG, Nogueira CW (2020) Ebselen reversed peripheral oxidative stress induced by a mouse model of sporadic Alzheimer’s disease. Mol Biol Rep 47(3):2205–2215. https://doi.org/10.1007/s11033-020-05326-5
Zhu W, Liu Y, Zhang W, Fan W, Wang S, Gu JH, Sun H, Liu F (2021) Selenomethionine protects hematopoietic stem/progenitor cells against cobalt nanoparticles by stimulating antioxidant actions and DNA repair functions. Aging 13:11705–11726. https://doi.org/10.18632/aging.202865
Reyes L, Bishop DP, Hawkins CL, Rayner BS (2019) Assessing the efficacy of Dietary Selenomethionine supplementation in the setting of Cardiac Ischemia/Reperfusion Injury. Antioxid (Basel Switzerland) 8:546. https://doi.org/10.3390/antiox8110546
Kajander EO, Harvima RJ, Kauppinen L, Akerman KK, Martikainen H, Pajula RL, Kärenlampi SO (1990) Effects of selenomethionine on cell growth and on S-adenosylmethionine metabolism in cultured malignant cells. Biochem J 267:767–774. https://doi.org/10.1042/bj2670767
Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, El-Deiry WS, Kennedy AR (2009) Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiat Res 172:175–186. https://doi.org/10.1667/RR1708.1
Nuth M, Kennedy AR (2013) Mitigating effects of L-selenomethionine on low-dose iron ion radiation-induced changes in gene expression associated with cellular stress. Oncol Lett 6:35–42. https://doi.org/10.3892/ol.2013.1362
Costa NS, Lima LS, Oliveira FAM, Galiciolli MEA, Manzano MI, Garlet QI, Irioda AC, Oliveira CS (2023) Antiproliferative Effect of Inorganic and Organic Selenium Compounds in breast cell lines. Biomedicines 11:1346. https://doi.org/10.3390/biomedicines11051346
Shi H, Liu S, Miyake M, Liu KJ (2006) Ebselen induced C6 glioma cell death in oxygen and glucose deprivation. Chem Res Toxicol 19:655–660. https://doi.org/10.1021/tx0502544
Song G, Zhang Z, Wen L, Chen C, Shi Q, Zhang Y, Ni J, Liu Q (2014) Selenomethionine ameliorates cognitive decline, reduces tau hyperphosphorylation, and reverses synaptic deficit in the triple transgenic mouse model of Alzheimer’s disease. J Alzheimer’s Dis 41:85–99. https://doi.org/10.3233/JAD-131805
Burk RF, Hill KE (2009) Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 1790:1441–1447. https://doi.org/10.1016/j.bbagen.2009.03.026
Xie Y, Tan Y, Zheng Y, Du X, Liu Q (2017) Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J Biol Inorg Chem 22:851–865. https://doi.org/10.1007/s00775-017-1463-2
Li X, Shi Q, Xu H, Xiong Y, Wang C, Le L, Lian J, Wu G, Peng F, Liu Q, Du X (2022) Ebselen interferes with Alzheimer’s disease by regulating mitochondrial function. Antioxidants 11:1350. https://doi.org/10.3390/antiox11071350
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933. https://doi.org/10.1093/brain/awy132
Farzi MA, Sadigh-Eteghad S, Ebrahimi K, Talebi M (2019) Exercise improves recognition memory and acetylcholinesterase activity in the beta amyloid-induced rat model of Alzheimer’s disease. Ann Neurosci 25:121–125. https://doi.org/10.1159/000488580
Campanari ML, García-Ayllón MS, Blazquez-Llorca L, Luk WK, Tsim K, Sáez-Valero J (2014) Acetylcholinesterase protein level is preserved in the Alzheimer’s brain. J Mol Neurosci 53:446–453. https://doi.org/10.1007/s12031-013-0183-5
García-Ayllón MS, Small DH, Avila J, Sáez-Valero J (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-Amyloid. Front Mol Neurosci 4:22. https://doi.org/10.3389/fnmol.2011.00022
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45(1):117–127. https://doi.org/10.1016/s0168-0102(02)00201-8
Martini F, Bruning CA, Soares SM, Nogueira CW, Zeni G (2015) Inhibitory effect of ebselen on cerebral acetylcholinesterase activity in vitro: kinetics and reversibility of inhibition. Curr Pharm Des 21:920–924. https://doi.org/10.2174/1381612820666141014124319
Aras M, Altaş M, Meydan S, Nacar E, Karcıoğlu M, Ulutaş KT, Serarslan Y (2014) Effects of ebselen on ischemia/reperfusion injury in rat brain. Int J Neurosci 124(10):771–776. https://doi.org/10.3109/00207454.2013.879581
Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML (1998) Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol 150:40–44. https://doi.org/10.1006/exnr.1997.6750
Angelucci F, Baiocco P, Brunori M, Gourlay L, Morea V, Bellelli A (2005) Insights into the catalytic mechanism of glutathione S-transferase: the lesson from Schistosoma haematobium. Structure 13:1241–1246. https://doi.org/10.1016/j.str.2005.06.007
Mazzetti AP, Fiorile MC, Primavera A, Lo Bello M (2015) Glutathione transferases and neurodegenerative diseases. Neurochem Int 82:10–18. https://doi.org/10.1016/j.neuint.2015.01.008
Ianiski FR, Alves CB, Souza AC, Pinton S, Roman SS, Rhoden CR, Alves MP, Luchese C (2012) Protective effect of meloxicam-loaded nanocapsules against amyloid-β peptide-induced damage in mice. Behav Brain Res 230(1):100–107. https://doi.org/10.1016/j.bbr.2012.01.055
Wilhelm EA, Bortolatto CF, Jesse CR, Luchese C (2014) Ebselen protects against behavioral and biochemical toxicities induced by 3-nitropropionic acid in rats: correlations between motor coordination, reactive species levels, and succinate dehydrogenase activity. Biol Trace Elem Res 162(1–3):200–210. https://doi.org/10.1007/s12011-014-0137-y
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957–1997. https://doi.org/10.1089/ars.2010.3586
Unsal C, Oran M, Albayrak Y, Aktas C, Erboga M, Topcu B, Uygur R, Tulubas F, Yanartas O, Ates O, Ozen OA (2016) Neuroprotective effect of ebselen against intracerebroventricular streptozotocin-induced neuronal apoptosis and oxidative stress in rats. Toxicol Ind Health 32(4):730–740. https://doi.org/10.1177/0748233713509429
Garlet QI, Haskel MVL, Pereira RP, da Silva WCFN, da Rocha JBT, Oliveira CS, Bonini JS (2019) Delta-aminolevulinate dehydratase and glutathione peroxidase activity in Alzheimer’s disease: a case-control study. EXCLI J 18:866–875. https://doi.org/10.17179/excli2019-1749
Martorell P, Bataller E, Llopis S, Gonzalez N, Alvarez B, Montón F, Ortiz P, Ramón D, Genovés S (2013) A cocoa peptide protects Caenorhabditis elegans from oxidative stress and β-amyloid peptide toxicity. PLoS ONE 8:e63283. https://doi.org/10.1371/journal.pone.0063283
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and the Instituto de Pesquisa Pelé Pequeno Príncipe (IPPPP).
Funding
Not applied.
Author information
Authors and Affiliations
Contributions
Study conception and design: MEP, ACI, and CSO; data collection: MEP and CSO; analysis and interpretation of results: MEP, LSL, NSC, JVS, JFS, ICG, and CSO; draft manuscript preparation: MEP, LSL, NSC, ACI, and CSO.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, M.E., Lima, L.S., Souza, J.V. et al. Evaluation of the Neuroprotective Effect of Organic Selenium Compounds: An in Vitro Model of Alzheimer’s Disease. Biol Trace Elem Res 202, 2954–2965 (2024). https://doi.org/10.1007/s12011-023-03893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03893-9