Abstract
Cholinergic transmission is critical to high-order brain functions such as memory, learning, and attention. Alzheimer’s disease (AD) is characterized by cognitive decline associated with a specific degeneration of cholinergic neurons. No effective treatment to prevent or reverse the symptoms is known. Part of this might be due to the lack of in vitro models that effectively mimic the relevant features of AD. Here, we describe the characterization of an AD in vitro model using the SH-SY5Y cell line. Exponentially growing cells were maintained in DMEM/F12 medium and differentiation was triggered by the combination of retinoic acid (RA) and BDNF. Both acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) enzymatic activities and immunocontent were determined. For mimicking tau and amyloid-β pathology, RA + BDNF-differentiated cells were challenged with okadaic acid (OA) or soluble oligomers of amyloid-β (AβOs) and neurotoxicity was evaluated. RA + BDNF-induced differentiation resulted in remarkable neuronal morphology alterations characterized by increased neurite density. Enhanced expression and enzymatic activities of cholinergic markers were observed compared to RA-differentiation only. Combination of sublethal doses of AβOs and OA resulted in decreased neurite densities, an in vitro marker of synaptopathy. Challenging RA + BDNF-differentiated SH-SY5Y cells with the combination of sublethal doses of OA and AβO, without causing considerable decrease of cell viability, provides an in vitro model which mimics the early-stage pathophysiology of cholinergic neurons affected by AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a disorder clinically characterized by progressive cognitive decline, including disruptions in memory and reasoning, leading to a state of dementia [1, 2]. This cognitive impairment is correlated with the dysfunction and degeneration of cholinergic neurons located in the basal forebrain complex (BFC), which is an early pathological event of the disease [3, 4]. Histologically, AD is characterized by two main pathological hallmarks: neurofibrillary tangles and extracellular deposits of Amyloid-β (Aβ) [5]. Neurofibrillary tangles are formed by unfolded protein aggregates constituted mainly of hyperphosphorylated tau protein [6]. The extracellular deposits consist of Aβ peptides which are products of an irregular cleavage of amyloid precursor protein (APP) [7, 8]. In AD, APP is abnormally cleaved, forming Aβ peptides that produce amyloid deposits known as amyloid plaques [8, 9]. Aβ is a 40 (Aβ1–40) or 42 (Aβ1–42) amino acids peptide, of which Aβ1–42 is the most toxic and faster-aggregating form [10]. However, the molecular mechanisms underlying the formation of toxic aggregates have not been fully elucidated. This might be due in part to the lack of suitable in vitro models resembling mature human cholinergic neurons [11, 12]. Most common in vitro models within AD research include cell lines that lack proper neurite structures and many of the features that define neurons, such as mature neuronal markers [12, 13]. Also, the use of primary rodent neurons derived from embryonic central nervous system tissue is limited by the fact that they do not express the human proteins most closely associated with neurodegenerative diseases [14]. Still, human stem cells offer high risks of mutations and, as well as 3D human neural cell culture, are time consuming, highly expensive [15], and are thus not suitable for high-throughput studies. However, differentiated neuronal-like cell lines can be used to overcome this limitation. The human neuroblastoma cell line SH-SY5Y is frequently used as an in vitro model for neurodegenerative disease studies. SH-SY5Y cells are derived from the sympathetic nervous system and considered to be derived from a neuronal lineage in its immature stage. This cell line is characterized by continuously proliferation, expression of immature neuronal proteins, and low abundance of neuronal markers [16, 17]. Many lines of evidence have indicated that, according to the protocol used, these cells are able to differentiate and acquire mature neuron-like features [18,19,20,21]. Following neuronal differentiation, SH-SY5Y neuroblastoma cells unfold a number of morphological and biochemical events, including a decrease in proliferation rate, formation and extension of neurites, and expression of mature neuronal markers, thus becoming phenotypically closer to primary neurons. Most importantly, SH-SY5Y cells express human proteins [17, 22].
The most commonly known differentiation protocol implemented is through addition of retinoic acid (RA) to the cell culture medium [17]. The reduction of media serum content to 1% plus supplementation with 10 μM RA results not only in neurite outgrowth, but also increase in expression of neuronal markers, such as tyrosine hydroxylase (TH), neuron-specific enolase (NSE), neuronal nuclei protein (NeuN), and the dopamine transporter (DAT) [21, 23]. A number of alternative differentiation methods have also been described. For instance, treatments with neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) have been shown to induce the differentiation of SH-SY5Y cells [14, 18]. RA differentiation induces the expression of TrkB receptor, which turn cells responsive to BDNF [24, 25].
It has also been described that growth factors play an important role in protection and maintenance of cholinergic neurons [26, 27]. Cholinergic neurons from BFC are involved in learning, memory, and sleep cycle [28,29,30] and display a selective vulnerability in AD [27, 31]. Also, a number of studies have shown that BDNF is essential to cholinergic differentiation in brain development [26, 32]. Hence, it is of crucial importance to also explore this hallmark in AD models. Although the combination of BDNF and RA for differentiating cells has been already described [11, 19], its role in a cholinergic neuronal phenotype in SH-SY5Y cells is yet to be exploited. Here, we present a method for the differentiation of SH-SY5Y cells into a cholinergic phenotype using a combination of RA and BDNF, with further challenges with okadaic acid and soluble Aβ oligomers which is suitable for AD-based studies.
Materials and Methods
Cell Culture and Differentiation
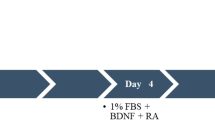
Exponentially growing human neuroblastoma cell line SH-SY5Y, obtained from ATCC (Manassas, VA, USA), was maintained at 37 °C in a humidified atmosphere of 5% of CO2. Cells were grown in a mixture of 1:1 of Ham’s F12 and Dulbecco Modified Eagle Medium (DMEM, Gibco®/Invitrogen, Sao Paulo, Brazil) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Cripion®, Sao Paulo, Brazil) and antibiotic/antimycotic (Gibco® 15240-062). Cell medium were replaced every 3 days and cells were sub-cultured once they reached 80% confluence. Only attached cells were maintained and floating cells were discarded. To evaluate the effects of BDNF, we designed two distinct differentiation protocols. A general description is depicted in Fig. 1. Neuronal differentiation was induced by treatment with RA (Enzo® Life Sciences, Lörrach, Germany) and BDNF (Human Recombinant, Prospec®, NJ, USA). After 24 h of plating, differentiation was initiated by lowering FBS in culture medium to 1% and supplementing with 10 μM RA for 7 days. This treatment was replaced every 3 days to replenish RA in the culture media. Same treatment was performed with the addition of 50 ng/mL BDNF on the fourth day of differentiation with RA. RA stock solutions were prepared in absolute ethanol and the concentration determined using EM (351 nm) = 45000 [33]. BDNF stock solution at a concentration of 100 μg/mL was prepared by dissolving it in a solution of 0.1% bovine serum albumin (BSA) according to the manufacturer.
Differentiation protocols. Proliferative SH-SY5Y cells are seeded and cultured in medium supplemented with 10% FBS for 24 h for complete adhesion. Then, biochemical and microarray analyses were performed. For RA treatment (upper panel), differentiation is induced after cell adhesion with the reduction of FBS to 1% and the addition of 10 μM of AR, which is considered the first day. Lower panel shows that 50 ng/mL BDNF is added on the fourth day combined with RA replenishment. Microarray analyses were also performed on day 4 and 7
RNA Isolation and Microarray Assay
To explore the effects of BDNF on genetic networks, total RNA samples were extracted using TRIzol™ reagent (Thermo Fisher Scientific®, Waltham, MA, USA) following purification (Qiagen RNeasy Mini Kit #74104 and #79254—Free RNase DNase set, Hilden, Germany). Microarray was performed using GeneChip® PrimeView™ Human Gene Expression Array (Affymetrix™). Samples were collected at day 0 (undifferentiated cells), day 4 (RA-differentiated cells), and day 7 (RA- and RA + BDNF-differentiated cells) as shown in Fig. 1. Raw data were deposited in the GEO repository (GEOID: GSE71817).
Raw microarray CEL files were analyzed using the R/Bioconductor pipeline. The data was normalized by Robust Multi-Array Average (RMA) in the AFFY package [34], log (base 2) transformed, and batch-corrected with ComBat in the SVA package [35].
Enrichment Analysis and Expression Values
Three gene sets were analyzed: Alzheimer’s disease network, cholinergic synapse, and neurotrophin signaling network (extracted from the Kyoto Encyclopedia of Genes and Genomes (KEGG) platform—KEGG Pathway Database, 2017: http://www.genome.jp/kegg/pathway.html). Gene set enrichment analysis (GSEA) considered experiments with genome-wide expression profiles from two classes of samples (e.g., 7-day-RA + BDNF-differentiated cells vs.7-day-RA-differentiated cells, and RA + BDNF-differentiated cells vs. undifferentiated cells). Genes were ranked based on the correlation between their expression and the class association. Given a prior defined network (e.g., cholinergic synapse), GSEA determines if the members of these sets of genes are randomly distributed or primarily found at the top or bottom of the ranking [36].
Morphological Analysis
To assess changes in morphological parameters between RA-differentiated and RA + BDNF-differentiated cells, we evaluated neuronal morphology and neurite densities. Firstly, neuronal morphology was assessed through scanning electron microscopy (SEM). Cells were seeded onto glass coverslips in 24-well plates at a density of 6 × 104 cells per well. Cells were fixed by immersion in 25% glutaraldehyde for 1 week. Next, cells were washed in 0.2 M phosphate buffer. For dehydration, sequential immersions in acetone 30% to 100% were performed. Drying was carried out in a Critical Point Dryer (Balzers CPD030). Metallization process used gold as metal target (Sputter Coater, Balzers SCD050).
Neurite Density
Neurite densities of RA + BDNF-differentiated cells, OA, Aβ, or combined treatments were evaluated using immunofluorescence. Cells were washed with phosphate buffered saline (PBS), fixed with 1:1 methanol:acetone (20% v/v) for 20 min at room temperature (RT), and permeabilized with 0.2% Triton X-100-supplemented PBS. Nonspecific binding was blocked with 1% BSA for 1 h at RT. After PBS washes, cells were incubated with conjugated anti-β-tubulin III (Alexa Fluor®488, 1:50, Abcam) for 2 h at RT followed by nuclear dye Hoechst 33342 (Molecular Probes® Life Technologies) incubation for 15 min (1 μg/μL). Ten microscopic fields (× 200 magnification) were selected randomly from each of three independent experiments (n = 3). Images were captured with NIS elements software, using an Olympus IX70 inverted microscope. Neurite density was assessed using the AutoQuant Neurite software (implemented in R language) and expressed as arbitrary units (A.U.).
RNA Isolation and Real-Time qPCR Assay
Gene expression analysis was performed using gene-specific primers designed with IDT Design Software (Integrated DNA Technologies Inc., CA, USA). Total RNA was isolated from SH-SY5Y cells using TRIzol™ Reagent (Thermo Fisher Scientific®, Waltham, MA, USA). Samples were transcribed into cDNA using random nonamers (Sigma-Aldrich®, St. Louis, MO, USA) and M-MLV Reverse Transcriptase (Sigma-Aldrich®). Real-time PCR reactions were carried out in Step One Plus real-time cycler (Applied-Biosystem®, NY, USA) using Taq polymerase (Sigma-Aldrich®) and SYBR green. Gene expression was quantified by the comparative cycle threshold method (ΔΔCT) and normalized using the housekeeping gene RACK1. Melting curves were used to monitor unspecific amplification products. The amplification reaction consisted of a hold of 10 min at 95 °C and 40 cycles with subsequent recording of primer melting curves. The primers sequences for amplification of the target genes were CDK5: HsCDK5Fwd: CGAGAAACTGGAAAAGATTGGG, and HsCDK5Rev: TTTCAGAGCCACGATCTCATG. PSEN1: HsPSEN1Fwd: GGTGAATATGGCAGAAGGAGAC, and HsPSEN1Rev: AGGGCTTCCCATTCCTCACTG. SCL18A: HsSCL18Fwd: GTCCTCGGAAGAGCATCG, and HsSCL18Rev: CACACGATAACAAGCACCAG.
Cholinergic Enzyme Activities
AChE (EC 3.1.1.7) enzymatic activity was determined by the colorimetric assay described by Ellman [37]. Cells were washed twice in PBS (pH 7.4) and total protein was extract with lysis buffer (20 mM HEPES, 150 mM NaCl and 1% NP-40) with the addition of protease and phosphatase inhibitors (Roche® Basel, Switzerland). Cell lysates were incubated for 5 min in 10 mM 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) (Sigma®). Acetylthiocholine (8 mM, Sigma®) was added to this mixture and absorbance was measured at 412 nm for 10 min. Results were expressed as μmol/min/mg of protein. The activity of the enzyme choline acetyltransferase (ChAT) (E.C. 2.3.1.6) was determined according to Chao & Wolfgram [38] with some minor modifications. Samples were incubated with reaction buffer (PBS pH 7.2, 6.2 mM acetylcoenzyme A, 1.0 M choline chloride, 0.76 mM neostigmine sulfate, 3 M NaCl and 1.1 mM EDTA). To initiate the reaction, 1 mM 4,4′-dithiodipyridine (4- PDS) was added and the absorbance was measured for 90 min at 324 nm using a SpectraMax® Microplate Reader (Molecular Devices®). Results were expressed in nmol/min/mg of protein based on the molar extinction coefficient of 1.98 × 104.
Western Blot
For western blot analysis, 3 × 106 cells were seeded into 75 cm2 flasks. After 24 h plating or after differentiation treatment, cells were washed with PBS and suspended in lysis buffer (1% (w/v) SDS, 10 mM TRIS, 1 mM EDTA) supplemented with protease and phosphatase inhibitors (Roche® Basel, Switzerland). Total protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) membrane. Thereafter, nonspecific binding was blocked with 5% of BSA in Tris-buffered saline 0.1% Tween 20 (TBST) for 1 h at RT. Membranes were then incubated overnight at 4 °C with mouse anti-DAT antibody (1:1000, Santa Cruz® Biotechnology, Dallas, Texas, USA), rabbit anti-ChAT (1:1000, Abcam), rabbit anti-Tau (1:500, Abcam), or mouse anti-phospho-Tau Ser202–199 (Invitrogen, Waltham, Massachusetts). After washing, the membrane was incubated with peroxidase-conjugated secondary antibodies (1:5000, Dako®, Glostrup, Denmark) for 2 h at RT. Bands were visualized with Super Signal West Pico Chemiluminescent Substrate (PIERCE®, Rockford, Il, USA). For the loading control, membranes were subsequently stripped and reprobed with rabbit anti-β-actin (1:5000, Santa Cruz Biotechnology, Inc.) followed by goat anti-rabbit peroxidase-conjugated secondary antibody (1:5000, Dako®). Protein content was measured using Lowry assay (Bradford, 1976).
Amyloid-β Oligomerization Protocol
Soluble AβOs were prepared according to Klein (2002) [39]. Human Aβ peptides (Abcam®) were diluted in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) (Sigma®) at a concentration of 1 mM and incubated at RT for 1 h. In order to evaporate the HFIP, samples were kept overnight in a laminar flow cabinet. Possible residues were removed in a SpeedVac device (SVR 2-18 Christ) for 10 min. Dried tubes were stored at − 20 °C. For each assay, an aliquot was thawed and diluted in DMSO at a concentration of 5 mM. This solution was further diluted in PBS and incubated at 4 °C for 24 h. Alternatively, incubation at 37 °C was also performed for fibrils formation. After incubation, the solution was centrifuged at 14000g for 10 min and the supernatant collected [39, 40]. To characterize the preparation, AβOs were separated by a 12% nondenaturing glycine polyacrylamide gel electrophoresis. Then, gel was stained with a Coomassie G250 solution (0.08% Coomassie (Sigma); 1.6% H3PO4; 8% (NH4)2SO4; 20% methanol).
Cytotoxicity Parameters
Cytotoxicity induced either by OA (Sigma-Aldrich®) or AβOs in RA + BDNF-differentiated cells was analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich®) assay. For this assay, SH-SY5Y cells were seeded in 24-well plates at density of 6 × 104 cells per well and treated with increasing amounts of OA or AβOs in order to determine sublethal doses. After 24 h treatment, cells were incubated with 0.5 mg/mL MTT for 1 h at 37 °C. DMSO was added to solubilize formazan crystals. The absorbance was measured at 560 nm and 630 nm in a plate reader (SoftMax Pro, Molecular Devices, USA).
Statistical Analysis
Band intensities of Western blots were quantified using ImageJ and expressed as relative values to the controls. Data were expressed as means ± SD from at least three independent experiments. Data from enzymatic analysis were expressed as percentage of untreated cells (mean ± SD) from at least four independent experiments. Multiple comparisons were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test, unless otherwise indicated. Differences were considered significant at p < 0.05. Statistical analyses were performed using the GraphPad® (San Diego, CA, USA, version 5.0).
Results
RA + BDNF-Differentiation Protocol Increased Neurite Density
The RA + BDNF-differentiation protocol is outlined in Fig. 1. Previously, our research group described that treatment with RA in combination with the reduction of FBS to 1% induced cell growth inhibition and neuronal morphology in SH-SY5Y cells, along with the expression of dopaminergic markers [21]. Based on this protocol, we added BDNF in the fourth and seventh day of differentiation. Although TrkB receptors are absent in undifferentiated SH-SY5Y cells, this cell line becomes responsive to BDNF treatment by previous incubation with RA [24]. Therefore, we analyzed the effect of RA differentiation protocol for 4 days on the expression of genes related to neurotrophin signaling network (Fig. 2a). Dozens of genes were upregulated by 4-day RA treatment including, as expected, the TrkB receptor gene (NTRK2). This molecular reprogramming justifies the remarkable neuronal morphology observed in cells treated with the RA + BDNF-differentiation protocol (Fig. 2b, c). When compared to RA treatment, BDNF-treated cells exhibited longer and more branched neurites, forming a robust neuritic network. Thus, our data confirm TrkB activation through RA treatment which allowed BDNF to induce a morphological differentiation. In light of this, we assessed the neurite density using immunocytochemistry via βIII-tubulin immunolabeling. Results revealed a twofold increase in neurite density in RA + BDNF-differentiated cells compared to RA alone treatment (Fig. 2d).
a Gene enrichment analysis was used to identify genes in neurotrophic signaling network which expression would be affected by the RA treatment on day 1 and 4. Morphometric Analysis: bLeft panel: representative segmented immunofluorescence images of proliferative SH-SY5Y cells, differentiated for 7 days with RA and co-treated with BDNF. Fluorescent labeling in green indicates βIII-tubulin (neuron-specific) evidencing neurites, superimposed on nuclear labeling with Hoechst 33342 (200X). Right panel: Histograms representing automated neurite quantification of segmented images generated by the software AutoQuant Neurite®. The statistics test used was Tukey’s (p < .001). c Representative images of scanning electronic microscopy of cells submitted to the three differentiation protocols. Ten microscopic fields (× 200 magnification) were selected from three independent experiments (n = 3)
Cholinergic Synapse Pathway Is Enriched in RA + BDNF-Differentiation Protocol
Severe synaptopathy and loss of cholinergic neurons are major hallmarks of AD. Therefore, an important feature for an in vitro model of AD is based on the generation of human cholinergic neurons that express AD-relevant genes. Our RA + BDNF-differentiation protocol triggered an enrichment of key elements from the cholinergic synapse and Alzheimer’s networks (gene list curated by KEGG—pathways) (Fig. 3). This analysis showed an increased expression of choline O-acetyltransferase (CHAT), acetylcholinesterase (ACHE), cholinergic receptors (CHRNA6, CHRM4, CHRM3 and CHRNA4) (Fig. 3a), and important key genes related to AD cascade such as the beta-site APP-cleaving enzyme 2 (BACE2), microtubule-associated protein Tau (MAPT), and ADAM metallopeptidase 10 (ADAM10) (Fig. 3b).
Effect of the differentiation protocol on gene expression. Differential expression of cholinergic synapse and Alzheimer’s Disease network genes mediated by treatment with RA + BDNF in human SH-SY5Y neuroblastoma lineage. Here are shown genes belonging to the gene interaction network of cholinergic synapse (a) and Alzheimer’s Disease network (b), from KEGG platform, and genes that showed significant modulation in their expression by treatment with RA and BDNF compared to treatment with RA. Genes were ranked based on the correlation between their expression and the class distinction
Cholinergic Markers Have Increased Expressions and Activities in RA + BDNF-Differentiated Cells
Effects of sequential RA + BDNF treatment on the enzymatic activities of cholinergic markers (such as AChE and ChAT) in each of the experimental groups were evaluated in order to characterize a potential cholinergic differentiation. AChE is the primary cholinesterase in the body and catalyzes the breakdown of acetylcholine (ACh) and other choline esters that function as neurotransmitters [3]. Even though RA treatment induced an increase in AChE activity in relation to nondifferentiated cells, the combination of RA with BDNF revealed a significant enhancement in AChE activity (p < 0.05) compared to other treatments (Fig. 4a). Similarly, ChAT activity (p < 0.01) (Fig. 4b) and protein levels (p < 0.01) (Fig. 4c) were significantly increased in RA + BDNF-treated cells. ChAT is a transferase responsible for the synthesis of ACh and its presence classifies the nerve cell as a cholinergic neuron [3, 41]. However, studies have shown that several other neuronal proteins have their expression increased by BDNF [25]. This raised the question whether BDNF, as a neurotrophic factor, caused an indiscriminate increase in neuronal markers. Therefore, we analyzed DAT expression by Western blot, a widely used dopaminergic marker [11] (Fig. 4d). Interestingly, DAT levels were increased only in RA-differentiated SH-SY5Y, as previously described by our group [22]. Moreover, qPCR analysis showed slightly increased cDNA levels of important AD genes in RA + BDNF-differentiated cells such as SCL18A, which encodes the vesicular acetylcholine transporter (vAChT) (p < 0.05), CDK5 (cyclin-dependent kinase 5) which dysregulation has been implicated in AD (p < 0.05), and PSEN1 (presenilin-1, a member of the gamma secretase complex, which has an important role in generation of Aβ from APP) (p < 0.05) (Fig. 4e). These data suggest that our differentiation protocol induces a predominantly cholinergic phenotype in SH-SY5Y with increased expression of AD-relevant proteins.
Effects of RA + BDNF-differentiation on cholinergic markers. a AChE activity determined by the kinetics of formation of sulfhydryl groups (-SH) released from acetylthiocholine degradation during 10 min. Data presented as mean ± SD for four independent experiments performed in triplicates (ANOVA, *p < .05, **p < .01, ***p < .001). b ChAT activity determined by the kinetics of formation of CoA and 4-TP conjugate for 90 min. Data presented as mean ± SD for four independent experiments performed in duplicates. (ANOVA, ***p < .001). c Densitometry and representative image of Western Blot main marker of cholinergic neurons. Data presented as mean ± SD of three experiments. (ANOVA, *p < .05). d Densitometry and Western blotting of the dopaminergic neuron marker. Analysis of the bands represented by mean ± SD of three independent experiments. e RT-qPCR from undifferentiated, RA-treated or BDNF+RA-treated SH-SY5Y cells for 7 days. mRNAs were isolated, transcribed into cDNAs, and RT-qPCR was performed as described. Gene expression was quantified by ΔΔCT method and normalized using RACK1 in three independent experiments. Multiple comparisons were analyzed by ANOVA followed by Mann-Whitney U test. Data was considered significant at *p < .05
Characterization of an AD-Like In vitro Model
The RA + BDNF-differentiated cells were then used for mimicking pathological events typically found in early stages of AD. To do so, we challenged our cells with OA and AβOs and evaluated neuronal cell viability (by MTT assay) and neurite densities as measurements of neurotoxic endpoints. OA is a serine/threonine phosphatase 1 (PP1) and 2A (PP2A) inhibitor that can lead to increased phosphorylated/dephosphorylated tau ratio, since dephosphorylation of this protein is mainly mediated by these phosphatases [42]. Neurites in RA + BDNF-differentiated SH-SY5Y cells were highly sensitive to OA treatment in the low nanomolar range (10–15 nM) (p < 0.01) (Fig. 5b). Moreover, treatment with 5 nM and 10 nM OA in RA + BDNF-differentiated cells, which was demonstrated to be in the sublethal range of the drug (EC50 = 36 nM) (Fig. 5b), was able to demonstrate tau hyperphosphorylated at Ser202 (p < 0.01) (Fig. 5c).
Characterization of an AD-like in vitro model. a Representative images of RA + BDNF-differentiated cells treated with distinct doses of OA or soluble Aβ oligomers. Overlapping of βIII-tubulin and Hoechst 33342 and further segmentation performed by AutoQuant Neurites Software. b Graph displaying quantification of segmented images (left axis) showing the effects of OA toxicity on neurite density. Five randomly selected images were captured from each of three independent experiments A.U., arbitrary units. On the right axis, the cytotoxicity of the drug was assessed by the MTT assay. Data presented as mean ± SD for four independent experiments performed in triplicates. (ANOVA *p < .05). c Densitometry and representative Western blot of the hyperphosphorylated Tau immunocontent. Membranes were tested for p-Tau Ser202. Analysis of the bands represented by mean ± SD of two independent experiments. d Graph representing, on the left axis, the neurite density per cell body, showing the neurotoxic effect of treatment with Aβ. On the right axis, treatment with soluble βA oligomers effects on cell viability determined by the MTT assay. Data presented as mean ± SD for three independent experiments performed in triplicates. (ANOVA, *p < .05). e Representative gel electrophoresis of the preparation of soluble oligomers from Aβ(1–42). f Effect of the combination of sublethal doses of OA and Aβ oligomers. Graphs showing neurite density and cell viability. Data presented as mean ± SD for three independent experiments performed in duplicates
Subsequently, we studied the neurotoxic effect of AβOs on RA + BDNF-differentiated SH-SY5Y cells. AβOs were highly synaptotoxic in RA + BDNF-differentiated SH-SY5Y cells, as measured by neurite density, even at concentrations as low as 0.01 nM (p < 0.05) (Fig. 5d). It is possible that the dehydrogenases that metabolize MTT to formazan salt were still active in the soma of neurons whereas AβOs toxic effects induced the retraction of neurites.
We then chose sublethal doses of each neurotoxin to characterize a cell model mimicking early stages of AD. As shown in Fig. 5f, SH-SY5Y cells were treated with a combination of selected OA and AβOs concentrations. We observed that, whereas the combination of 10 nM OA with 0.1 nM AβOs induced a low death rate, neuritic density was drastic reduced (p < .0001).
Discussion
This study reveals a significant increase in neurite density when BDNF was added to the RA-differentiation protocol, indicating a switch to a neuronal phenotype resembling a highly connected synaptic network. Moreover, we observed higher expression and enzymatic activities of cholinergic markers in RA + BDNF-treated cells. These findings suggest that this differentiation protocol induces a shift to a neuronal phenotype with predominantly cholinergic features. Next, differentiated cells were exposed to sublethal doses of OA and AβOs (Fig. 5a–f) which might recapitulate early stages of tau and amyloid pathology, respectively. The combination of sublethal doses of OA and AβOs in the treatment of differentiated SH-SY5Y cells provides an in vitro model resembling the pathophysiology of cholinergic neurons initially affected by AD. In general, detection of AD symptoms occurs in a very advanced stage of disease where the inhibition or reversal of the disease progression is a great challenge [43]. In addition, as few AD models have clear cholinergic loss, the establishment of a cholinergic differentiation protocol for SH-SY5Y cells seems an important step for characterizing a suitable AD in vitro model.
TrkB receptors are expressed under the RA-inducing activity, switching on the TrkB-centered signaling pathways which eventually affects cell survival, axonal outgrowth, and cell differentiation. Thus, the RA-differentiation effect upon SH-SY5Y cells can be potentiated by the addition of BDNF [24]. The addition of BDNF on the fourth day of RA-treated cells produced morphological alterations indicating that RA was able to induce the expression of TrkB receptors early in treated neuroblastoma SH-SY5Y cells [25]. The heat map diagram of differential gene expression (Fig. 2a) showed enrichment of NTRK2 gene. Moreover, the expression of a number of downstream genes of the TrkB signaling cascade was also enhanced, such as SHC, AKT, and genes encoding subunits of PI3K.
At the gene expression level, cholinergic synapse and AD networks are enriched following addition of BDNF to RA-treated cells. Biochemical analysis also showed higher cholinergic protein expression and activity under same treatment conditions. Cholinergic neurons are especially vulnerable in AD playing a significant role in cognitive impairment [4, 41, 44]. Therefore, studies focusing on cholinergic markers provide insight into the pathophysiological conditions of the disease. Indeed, anticholinesterase inhibitors are currently approved for treating AD and are temporarily effective for attenuating cognitive symptoms [45]. Both microarray and RT-PCR analysis demonstrated that the RA + BDNF-differentiation protocol promoted the expression of CHAT, ACHE, and important cholinergic receptors (Fig. 2). The expression of SLC18A3 gene, which encodes the transmembrane protein vAChT, is also increased. vAChT is responsible for the transportation of ACh into secretory vesicles to be released into the extracellular space [46]. Interestingly, AD network was found enriched in RA + BDNF-treated cells. Important genes related to AD pathophysiology such as PSEN1, BACE2, MAPT, and ADAM10 were found to have its expression enriched. BACE2 gene, a BACE1 homolog, is also responsible for the proteolytic processing of the APP and it is associated to AD pathology [47, 48]. PSEN1 encodes the protein presenilin-1, which is a subunit of the gamma-(γ)-secretase complex responsible for the cleavage of a variety of transmembrane proteins. Mutations in the PSEN1 gene are the most common cause of early-onset AD, accounting for up to 70% of cases [49]. A disintegrin and metalloproteinase domain-containing protein 10 is the protein encoded by the ADAM10 gene that cleaves several membrane proteins at the cellular surface, including APP. This is the main α-secretase in the brain and it accounts for the releasing of neuroprotective soluble APPα fragments [50]. Further, results from RT-PCR have shown enhanced expression of CDK5 gene. CDK5 is involved in cell survival pathways and its deregulation contributes to the development of AD neurodegenerative features. This proline-directed serine/threonine protein kinase is implicated in mitochondrial dysfunction and induction of Aβ production and accumulation [51]. Taken together, our differentiation protocol induces the expression of cholinergic markers and genes already reported to be associated with AD. With this in mind, this cell model enables the investigation of mechanisms linked to AD pathophysiology.
Neuronal information processing is highly dependent upon synaptic connectivity. Therefore, neuronal arborization is a crucial morphological parameter for determining neuronal survival [52]. When cells were treated with OA, we observed that the reduction in neurite density and cell viability occurred in a dose-dependent manner. In addition, an increase in phosphorylated tau at Ser202 (Fig. 5c). OA inhibits the action of phosphatases 1 and 2A responsible for the dephosphorylation of tau protein. Abnormal phosphorylation might initially occur at Ser202 site in dystrophic neurites [53]. This also corroborates with data in the literature indicating that hyperphosphorylation of Tau and its subsequent deposition are related to the degeneration of neurons in brains of AD patients [6].
Regarding the role played by Aβ in AD, recent studies suggest that AβOs can alter neurotransmitter release such as ACh [54]. Interestingly, low doses of OA and AβOs induced a severe decrease in neurite density but only a slight decrease in cell viability (Fig. 5d). Many neurotoxic insults can cause neurite retraction. Studies indicate that altered retraction and elongation might disturb neurite outgrowth homeostasis and induce tau pathology [55, 56], which correlates with neurodegeneration [57]. Aβ oligomers have been identified in different stages of AD, and studies suggest that they could be used as biomarkers in the early preclinical stages of the disease [58]. Here, we described the characterization of an AD-like in vitro model using SH-SY5Y cells and challenged this cell line with AβOs and okadaic acid. Low doses of neurotoxins used in the treatment of SH-SY5Y cells were chosen with the purpose of subsequently selecting one that does not compromise drastically the basic functionality of the cells, in order to study mechanisms that lead to early synaptic dysfunction.
Age-related neurodegenerative diseases such as Alzheimer’s are largely human-specific diseases and represent the interface of environmental factors, genetics, epigenetics, and the aging process per se [2, 5]. Even though most of the current models only take into account one of these factors and therefore do not reproduce the complexity of the diseases, cell models, such as the described here, provide a simple, inexpensive, and potentially useful tool for the dissection of the basic disease mechanisms and the screening of compounds targeting specific mechanisms involved in AD.
Conclusion
Few AD models reproduce the cholinergic loss found in the disease [45]. In this work, human neuroblastoma SH-SY5Y cells were differentiated into a neuronal phenotype with cholinergic features. While it is not clear yet how AD early signs are developed, it is known that pathophysiological abnormalities precede clear clinical symptoms [43]. By challenging these cells, we attempted to mimic initial stages of neuronal death by Aβ and tau pathology. Our preliminary results highlighted the potential applicability of this cell model as a useful tool for AD research. The in vitro model proposed here might be convenient for performing fast high-throughput neuroprotective drugs screening for reversing or inhibiting damage caused by neurotoxins involved in AD pathology.
Abbreviations
- AD:
-
Alzheimer’s disease
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- FBS:
-
Fetal bovine serum
- RA:
-
Retinoic acid
- BDNF:
-
Brain-derived neurotrophic factor
- AChE:
-
Acetylcholinesterase
- ChAT:
-
Choline acetyltransferase
- AβOs:
-
Amyloid-β oligomers
- AO:
-
Okadaic acid
- MTT:
-
3-(4,5-Dimethylthiazol-2il)-2,5-diphenyltetrazolium bromide
- DAT:
-
Dopamine transporter
- Aβ:
-
Amyloid-β
- BFC:
-
Basal forebrain complex
- APP:
-
Amyloid precursor protein
- ACh:
-
Acetylcholine
- CDK5:
-
Cyclin-dependent kinase
References
Moller HJ, Graeber MB (1998) The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. Eur Arch Psychiatry Clin Neurosci 248:111–122
Forman MS, Trojanowski JQ, Lee VM (2004) Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med 10:1055–1063
Oda Y, Nakanishi I (2000) The distribution of cholinergic neurons in the human central nervous system. Histol Histopathol 15:825–834
Nyakas C, Granic I, Halmy LG, Banerjee P, Luiten PGM (2011) The basal forebrain cholinergic system in aging and dementia. Rescuing cholinergic neurons from neurotoxic amyloid-β42 with memantine. Behav Brain Res 221:594–603
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640–651
Adalbert R, Gilley J, Coleman MP (2007) Aβ, tau and ApoE4 in Alzheimer’s disease: the axonal connection. Trends Mol Med 13:135–142
Pagani L, Eckert A (2011) Amyloid-β interaction with mitochondria. Int J Alzheimers Dis 2011:925050
Deshpande A, Mina E, Glabe C, Busciglio J (2006) Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 26:6011–6018
Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal amyloid-β accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol 119:523–541
Wang H-W, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B et al (2002) Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res 924:133–140
Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M, Kgedal K et al (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis 20:1069–1082
Carolindah MN, Rosli R, Adam A, Nordin N (2013) An overview of in vitro research models for Alzheimer’s disease. Regen Res 2:8–13
Gu H, Li L, Cui C, Zhao Z, Song G (2017) Overexpression of let-7a increases neurotoxicity in a PC12 cell model of Alzheimer’s disease via regulating autophagy. Exp Ther Med 14:3688–3698
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21
Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C et al (2014) A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature.
Biedler JL, Roffler-tarlov S, Schachner M, Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res:3751–3757
Påhlman S, Ruusala a I, Abrahamsson L, Mattsson ME, Esscher T (1984) Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ 14:135–144
Påhlman S, Hoehner JC, Nånberg E, Hedborg F, Fagerström S, Gestblom C et al (1995) Differentiation and survival influences of growth factors in human neuroblastoma. Eur J Cancer 31A:453–458
Arcangeli A, Rosati B, Crociani O, Cherubini A, Fontana L, Passani B et al (1999) Modulation of HERG current and herg gene expression during retinoic acid treatment of human neuroblastoma cells: potentiating effects of BDNF. J Neurobiol 40:214–225
Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cen V et al (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated , neurotrophic factor-dependent. J Neurochem 75:991–1003
Lopes FM, Schröder R, da Frota MLC, Zanotto-Filho A, Müller CB, Pires AS et al (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94
Constantinescu R, Constantinescu AT, Reichmann H, Janetzky B (2007) Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J Neural Transm:17–28
Lopes FM, Londero GF, de Medeiros LM, da Motta LL, Behr GA, de Oliveira VA et al (2012) Evaluation of the neurotoxic/neuroprotective role of organoselenides using differentiated human neuroblastoma SH-SY5Y cell line challenged with 6-hydroxydopamine. Neurotox Res 22:138–149
Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ (1993) Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron Neuron, Cell Press 11:321–331
Edsjö A, Lavenius E, Nilsson H, Hoehner JC, Simonsson P, Culp LA et al (2003) Expression of trkB in human neuroblastoma in relation to MYCN expression and retinoic acid treatment. Lab Investig 83:813–823
Ward NL, Hagg T (2000) BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp Neurol 162:297–310
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221:555–563
Paul S, Jeon WK, Bizon JL, Han J-S (2015) Interaction of basal forebrain cholinergic neurons with the glucocorticoid system in stress regulation and cognitive impairment. Front Aging Neurosci 7:1–11
Ozen Irmak S, de Lecea L (2014) Basal forebrain cholinergic modulation of sleep transitions. Sleep 37:1941–1951
Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J Neurochem 142:111–121
Grothe MJ, Schuster C, Bauer F, Heinsen H, Prudlo J, Teipel SJ (2014) Atrophy of the cholinergic basal forebrain in dementia with Lewy bodies and Alzheimer’s disease dementia. J Neurol:71–73
Nilbratt M, Porras O, Marutle A, Hovatta O, Nordberg A (2010) Neurotrophic factors promote cholinergic differentiation in human embryonic stem cell-derived neurons. J Cell Mol Med 14:1476–1484
Sharow KA, Temkin B, Asson-Batres MA (2012) Retinoic acid stability in stem cell cultures. Int J Dev Biol 56:273–278
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28:882–883
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide. PNAS 102:15545–15550
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Chao L, Wolfgram F (1972) Spectrophotometric for choline acetyltransferase. Anal Biochem 46:114–118
Klein WL (2002) Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int 41:345–352
Stine WB, Dahlgren KN, G a K, LaDu MJ (2003) In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem 278:11612–11622
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49:921–937
Kamat PK, Tota S, Saxena G, Shukla R, Nath C (2010) Okadaic acid (ICV) induced memory impairment in rats: a suitable experimental model to test anti-dementia activity. Brain Res 1309:66–74
Jack CR, Holtzman DM (2013) Biomarker modeling of Alzheimer’s disease. Neuron 80:1347–1358
Schliebs R, Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113:1625–1644
Douchamps V, Mathis C (2017) A second wind for the cholinergic system in Alzheimer’s therapy. Behav Pharmacol 28:112–123
Butcher LL, Oh JD, Woolf NJ (1993) Cholinergic neurons identified by in sity hybridization histochemistry. In: Cholinergic Function and Dysfunction, AC Cuello, Ed., pp.1–8, Elsevier, Amsterdam, 1993
Mok KY, Jones EL, Hanney M, Harold D, Sims R, Williams J et al (2014) Polymorphisms in BACE2 may affect the age of onset Alzheimer’s dementia in down syndrome. Neurobiol Aging 35:1513.e1–1513.e5
Ma Z, Jiang W, Zhang EE (2016) Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer’s disease-risk genes. Sci Rep 6:36035
Kelleher RJ, Shen J (2017) Presenilin-1 mutations and Alzheimer’s disease. Proc Natl Acad Sci 114:629–631
Endres K, Deller T (2017) Regulation of alpha-secretase ADAM10 in vitro and in vivo: genetic, epigenetic, and protein-based mechanisms. Front Mol Neurosci 10:1–18
Liu SL, Wang C, Jiang T, Tan L, Xing A, Yu JT (2016) The role of Cdk5 in Alzheimer’s disease. Mol Neurobiol 53:4328–4342
van Pelt J, van Ooyen A, Uylings HBM (2014) Axonal and dendritic density field estimation from incomplete single-slice neuronal reconstructions. Front Neuroanat 8:1–16
Su JH, Cummings BJ, Cotman CW (1994) Early phosphorylation of tau in Alzheimer’s disease occurs at Ser-202 and is preferentially located within neurites. Neuroreport 5:2358–2362
Olivero G, Grilli M, Chen J, Preda S, Mura E, Govoni S et al (2014) Effects of soluble β-amyloid on the release of neurotransmitters from rat brain synaptosomes. Front Aging Neurosci 6:166
Franze K, Gerdelmann J, Weick M, Betz T, Pawlizak S, Lakadamyali M et al (2009) Neurite branch retraction is caused by a threshold-dependent mechanical impact. Biophys J 97:1883–1890
Klein WL (2013) Synaptotoxic amyloid-beta oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? J Alzheimers Dis 33(Suppl 1):S49–S65
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Sarmiento J, Troncoso J, Jackson GR et al (2012) Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J 26:1946–1959
Amaro M, Kubiak-Ossowska K, Birch DJS, Rolinski OJ (2013) Initial stages of beta-amyloid Aβ 1−40 and Aβ 1−42 oligomerization observed using fluorescence decay and molecular dynamics analyses of tyrosine. Methods Appl Fluoresc 1:15006
Funding
This study was supported by the Brazilian funds MCTI/CNPq INCT-TM/CAPES/FAPESP (465458/2014-9), CNPq/MS/SCTIE/DECIT - Pesquisas Sobre Doenças Neurodegenerativas (466989/2014-8), and PRONEX/FAPERGS (16/2551-0000499-4). FK received a fellowship from MCT/CNPq [306439/2014-0]. ERZ receives financial support from CAPES [88881.141186/2017-01].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Medeiros, L.M., De Bastiani, M.A., Rico, E.P. et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol Neurobiol 56, 7355–7367 (2019). https://doi.org/10.1007/s12035-019-1605-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1605-3