Abstract
Zinc is an essential micronutrient for organisms involved in regulating various biological processes. This study evaluated the effects of dietary zinc on growth performance, digestive enzyme activities, antioxidant status, and immune responses of sea cucumber Apostichopus japonicus. Five experimental diets were formulated with graded levels of zinc (0, 20, 40, 60, and 80 mg/kg, respectively), and the actual dietary zinc values were 31.4, 51.0, 68.2, 91.9, and 110.8 mg/kg diet, respectively. Sea cucumbers were fed with diets for 2 months. The results showed the growth performance, amylase, and trypsin activities of sea cucumber increased significantly with zinc supplementation, and the best growth performance and enzyme activities were observed at 40 mg/kg zinc diet. Zinc supplementation significantly increased activities of superoxide dismutase, catalase, anti-superoxide anion, and inhibiting hydroxyl radical, while significantly reduced the malondialdehyde content. Furthermore, the higher zinc supplementation levels resulted in significantly upregulated immune-related genes of hsp90, p105, rel, and lsz, suggesting that excessive zinc caused oxidative stress. The broken-line regression analysis of specific growth rate indicated dietary zinc requirement in juvenile sea cucumber was ~ 66.3 mg/kg diet. Overall, dietary zinc contributes to the growth and immune resistance of juvenile sea cucumber, and our study will provide insights into the rational use of dietary zinc in aquaculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea cucumber Apostichopus japonicus, widely distributed in the northwest Pacific coast, is an economic fishery species with high nutritional and commercial value [1]. It is one of the pillar species of aquaculture industry in Asia, with a total production of 222,707 tons in 2021 Bureau of Fisheries of the Ministry of Agriculture, [2]. With the expansion of breeding scale, ecological problems caused by excessive breeding density and environmental pollution have become increasingly prominent. The ecological problems not only restrict the growth of sea cucumber, but also stimulate the organism to produce excessive reactive oxygen to attack the cellular defense system, resulting in oxidative damage to cells and a variety of diseases [3, 4]. Therefore, new strategies have been exploring to improve the antioxidant capacity and growth performance of sea cucumber.
Zinc (Zn) is an essential micronutrient for animal growth, development, and metabolism. Zn plays an important role in regulating cellular processes such as cell proliferation, immune regulation, and antioxidant defense [5, 6]. Meanwhile, Zn acts as a coenzyme factor for more than 200 biological enzymes and is involved in biological processes of protein synthesis and metabolism, scavenging of free radicals, and protection of cell membranes from oxidative damage [6,7,8]. Zn deficiency leads to symptoms such as stunted growth, skin erosion, and decreased immunity [6, 9], while Zn supplementation can effectively alleviate and eliminate the symptoms caused by Zn deficiency [10, 11]. The presence and chemical form of Zn are crucial for the absorption of Zn in feeds [12]. Previous studies confirmed that organic Zn could be better absorbed and more bioavailable when compared to inorganic Zn [13, 14]. Therefore, supplementing an appropriate amount of organic Zn in feed is an effective approach to promote growth performance and resist oxidative damage.
Recently, studies on Zn in aquatic species have attracted extensive attentions. It has been reported that Zn plays a vital part in modulating growth and development, immune responses, and stressful regulation in aquatic animals including fish and crustaceans [15,16,17]. Kishawy et al. [15] showed that 40 mg/kg of Zn supplementation significantly increased the weight gain rate, antioxidant enzyme activity, and bacterial inhibition of Nile tilapia (Oreochromis niloticus). Zhang et al. [18] found that dietary Zn ranging from 50 to 81 mg/kg has a positive impact on growth, immunity, and reproductive performance of female prawn (Macrobrachium nipponense). Shi et al. [19] demonstrated that Zn burst in hemolymph of Fujian oyster (Crassostrea angulata) can inhibit bacterial growth and the accumulation of Zn increases host resistance to pathogens. However, the effects of dietary Zn supplementation on sea cucumber were not clear. Therefore, the objective of this study was to investigate the effects of dietary supplementation of Zn on growth performance, digestive enzyme activity, antioxidant levels, and immune-related gene expression of sea cucumber.
Materials and Methods
Diet Preparation
Based on previous study on the feed for sea cucumber [20], this experiment made some modifications to the formulate and configured a basic diet with 20.49% crude protein and 2.69% crude lipid content using sargassum and sea mud as the main ingredients, as shown in Table 1. Zinc glycinate (Shanghai Yuanye Bio-Technology Co., Shanghai, China) was supplemented to the basal diet as Zn source in a gradient of 0, 20, 40, 60, and 80 mg/kg Zn content, of which the 0 mg/kg was the control group. The actual levels of Zn in each group were 31.4, 51.0, 68.2, 91.9, and 110.8 mg/kg, respectively, as shown in Table S1. All feed ingredients were sifted, mixed, granulated, and dried to form experimental feed. The dried feed pellets were stored at -20 ℃ for future use.
Study Design and Sample Collection
The experiment was conducted at the laboratory of Qingdao National Ocean Science Research Center, Ocean University of China, for 2 months. The healthy and vigorous juvenile sea cucumbers, with average initial body mass of 8.5 g, were collected from a local farm (Qingdao, Shandong, China). Prior to the experiment, all sea cucumbers were acclimated for 2 weeks in the culture environment. After acclimatization, the sea cucumbers were starved for 24 h and randomly assigned to 15 glass aquariums (60 × 30 × 40 cm of L × W × H) with the density of 8 individuals per tank. The 15 glass aquariums were divided into 5 groups and marked with 3 replicates for each group. One third of water was changed at 16:00 every day while the residual bait and feces were collected. Feeding was performed once per day at 17:00, with about 4% of the body weight. During the experiment period, oxygen was supplied continuously for 24 h, water temperature was kept at 16 ± 0.5 ℃, and salinity was 30–32.
At the end of the experiment, sea cucumbers were starved for 24 h and weighed. The coelomic fluid was collected with a sterile syringe and stored in 2 ml sterile centrifuge tubes. The intestinal tissue was separated with scissors and tweezers, washed with sterile water, and put into the marked sterile tubes. Samples from each tank were stored in a mixed sample tube, and three sample tubes from three tanks in each group were used as biological replicates. All the above operations were completed on ice. All the experimental tools used during sample collection had been ultrasonically cleaned and autoclaved. Samples were immediately transferred to liquid nitrogen and stored at − 80 °C for further analysis.
Determination of Feed Components
Moisture, crude protein, crude lipid, and Zn content were analyzed according to the standard procedures of AOAC [21]. Feed powder was dried to constant weight in 110 °C oven to determine moisture. Crude protein (%) was calculated by Kjeldahl nitrogen determination method, i.e., N × 6.25. Crude lipid (%) was obtained with the method of Soxhlet extraction. The feed powder was fully decomposed in strong acid under high temperature and pressure. After constant volume and filtration, the Zn content was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES).
Determination of Enzyme Activities
The intestinal samples were transferred from − 80 ℃ to ice, and appropriate amounts of incompletely melted intestinal tissues were homogenized thoroughly at a ratio of 1:9 with the corresponding homogenization medium and centrifuged at 4 °C for 10 min (2500 r/min). The supernatant was collected to evaluate the activities of digestive enzymes, including amylase, lipase, and trypsin. Antioxidant indicators were measured by coelomic fluid, including the activities of superoxide dismutase (SOD), catalase (CAT), anti-superoxide anion (ASA), inhibiting hydroxyl radical (IHR), and the content of malondialdehyde (MAD). All enzyme activities were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, China).
Gene Expression Analysis by Quantitative PCR
Intestinal tissue sample was freeze-ground and added to the lysate buffer. The total RNA of each sample was extracted using FastPure® Tissue Total RNA Isolation Kit (Vazyme Biotech Co., Ltd, Nanjing, China). The extracted RNA was run in 1% agarose gel electrophoresis to verify the quality. The concentration of the extracted RNA and its absorbance values at 260 nm and 280 nm were measured using a NanoDrop 300 spectrophotometer (Hangzhou Allsheng Instruments Co., Ltd) as a basis for determining its purity. Following that, the intact and pure RNA was reverse-transcribed in a thermal cycle using HiScript® III RT SuperMix for qPCR (+ gDNA wiper) (Vazyme Biotech Co., Ltd, Nanjing, China) to synthesize cDNA. The final cDNA product was stored at − 20 ℃ for subsequent quantitative PCR. Primer sequences of β-actin, heat shock protein 90 (hsp90), lysozyme (lsz), NF-kappa-B1 (p105), and NF-kappa-B transcript factor p65 (rel) were referenced from the publication [22], presented in Table 2. The expression analysis was performed in a real-time quantitative PCR using ChamQ SYBR Color qPCR Master Mix (Low ROX Premixed) (Vazyme Biotech Co., Ltd, Nanjing, China), and the specific reaction procedure and system were as described in the article [23]. In our experiment, three replicates of real-time quantitative PCR were performed for each sample, and the amplification efficiency of all samples was between 90 and 110%. The relative expression of genes was calculated by the 2−∆∆t method [24].
Statistical Analysis
The specific growth rate (SGR), weight gain rate (WGR), feed conversion ratio (FCR), and protein efficiency ratio (PER) were used to measure the growth of sea cucumber fed diets with different Zn levels, calculated as follows:
where IBW means the initial body weight (g), FBW means the final body weight (g), FI refers to feed intake, and FPC stands for the feed protein content.
Results were presented as mean ± S.D. Statistical analysis was performed using IBM SPSS Statistic 22 (SPSS Inc., USA). Data from each group were subjected to one-way analysis of variance (ANOVA). When overall differences were significant (P < 0.05), Duncan’s multiple range test was used to compare the mean values among the groups. The dietary Zn requirement of sea cucumber was estimated by broken-line regression analysis based on SGR. The equation of broken-line regression model is \(y=\beta0-\beta1\left(\mathrm X-x\right)\;\mathrm{if}\;x<\mathrm X,\mathrm{else}\;y=\beta0+\beta2(\mathrm X-x)\;\mathrm{when}\;x\geq\mathrm X.\) X represents the optimal dietary Zn level for the maximum SGR.
Result
Growth Performance
Growth performance of sea cucumber fed diets with different levels of Zn are shown in Table 3. The survival rate of each group was 100%. SGR and WGR were significantly improved by the supplementation of Zn and showed an increased trend followed by decreasing with the increase of Zn level. The highest SGR and WGR were observed in the 40 mg/kg Zn group (P < 0.05). FCR and PER in the 20–80 mg/kg Zn groups were significantly higher than control (P < 0.05). The broken-line regression analysis of SGR showed the optimum Zn content for sea cucumber was 66.3 mg/kg (Fig. 1).
Activities of Digestive Enzymes
The effect of Zn on the digestive enzyme activities of sea cucumber is shown in Fig. 2. Compared to the control group, amylase activity was significantly increased in the 20–80 mg/kg Zn groups (P < 0.05), with the highest value reached at 40 mg/kg followed by 20 mg/kg Zn group. No significant change of lipase activity was observed among the groups (P > 0.05). Increasing dietary Zn level markedly enhanced trypsin activity (P < 0.05). Nevertheless, when the Zn supplementation content exceeded 40 mg/kg, the trypsin activity showed a decreasing trend with the gradual increase in Zn level.
Activities of Antioxidant Enzymes
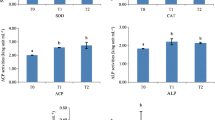
The effect of Zn on the antioxidant enzyme activities of sea cucumber is shown in Fig. 3. The activity of SOD was highest in the 20 mg/kg Zn group, followed by the 40 mg/kg and 80 mg/kg Zn groups, which were significantly higher than the control group (P < 0.05). The CAT activity was significantly increased and showed a decreasing trend with 20–60 mg/kg Zn groups compared to the control (P < 0.05). The ASA activity increased significantly at 20 mg/kg Zn group (P < 0.05), and no significant difference was observed with a further increase of Zn levels (P > 0.05). The IHR activity was significantly higher in the 20 mg/kg Zn group than the control and gradually decreased with increasing Zn content (P < 0.05); except for the 20 mg/kg Zn group, there was no significant difference between the other groups and the control group (P > 0.05). The MDA content was significantly reduced by the supplementation of 20–80 mg/kg Zn compared with the control group (P < 0.05), with the lowest at 20 mg/kg Zn group and significantly increased with the supplementation of 60–80 mg/kg Zn (P < 0.05).
Antioxidant enzyme activities of sea cucumber (Apostichopus japonicus) fed different Zn diets. SOD, superoxide dismutase; CAT, catalase; ASA, anti-superoxide anion; IHR, inhibiting hydroxyl radical, and MAD, malondialdehyde. Data were presented as mean ± SD (n = 3). Different letters represent significant differences between diets at the significance level of 0.05 (P < 0.05)
Transcription of Immune-Related Genes
The expression of immune-related genes in sea cucumber fed diets with different levels of Zn is shown in Fig. 4. The relative expression of hsp90 was increased significantly when Zn supplementation reached 60 mg/kg (P < 0.05). The relative expression of p105 showed significant upregulation with the increase of Zn supplementation, which was significantly higher than that of the control group except for the 20 mg/kg Zn group (P < 0.05). Compared with the control group, the rel gene relative expression was significantly higher when Zn was added up to 40 mg/kg and exerted an increased trend with increased Zn content (P < 0.05). The relative expression of lsz in the 60 and 80 mg/kg Zn groups was significantly upregulated when compared to other groups.
Discussion
Zn is a trace element in the biological body second only to iron, widely distributed in various tissues and organs, with catalytic, structural, and regulatory functions. Previous studies have shown that Zn is involved in regulating growth and development, oxidative stress, and immune defense of various aquaculture animals, including fish and crustaceans [6, 10]. However, there has been no report for Apostichopus japonicus. Therefore, this experiment explored the effects of dietary Zn on growth performance, digestive enzyme activities, antioxidant status, and immune response of Apostichopus japonicus.
Our results showed the survival rate of each group was 100% and the growth exhibited a certain degree of improvement after 2 months of cultivation, which could be attributed to the excellent conditions and nutrition supply provided by the experiment. The Zn supplemented group showed significantly higher WGR and SRG compared to the control group, especially at a maximum of 40 mg/kg Zn group, indicating an appropriate amount of Zn supplementation promote the growth of sea cucumber. In addition, FCR and PER were found significantly increased in the Zn supplemented group, further confirming the positive effects of Zn on the growth of sea cucumber. Our results were consistent with previous studies in aquatic animals, including African catfish (Clarias gariepinus) [25], blunt snout bream (Megalobrama amblycephala) [10], and Siberian sturgeon (Acipenser baerii) [26]. Meanwhile, growth performance was significantly decreased when Zn supplements were more than 40 mg/kg. This is probably due to the negative effects of excess Zn in the diet [27]. Previous studies reported that high doses of Zn produce excess reactive oxygen species, which activate immune-related signaling pathways and induce oxidative stress and inflammation. The body’s energy is allocated to deal with these uncomfortable reactions, and relatively less is devoted to growth, resulting in slower growth [28].
Digestive enzymes are essential catalysts for the hydrolysis of carbohydrates, lipids, and proteins, and the activity of digestive enzymes is an important indicator which reflects the digestive capacity [29, 30]. In the present study, dietary Zn supplementation dramatically increased the activities of amylase and trypsin, suggesting that Zn can promote the intestinal digestive capacity of sea cucumber. The maximum enzyme activities were observed with Zn supplementation of 40 mg/kg, which was consistent with the growth performance. Consistently, the improvement of Zn on digestive enzyme activity has been demonstrated in other species. Li et al. [31] found that the supplementation of 50 mg/kg Zn to the basal diet enhanced the hepatopancreatic amylase activity of hybrid tilapia (Oreochromis niloticus) by 70% and the intestinal amylase and lipase activities by 48.5% and 84%, respectively. Muralisankar et al. [32] also showed that Zn could significantly increase the activities of amylase, protease, and lipase in giant freshwater prawn (Macrobrachium rosenbergii). Consistent with the above results, in our experiment, the activities of amylase and trypsin of sea cucumber were increased with the supplementation of Zn, but no significant difference was observed in the activity of lipase among all groups, which may be due to the low lipid requirements of sea cucumber [33].
Zn is a coenzyme factor of various enzymes in organisms including SOD, which widely participates in the metabolic process of living organisms [17, 34]. In the present study, SOD activity was significantly increased in the Zn supplemented group and then exerted a decreased trend when compared with the control group, indicating that adding appropriate amount of Zn in the diet can effectively improve the antioxidant level of the body. However, when the Zn supplementation content reached 80 mg/kg, SOD activity showed an upward trend. Considering the growth performance of sea cucumber, we proposed that excessive Zn supplementation exerted toxicity to sea cucumber. There is evidence that excessive Zn can cause oxidative stress in the organism and induce genotoxicity, which can inhibit growth. Consistently, this toxic effect had previously been described in freshwater prawn (Macrobrachium rosenbergii) [32] and goldfish (Carassius auratus) [35]. SOD can dismutate superoxide anions to produce oxygen and hydrogen peroxide. Hydrogen peroxide reacts with the hydrogen ions under the action of CAT to convert into water and oxygen [36, 37]. Our results showed that the intake of 20–60 mg/kg Zn supplementation significantly improved the CAT activity, indicating that Zn can promote the decomposition of hydrogen peroxide to weaken the attack of reactive oxygen species at this concentration. Consistently, the increase of SOD and CAT activities by dietary Zn supplementation has also been reported in blunt snout bream (Megalobrama amblycephala) studied by Jiang et al. [10] and juvenile Jian carp (Cyprinus carpio var. Jian) studied by Feng et al. [7], which are in agreement with the present investigation. On the other hand, Hidalgo et al. [38] have also reported that Zn deficiency reduced the CAT activity of rainbow trout (Oncorhynchus mykiss), thus reducing the defense ability to reactive oxygen species. In general, dietary Zn supplementation improved the antioxidant capacity by increasing SOD and CAT activities of the organism. In this study, 20–60 mg/kg Zn supplementation is more beneficial to the antioxidant defense of sea cucumber, while 80 mg/kg Zn supplementation leads to toxic effects.
Hydroxyl free radicals and superoxide anions have strong oxidability, which can attack biological membranes and peroxidation lipid [36, 39]. MDA, acting as an end product of lipid peroxidation, has been widely used to evaluate the degree of lipid oxidation [40]. In the current study, supplementation of 20 mg/kg Zn in the diet remarkably raised the ASA and IHR activities and achieved the lowest MDA content, which may be correlated with the higher SOD and CAT activities in this gradient. These results further support the inference that Zn can improve the antioxidant status of the sea cucumber, while the mechanism of antioxidant role played by Zn needs to be further explored.
Heat shock proteins are a kind of stress proteins widely existing in organisms. Some family members can act as “danger”-signaling molecules to directly stimulate the innate immune system of sea cucumber [41, 42]. It has been demonstrated that external stimuli can induce heat shock proteins in cells [41, 43]. In this experiment, when the Zn supplementation content was over 60 mg/kg, the relative expression level of hsp90 was significantly upregulated, indicating that high concentration of Zn produces oxidative stimulation to the body. As transcription factors, the NF-kappa-B family participates in the regulation of non-specific immune responses of sea cucumber and can regulate the expression of multiple target genes including lsz [22, 44]. Our results showed that the relative expression levels of p105, rel, and lsz genes were significantly elevated at higher Zn concentrations, which further confirmed the NF-kappa-B signaling pathway in sea cucumber.
Conclusion
In conclusion, the supplementation of Zn in diets can significantly improve the growth performance and digestive capacity of sea cucumber Apostichopus japonicus. Dietary Zn supplementation can improve the antioxidant capacity of sea cucumber Apostichopus japonicus by increasing the activity of antioxidant enzymes. Excess Zn causes oxidative stress that induces immune response. Based on the specific growth rate, the present study indicated that the optimum requirement of Zn for juvenile A. japonicus was ~ 66.3 mg/kg.
Data Availability
Data and materials supporting the results are available upon reasonable request from the corresponding author.
References
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods-a review. Mar Drugs 9:1761–1805. https://doi.org/10.3390/md9101761
Bureau of Fisheries of the Ministry of Agriculture (2021) Fisheries Statistical Yearbook of China. China Agricultural Press, Beijing
Gutteridge JMC, Halliwell B (1992) Comments on review of free-radicals in biology and medicine. Free Radical Biol Med 12:93–95. https://doi.org/10.1016/0891-5849(92)90062-l
Ren Y, Liu W, Pearce CM (2018) Effects of stocking density, ration and temperature on growth, survival and metamorphosis of auricularia larvae of the California sea cucumber, Parastichopus californicus. Aquac Res 49:517–525. https://doi.org/10.1111/are.13482
Bray TM, Bettger WJ (1990) The physiological role zinc as an antioxidant. Free Radical Biol Med 8:281–291. https://doi.org/10.1016/0891-5849(90)90076-u
Dawood MAO, Alagawany M, Sewilam H (2022) The role of zinc microelement in aquaculture: a review. Biol Trace Elem Res 200:3841–3853. https://doi.org/10.1007/s12011-021-02958-x
Feng L, Tan LN, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr 17:E875–E882. https://doi.org/10.1111/j.1365-2095.2011.00858.x
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151:185–207. https://doi.org/10.1016/s0044-8486(96)01503-7
Shukry M, Albogami S, Gewaily M, Amer AA, Soliman AA, Alsaiad SM, El-Shehawi AM, Dawood MAO (2022) Growth performance, antioxidative capacity, and intestinal histomorphology of grey mullet (Liza ramada)-fed dietary zinc nanoparticles. Biol Trace Elem Res 200:2406–2415. https://doi.org/10.1007/s12011-021-02844-6
Jiang M, Wu F, Huang F, Wen H, Liu W, Tian J, Yang C, Wang W (2016) Effects of dietary Zn on growth performance, antioxidant responses, and sperm motility of adult blunt snout bream, Megalobrama amblycephala. Aquaculture 464:121–128. https://doi.org/10.1016/j.aquaculture.2016.06.025
Tan LN, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquac Nutr 17:338–345. https://doi.org/10.1111/j.1365-2095.2010.00793.x
Mohammady EY, Soaudy MR, Abdel-Rahman A, Abdel-Tawwab M, Hassaan MS (2021) Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia, Oreochromis niloticus. Aquaculture 532. https://doi.org/10.1016/j.aquaculture.2020.736006
Tan BP, Mai KS (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 192:67–84. https://doi.org/10.1016/s0044-8486(00)00435-x
Yuan Y, Luo J, Zhu T, Jin M, Jiao L, Sun P, Ward TL, Ji F, Xu G, Zhou Q (2020) Alteration of growth performance, meat quality, antioxidant and immune capacity of juvenile Litopenaeus vannamei in response to different dietary dosage forms of zinc: comparative advantages of zinc amino acid complex. Aquaculture 522. https://doi.org/10.1016/j.aquaculture.2020.735120
Kishawy ATY, Roushdy EM, Hassan FAM, Mohammed HA, Abdelhakim TMN (2020) Comparing the effect of diet supplementation with different zinc sources and levels on growth performance, immune response and antioxidant activity of tilapia, Oreochromis niloticus. Aquac Nutr 26:1926–1942. https://doi.org/10.1111/anu.13135
Maage A, Julshamn K (1993) Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture 117:179–191. https://doi.org/10.1016/0044-8486(93)90134-k
Shiau SY, Jiang LC (2006) Dietary zinc requirements of grass shrimp, Penaeus monodon, and effects on immune responses. Aquaculture 254:476–482. https://doi.org/10.1016/j.aquaculture.2005.10.033
Zhang M, Huang Y, Li Y, Cai M, Zhao Y (2021) The effects of dietary zinc on growth, immunity and reproductive performance of female Macrobrachium nipponense prawn. Aquac Res 52:1585–1593. https://doi.org/10.1111/are.15010
Shi B, Wang T, Zeng Z, Zhou L, You W, Ke C (2019) The role of copper and zinc accumulation in defense against bacterial pathogen in the Fujian oyster (Crassostrea angulata). Fish Shellfish Immunol 92:72–82. https://doi.org/10.1016/j.fsi.2019.05.049
Yu H, Gao Q, Dong S, Hou Y, Wen B (2016) Change of digestive physiology in sea cucumber Apostichopus japonicus (Selenka) induced by corn kernels meal and soybean meal in diets. J Ocean Univ China 15:697–703. https://doi.org/10.1007/s11802-016-2985-x
AOAC (2006) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Arlington, VA, USA
Chen J, Ren Y, Wang G, Xia B, Li Y (2018) Dietary supplementation of biofloc influences growth performance, physiological stress, antioxidant status and immune response of juvenile sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immunol 72:143–152. https://doi.org/10.1016/j.fsi.2017.10.061
Mei Y, Tian Y, Gao Q, Dong S, Li X, Xu Y (2022) Effects of different stocking densities on the CO2 fluxes at water-air interface and the respiration metabolism in sea cucumber Apostichopus japonicus (Selenka). Front Mar Sci 9. https://doi.org/10.3389/fmars.2022.965700
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mahboub HH, Shahin K, Zaglool AW, Roushdy EM, Ahmed SAA (2020) Efficacy of nano zinc oxide dietary supplements on growth performance, immunomodulation and disease resistance of African catfish Clarias gariepinus. Dis Aquat Org 142:147–160. https://doi.org/10.3354/dao03531
Moazenzadeh K, Islami HR, Zamini A, Soltani M (2018) Effects of dietary zinc level on performance, zinc status, tissue composition and enzyme activities of juvenile Siberian sturgeon, Acipenser baerii (Brandt 1869). Aquac Nutr 24:1330–1339. https://doi.org/10.1111/anu.12670
Puar P, Niyogi S, Kwong RWM (2020) Regulation of metal homeostasis and zinc transporters in early-life stage zebrafish following sublethal waterborne zinc exposure. Aquat Toxic 225. https://doi.org/10.1016/j.aquatox.2020.105524
Ma S, Shu X, Wang WX (2022) Multi-omics reveals the regulatory mechanisms of zinc exposure on the intestine-liver axis of golden pompano Trachinotus ovatus. Sci Total Environ 816. https://doi.org/10.1016/j.scitotenv.2021.151497
Li B, Wang L, Wang J, Sun Y, Wang S, Jiang L, Huang B (2020) Requirement of vitamin E of growing sea cucumber Apostichopus japonicus Selenka. Aquac Res 51:1284–1292. https://doi.org/10.1111/are.14479
Wang X, Jiang J, Guan X, Zhao Z, Dong Y, Wang J, Li S, Jiang B, Liu G, Sun H, Gao S, Jiang P, Wang X, Zhou Z (2021) Effects of Lactobacillus acidophilus and tussah immunoreactive substances on disease resistance of sea cucumber (Apostichopus japonicus) against Vibrio splendidus. Aquac Res 52:4601–4612. https://doi.org/10.1111/are.15293
Li JS, Li JL, Wu TT (2007) The effects of copper, iron and zinc on digestive enzyme activity in the hybrid tilapia Oreochromis niloticus (L.) x Oreochromis aureus (Steindachner). J Fish Biol 71:1788–1798. https://doi.org/10.1111/j.1095-8649.2007.01643.x
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Srinivasan V, Santhanam P (2015) Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 448:98–104. https://doi.org/10.1016/j.aquaculture.2015.05.045
Liao ML, Ren TJ, Chen W, Han YZ, Liu CM, Jiang ZQ, Wang FQ (2017) Effects of dietary lipid level on growth performance, body composition and digestive enzymes activity of juvenile sea cucumber, Apostichopus japonicus. Aquac Res 48:92–101. https://doi.org/10.1111/are.12864
Luo Z, Tan XY, Zheng JL, Chen QL, Liu CX (2011) Quantitative dietary zinc requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture 319:150–155. https://doi.org/10.1016/j.aquaculture.2011.06.047
Hasnat A, Rani B, Kohli MPS, Chandraprakash G (2012) Zinc supplementation and its effect on thermal stress resistance in Carassius auratus fry. Isr J Aquacult-Bamidgeh 64:779–786
Nyska A, Kohen R (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. https://doi.org/10.1080/01926230290166724
Winston GW, Digiulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161. https://doi.org/10.1016/0166-445x(91)90033-6
Hidalgo MC, Exposito A, Palma JM, de la Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193. https://doi.org/10.1016/s1357-2725(01)00105-4
Weissman L, De Souza-Pinto NC, Stevnsner T, Bohr VA (2007) DNA repair, mitochondria, and neurodegeneration. Neuroscience 145:1318–1329. https://doi.org/10.1016/j.neuroscience.2006.08.061
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med 9:515–540. https://doi.org/10.1016/0891-5849(90)90131-2
Wallin RPA, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG (2002) Heat-shock proteins as activators of the innate immune system. Trends Immunol 23:130–135. https://doi.org/10.1016/s1471-4906(01)02168-8
Yang A, Zhou Z, Dong Y, Jiang B, Wang X, Chen Z, Guan X, Wang B, Sun D (2010) Expression of immune-related genes in embryos and larvae of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 29:839–845. https://doi.org/10.1016/j.fsi.2010.07.023
Wang QL, Yu SS, Qin CX, Dong SL, Dong YW (2014) Combined effects of acute thermal and hypo-osmotic stresses on osmolality and hsp70, hsp90 and sod expression in the sea cucumber Apostichopus japonicus Selenka. Aquacult Int 22:1149–1161. https://doi.org/10.1007/s10499-013-9734-6
Wang T, Sun Y, Jin L, Thacker P, Li S, Xu Y (2013) Aj-rel and Aj-p105, two evolutionary conserved NF-kappa B homologues in sea cucumber (Apostichopus japonicus) and their involvement in LPS induced immunity. Fish Shellfish Immunol 34:17–22. https://doi.org/10.1016/j.fsi.2012.09.006
Acknowledgements
The authors would like to express their sincere thanks to the staff of laboratory for their help and logistic support during the experiment.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Project Nos. 31672657).
Author information
Authors and Affiliations
Contributions
Yuling Xu, Qinfeng Gao, and Shulin Dong conceived and designed the experiments; Yaoping Mei, Xueqi Li, Kang Dong, and Zhao Li participated in the collection and determination of the experimental samples; Yuling Xu wrote the article; Qinfeng Gao and Zhishuai Hou revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of Ocean University of China (Permit Number: SCXY-B20230203). The field studies did not involve endangered or protected species.
Competing Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Gao, Q., Dong, S. et al. Effects of Dietary Zinc on Growth Performance, Digestive Enzyme Activities, Antioxidant Status, and Immune Responses of Sea Cucumber Apostichopus japonicus. Biol Trace Elem Res 202, 1767–1775 (2024). https://doi.org/10.1007/s12011-023-03766-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03766-1