Abstract
Astaxanthin has potent antioxidant activity and is frequently used for feed supplementation. This study investigated the effects of dietary astaxanthin on the growth performance and innate immune index of juvenile sea cucumbers (Apostichopus japonicas). Astaxanthin was replaced 0 g/kg, 0.1 g/kg, 0.3 g/kg, and 0.5 g/kg of cellulose to formulate four kinds of test feeds with equivalent nitrogen and energy. Dietary astaxanthin supplementation improved body weight gain, specific growth rate, feed efficiency, intestinal trypsin, and amylase and lipase activities and enhanced the activities of lysozyme, alkaline phosphatase, and acid phosphatase in the coelomic fluid and the expression of intestinal immunity-related NF-κB p105, p50, and rel and lysozyme gene lys and disease resistance ability of juvenile sea cucumbers against the pathogen, Vibrio splendidus. The optimal dose of dietary astaxanthin supplementation required for the maximal growth of sea cucumbers was 0.29 g/kg, which was obtained by quadratic regression analysis. The results indicate that astaxanthin may be a promising feed supplementation for sea cucumbers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea cucumber (Apostichopus japonicus) belongs to the Echinodermata phylum, Apostichopus class, and Apostichopus family (Chan et al. 2022). This species is distributed in countries along the Pacific Northwest, including Russia, Japan, and South Korea (Bai et al. 2017). In China, sea cucumber is mainly produced in the intertidal zone along the coast of the Yellow Sea and Bohai Sea and one of the main economic species of aquaculture in coastal areas (Chen et al. 2018). Sea cucumber has rich nutritional value, unique flavor, and high medical and health care efficacy (Wang et al. 2021).
With the depletion of fishery resources and the impact of high market demand in recent years, cultivation of sea cucumber has rapidly intensified, and the scale has expanded quickly, gradually replacing the natural fishing industry (Chan et al. 2022). However, sea cucumbers are prone to outbreaks of infectious diseases due to the long breeding cycle and the lack of specific immunity (Song et al. 2019). In addition, the rapid development of the scale of sea cucumber imitation breeding and the backward breeding methods make it difficult for farmers to prevent infectious diseases, a situation which seriously limits the development of artificial culture of sea cucumber imitation (Ma et al. 2019). At present, feed additives such as antibiotics and other chemical drugs are used to prevent and treat aquaculture diseases will cause an imbalance of animal intestinal flora, reduce the activity of digestive enzymes, damage intestinal tissues, and increase bacterial infiltration and other hazards, thereby causing a series of environmental and social problems (Yu et al. 2016). Prebiotics, as feed additives, can improve the intestinal health of aquatic animals and promote their ability to resist disease and infection. With the transformation of the aquaculture mode, the pursuit of green and pollution-free aquatic products has gained popularity, and the application of prebiotics in artificial aquaculture has broad prospects (Yu et al. 2016). Many natural substances, such as ascorbic acid (Bai et al. 2017), biofloc (Chen et al. 2018), probiotic yeast (Ma et al. 2019), astragalus polysaccharide (Song et al. 2019), n-3 highly unsaturated fatty acids (Yu et al. 2016), and β-glucan (Zhao et al. 2011), have been explored recently for application as prebiotics to ameliorate the growth and/or immunity of sea cucumber.
Astaxanthin is a keto carotenoid with the structure of 3,3′- dihydroxy-4,4′ - diketo group- β,β′- carotene. It exists widely in the biological world, especially exists in aquatic animals such as crabs, shrimp, and fish and in the feathers of birds. Moreover, astaxanthin plays a role in color rendering. Astaxanthin is also a chain-breaking antioxidant. It has varying physiological functions, including strong antioxidant activity, hypolipidemic features, immunomodulatory activity, and anticancer activity (Chew et al. 1999; Rajesh et al. 2017; Sakir et al. 2023; Sun et al 2022). Astaxanthin has also been used to ameliorate the growth and immunity of varying aquatic animals such as the red swamp crayfish Procambarus clarkia (Cheng and Wu 2019), post-larval kuruma shrimp (Wang et al. 2018), white shrimp (Litopenaeus vannamei) (Liu et al. 2022), juvenile swimming crab (Han et al. 2018), blood parrot (Li et al. 2018), and pufferfish (Cheng et al. 2018).
Given these data, dietary astaxanthin supplementation may ameliorate the growth and immunity of sea cucumber. At the same time, data regarding the growth and immunity of sea cucumber are limited. Hence, this study aims to explore the effects of dietary astaxanthin supplementation on the growth, digestive enzyme activity, and innate immunity of sea cucumber.
Materials and methods
Diet preparation
The ingredient compositions of the experimental diets of sea cucumber are presented in Table 1. Astaxanthin (purity 10%, BASF, Germany) was replaced with cellulose in the basal diet to formulate the test diets at doses of 0.1, 0.3, and 0.5 g/kg (Cheng and Wu 2019). Each ingredient was crushed into fine powders, passed through a 180-micron mesh, and then mixed with water. The granulated feed with a diameter of 1.88 mm was prepared using a granulator (Pinzheng Equipment Co., Ltd., Changzhou, China), dried at 45 °C for 24 h and stored at −20 °C (Chan et al. 2022).
Sea cucumber culture
Sea cucumbers with average body weights of 2.28 ± 0.01 g were purchased from Lianyungang Hongde Breeding Co., Ltd, Jiangsu, China. Before the experiment, sea cucumbers were temporarily acclimated with basic feed for 1 week, during which the daily feeding amount was 3–5% of their body weight. The specific feeding amount depended on the feeding situation of the sea cucumbers to ensure satiety feeding. The sea cucumbers were fed at 17:00 every day. Before feeding, the residual bait and feces are siphoned promptly, and seawater was used to replace 30% of the water in the tank every day to maintain water quality.
Before the feeding test, 720 young ginseng individuals with good development, symmetrical body shape, healthy, and disease-free condition were selected and randomly allocated to 12 cages (1 m × 1 m × 1 m) with 60 young ginseng in each tank. Each feed was randomly fed with one of the three groups of sea cucumber twice a day (8:00 and 18:00) for apparent satiety feeding. Before feeding, the residual bait and feces are siphoned promptly. During the feeding period of 8 weeks, natural lighting conditions were maintained, and an air pump was employed for aeration to maintain sufficient oxygen content in the water. The water body indicators were as follows: water temperature 16 ± 0.5 °C, salinity 31.5 ± 0.5, pH 8.0 ± 0.1. Fresh seawater was used to replace 30% of the water in the tank every day to maintain water quality.
Growth performance
After the breeding experiment, the sea cucumber was starved for 48 h. The sea cucumbers in each cage were counted and weighed. The body weight gain (BWG), specific growth rate (SGR), feed efficiency (FE), and survival rate of the sea cucumbers were calculated according to the following equations:
where Wt is the final body weight of the sea cucumber (g), W0 is the initial body weight of sea cucumber (g), W1 is the total weight of the dried diet consumed (g), Nt is the final tail number of the sea cucumber, N0 is the initial tail number of sea cucumber, and t is the number of days of sea cucumber cultivation (d).
Sampling
Six sea cucumbers were placed on an ice plate. The sea cucumbers were cut 0.5 cm along the cloaca with a scalpel and immediately placed above the prepared 10-mL centrifuge tube to collect the body cavity fluid, which was centrifuged at 4 °C and 4000 × g (centrifugation radius 10 cm) for 10 min. The resulting supernatant was sub-packed in 0.5-mL centrifuge tubes and stored at −80 °C for the determination of various immune indicators.
After the body cavity fluid of the sea cucumber was collected, the digestive tract was taken out, and the respiratory tree connected to the end of the digestive tract was removed. After being washed with pre-cooled pH 7.0 phosphate buffer solution (Na2HPO4-NaH2PO4), the water on the surface of the digestive tract was gently sucked with a filter paper. The intestinal body was weighed and stored at −80 °C for the determination of intestinal digestive enzyme activities and immunity-related gene expression levels.
Enzyme determination assays
ELISA is a highly sensitive experimental technique that combines the specific reactions of antigens and antibodies with the high catalytic efficiency of enzymes on substrates, based on immunological reactions. The intestinal amylase (C016-1-1, 0.3–100 U/mL), trypsin (A080-2-2, 0.15–120 U/mL), and lipase (A054-1-1, 5–2000 U/L) activities as well as the plasma acid phosphatase (ACP, A080-1-1, 0.15–120 U/mL), alkaline phosphatase (AKP, A059-1-1, 0.05–50.00 king unit/100 mL) and lysozyme (LYZ, A050-1-1, 0.2–100 U/mL) activities were determined by using ELISA kits (Nanjing Jiancheng Biological Company Research Institute, Nanjing, Jiangsu, China) as previously described (Bai et al. 2017).
Relative expression of genes
The total RNA from the intestinal tissue of sea cucumber was extracted by RNAisoPlus (TaKaRa). The integrity and purity of the total RNA were detected by gel electrophoresis. The first strand of cDNA was synthesized according to the operating instructions of the PrimeScript TMRT kit (TaKaRa). The relative expression of immune genes (p105, p50, rel, and lys) was detected by the SYBR Green method using a fluorescence quantitative PCR instrument (HM-PCR1, Shandong Hengmei Electronic Technology Co., Ltd., Shandong, China). The internal reference genes were cytochrome B and β-actin. The sequences of the gene primers were shown in Table 2. The quantitative system of PCR included the following: 0.5 μL upstream and downstream primers (10 μmol/L), 1 μL first strand cDNA (5 μmol/L ), 5 μL 2×SYBR Premium Rx Taq II, and 3 μL Ultrapure water treated with diethyl pyrocarbonate. One cycle was performed at 95 °C for 30 s. The thermal cycling was carried out in accordance with the procedure as follows: 95 °C for 10 s, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s. The fusion curve was plotted to verify that there was only 1 PCR per PCR reaction. The relative expression of the target gene was calculated using the 2-ΔΔCt method (Li et al. 2022).
Challenge test
After the breeding experiment, 30 sea cucumbers from each group were randomly selected for the challenge test. Vibrio splendidus, the experimental strain, has a half-lethal concentration (LD50) of 2 × 107 CFU/mL. The bacterial concentration used was ~1 × 108 CFU/mL (Chen et al. 2018). The infection experiment was performed by the intracavity injection method with the injection volume of 0.1 mL/head. The status and death of the simulated sea cucumbers were observed and recorded daily.
Statistical analysis
All experiments were performed in sextuplicate. All data were diagnosed for normality of distribution and homogeneity of variance with the Kolmogorov-Smirnov test and Levene’s test, respectively. SPSS Statistics 17.0 for Windows was used for statistical analysis. The differences between the two means were analyzed by using one-way ANOVA and tested by using Duncan’s multiple-range test. Significant difference was set at p < 0.05. The orthogonal polynomial contrast was used to check the linear and quadratic effects of dietary levels of astaxanthin.
Results
Survival rate and growth performance
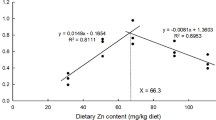
During the feeding experiment, the death rate of the sea cucumbers in each group was zero (Table 3). However, the BWG, SGR, food intake (FI), and FE of the sea cucumbers fed with dietary supplementation containing astaxanthin were higher than those fed with the basal diet (p < 0.05). Nevertheless, compared with a moderate dose (0.3 g/kg), a high dose (0.5 g/kg) of dietary astaxanthin supplementation decreased the BWG, SGR, and FI (p < 0.05). The dose of dietary astaxanthin supplementation required for the optimal growth of juvenile sea cucumbers was determined as 0.29 g/kg by using a polynomial test and regression analysis (Fig. 1).
Intestinal digestive enzyme activity
The sea cucumbers fed with dietary supplementation containing astaxanthin had higher activities of intestinal trypsin, amylase, and lipase activities than those in the control group (Table 4, p < 0.05). However, compared with a moderate dose (0.3 g/kg), a high dose (0.5 g/kg) of dietary astaxanthin supplementation decreased the intestinal trypsin, amylase, and lipase activities (p < 0.05).
Serum immunological indicators
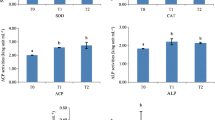
Different concentrations of astaxanthin can improve the non-specific immune enzyme activity of sea cucumbers in different degrees (Table 5). Dietary astaxanthin supplementation increased the serum ACP, AKP, and LYZ activities in a dose-dependent manner.
Gene expression level
The sea cucumbers fed with dietary supplementation containing astaxanthin had higher levels of intestinal p105, p50, rel, and lys than those in the control group (Table 6, p < 0.05). However, there were no differences in the levels of intestinal p105, p50, and rel among all treatment groups (p > 0.05).
Challenge test
The sea cucumbers in the control group began to die on the 3rd day after injection, and the survival rate dropped to 52.14% on the 7th day (Fig. 2). The sea cucumbers in the 0.1 g/kg group also began to die from the 3rd day, and the survival rate was 76.87% at the 7th day, a value which was significantly higher than that of the control group (p < 0.05). The sea cucumbers in the 0.3 g/kg and 0.5 g/kg groups also began to die on the 7th day, with survival rates of 86.47% and 87.15%, respectively, which were higher than the counterparts in the control and 0.1 g/kg groups (Fig. 2, p < 0.05).
Discussion
Dietary astaxanthin supplementation was reported to stimulate the growth performance of varying aquatic animals such as red swamp crayfish P. clarkia (Cheng and Wu 2019), post-larval kuruma shrimp (Wang et al. 2018), white shrimp (L. vannamei) (Liu et al. 2022), juvenile swimming crab (Han et al. 2018), blood parrot (Li et al. 2018), and pufferfish (Cheng et al. 2018), but its effects on sea cucumbers remain unclear. In this study, dietary astaxanthin supplementation increased the BWG, SGR, FI, and FE values of the sea cucumbers compared with those in the control group. The biosynthesis of protein, sugar, and lipids requires a reductive internal environment. Astaxanthin has potent antioxidant activity and helps to maintain a reductive internal environment, thus promoting the biosynthesis of protein, sugar, and lipids and improving the growth performance of sea cucumbers. However, a high dose of astaxanthin (0.5 g/kg) decreased the efficiency compared with a moderate dose (0.3 g/kg). High doses of astaxanthin presumably exhibited mainly hypolipidemic activity and suppressed the BWG, SGR, FI, and FE compared with a moderate dose (Rajesh et al. 2017). In contrast, the optimum dosage of astaxanthin used for swimming crab (P. trituberculatus), white shrimp (L. vannamei), red swamp crayfish (P. clarkia), and post-larval kuruma shrimp is 30–60 mg/kg (Han et al. 2018), 250 mg/kg (Liu et al. 2022), 200–400 mg/kg (Cheng and Wu 2019), and 600 mg/kg (Wang et al. 2018), respectively. The difference in the optimal dose of dietary astaxanthin supplementation required for the maximal growth of different aquatic animals could be due to different aquatic animal species.
Nutrients such as protein, sugar, and lipids need to be hydrolyzed by digestive enzymes such as trypsin, amylase, and lipase in the intestine; moreover, the hydrolysates of these enzymes can be absorbed by the intestine, thereby making the activities of these enzymes vital indices for the aquatic breeding industry (Chen and Zhang 2019; Chen and Chen 2019; Cheng 2019; Gao et al. 2019; Zhang 2019; Zhang 2018). The digestive system of the sea cucumber is poorly differentiated and lacks specific digestive glands. Its intestinal tract can secrete digestive fluid to play the role of a digestive gland. Consequently, the activity of the digestive enzymes in its intestinal tract can reflect the ability of the sea cucumber to digestive ability. Adding astaxanthin in the feed can significantly improve the intestinal trypsin, amylase, and lipase activities of young ginseng; helps young ginseng digest and absorb protein, sugar, and lipids; and then affects the growth of sea cucumbers. As discussed, astaxanthin can promote the biosynthesis of protein, thereby also promoting the biosynthesis of intestinal trypsin, amylase, and lipase.
The activities of ACP, AKP, and LYZ in the body cavity fluid of sea cucumber increased with the increase of the astaxanthin level, indicating that astaxanthin has a strong scavenging effect on reactive oxygen free radicals, a feature which is beneficial to improve the anti-oxidation ability of the sea cucumber and enhance its non-specific immunity. Given that sea cucumber only has non-specific immunity, humora plays a key role in cellular and humoral immunity (Chen et al. 2018). These immune and antioxidant indexes in body fluid play an irreplaceable role in the humoral immunity of the sea cucumber. ACP, AKP, and LYZ can kill invasive pathogens and change the surface of pathogenic molecules to accelerate their phagocytosis and degradation. These enzymes play a crucial role in the disease resistance, immune response, cell damage, and repair of sea cucumbers (Song et al. 2019). The results showed that dietary astaxanthin supplementation increased the activities of ACP, AKP, and LYZ in juvenile sea cucumbers compared with the control group; furthermore, this finding could be ascribed to the immuno-stimulating activitiy of astaxanthin (Sun et al 2022).
P105, p50, and rel in sea cucumber are important genes in the signaling pathway NF-κB. The signal pathway NF-κB can activate non-specific immune responses in the body. The transcription factors in the pathway can be activated by a variety of stimuli, thereby participating in inducing the expression of more than 150 target genes, including lysozyme (Pahl 1999). The transcription factors p105, p50, and rel have N-terminal rel homologous domains that can bind to DNA and regulate the expression of various downstream important immune factors such as chemokines, cytokines, lysozyme, and adhesion factors (Yang et al. 2015). The results indicated that dietary astaxanthin can significantly increase the relative expression of p105, p50, and rel in the intestinal tissue of sea cucumber. In the signal pathway NF-κB of sea cucumber, the transcription factor rel can form dimers with p105 and p50, respectively, to regulate the expression of related genes (Wang et al. 2013). Lysozymes can cut off the linkage (β-1,4 glycosidic bond) between N-acetyl cell wall acid and N-acetylglucosamine in peptidoglycans and accelerate bacterial dissolution and death, thereby eliminating pathogenic bacteria that invade the body (Lee and Brey 1995). Dietary astaxanthin increased the expression of the lysozyme gene lys in the intestine of sea cucumber, indicating that astaxanthin can enhance the transduction of the signal pathway NF-κB, regulate the expression of the downstream of the related immune genes, including lys, to improve the immune response of the intestinal tract, ultimately achieving the role of enhancing the non-specific immunity of sea cucumber.
The disease resistance of aquatic animals against pathogens is an important index for the aquatic breeding industry. During the challenge test against V. splendidus, dietary astaxanthin supplementation increased the disease resistance of the sea cucumbers compared with the control group. The decreased susceptibility could also arise from the immunomodulatory activity of astaxanthin (Sun et al 2016).
In conclusion, dietary astaxanthin supplementation increased the growth performance, intestinal digestive enzyme activities, and immunity of juvenile sea cucumbers. However, high dose of astaxanthin (0.5 g/kg) supplementation decreased the growth performance of juvenile sea cucumbers compared with a moderate dose (0.3 g/kg). The optimum dose of dietary astaxanthin required for the maximal growth of juvenile sea cucumbers was 0.29 g/kg. The results of this study can provide basic data for the promotion and application of astaxanthin in aquaculture, especially in the cultivation of sea cucumber.
Data availability
The data used to support the findings of this study are included within the article.
References
Bai YC, Zhang LB, Xia SD et al (2017) Effects of dietary ascorbic acid levels on the growth, energy budget, and immunological performance of green, white, and purple color morphs of the sea cucumber, Apostichopus japonicus. Anim Feed Sci Technol 226:1–11. https://doi.org/10.1016/j.anifeedsci.2017.02.006
Chan QX, Wang FQ, Han YZ et al (2022) An investigation on dietary chromium picolinate supplementation in the juvenile sea cucumber Apostichopus japonicus: Growth, digestive enzyme activity, growth-related genes expression, immune and antioxidant capacity. Aquac Rep. https://doi.org/10.1016/j.aqrep.2022.101099
Chen L, Zhang YP (2019) The growth performance and nonspecific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary Porphyrayezoensis polysaccharide supplementation. Fish Shellfish Immunol 87:615–619. https://doi.org/10.1016/j.fsi.2019.02.013
Cheng YX, Wu SJ (2019) Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia. Aquaculture. https://doi.org/10.1016/j.aquaculture.2019.734341
Chen J, Chen L (2019) Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnusanguillicadatus). Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115227
Cheng YX (2019) The growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia affected by dietary Rhodiola rosea polysaccharide. Fish Shellfish Immunol 93:769–800. https://doi.org/10.1016/j.fsi.2019.08.046
Cheng CH, Guo ZX, Ye CX et al (2018) Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol Biochem 44:209–218. https://doi.org/10.1007/s10695-017-0425-5
Chen JH, Ren YC, Wang GD et al (2018) Dietary supplementation of biofloc influences growth performance, physiological stress, antioxidant status and immune response of juvenile sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immunol 72:143–152 https://doi.org/10.1016/j.fsi.2017.10.061
Chew BP, Park JS, Wong MW et al (1999) A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res 19:1849–1853
Gao RJ, Chen L, Zhang W et al (2019) Effect of dietary Antarctic krill Euphausia superba on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia. Fish Shellfish Immunol 96:122–125. https://doi.org/10.1016/j.fsi.2019.12.004
Han T, Li XY, Wang JT et al (2018) Effects of dietary astaxanthin (AX) supplementation on pigmentation, antioxidant capacity and nutritional value of swimming crab, Portunus trituberculatus. Aquaculture 490:169–177. https://doi.org/10.1016/j.aquaculture.2018.02.030
Liu L, Li J, Cai XN et al (2022) Dietary supplementation of astaxanthin is superior to its combination with Lactococcus lactis in improving the growth performance, antioxidant capacity, immunity and disease resistance of white shrimp (Litopenaeus vannamei). Aquac Rep. https://doi.org/10.1016/j.aqrep.2022.101124
Li X, Wang Y, Jiang XD et al (2022) Effects pf peptide supplemental levels on growth performance, immunity and expression of intestine functional genes in sea cucumber (Apostichopus japonicas). Chin J Anim Nutr 34:1853–1863. https://doi.org/10.3969/j.issn.1006-267x.2022.03.045
Li F, Huang SY, Lu XX et al (2018) Effects of dietary supplementation with algal astaxanthin on growth, pigmentation, and antioxidant capacity of the blood parrot ( Cichlasoma citrinellum × Cichlasoma synspilum ). J Oceanol Limnol 36:1851–1859. https://doi.org/10.1007/s00343-019-7172-7
Lee WJ, Brey PT (1995) Isolation and characterization of the lysozyme-encoding gene from the silkworm Bombyx mori. Gene 161:199–203. https://doi.org/10.1016/0378-1119(95)00199-G
Ma YX, Li LY, Li M et al (2019) Effects of dietary probiotic yeast on growth parameters in juvenile sea cucumber, Apostichopus japonicus. Aquaculture 499:203–211. https://doi.org/10.1016/j.aquaculture.2018.09.043
Pahl HL (1999) Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866. https://doi.org/10.1038/sj.onc.1203239
Rajesh K, Janardan SK, Manimekalai K (2017) Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn Res 9:161–167. https://doi.org/10.1016/10.4103/0974-8490.204654
Sakir YA, Bartu B, Nilufer E et al (2023) The effect of antioxidant astaxanthin on intestinal ischemia reperfusion damage in rats. J Invest Surg. https://doi.org/10.1080/08941939.2023.2182930
Sun L, Kim S, Mori R et al (2022) Astaxanthin exerts immunomodulatory effect by regulating SDH-HIF-1α axis and reprogramming mitochondrial metabolism in LPS-stimulated RAW264.7 cells. Mar Drugs 20:660–666. https://doi.org/10.3390/MD20110660
Song XJ, Feng ZF, Zhang YP et al (2019) Regulation of dietary astragalus polysaccharide (APS) supplementation on the non-specific immune response and intestinal microbiota of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 94:517–524. https://doi.org/10.1016/j.fsi.2019.09.049
Wang XD, Zhou ZC, Guan XY et al (2021) Effects of dietary Lactobacillus acidophilus and tussah immunoreactive substances supplementation on physiological and immune characteristics of sea cucumber (Apostichopus japonicus). Fish Physiol Biochem 44:209–218. https://doi.org/10.1007/s10695-017-0425-5
Wang WL, Ishikawa M, Koshio S et al (2018) Effects of dietary astaxanthin supplementation on survival, growth and stress resistance in larval and post-larval kuruma shrimp, Marsupenaeus japonicus. Aquac Res 49:2225–2232. https://doi.org/10.1111/are.13679
Wang TT, Sun YX, Jin LJ et al (2013) Aj-rel and Aj-p105, two evolutionary conserved NF-kB homologues in sea cucumber (Apostichopus japonicus) and their involvement in LPS induced immunity. Fish Shellfish Immunol 34:17–22. https://doi.org/10.1016/j.fsi.2012.09.006
Yu HB, Gao QF, Dong SL et al (2016) Effects of dietary n-3 highly unsaturated fatty acids (HUFAs) on growth, fatty acid profiles, antioxidant capacity and immunity of sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immunol 54:211–219 https://doi.org/10.1016/j.fsi.2016.04.013
Yang G, Tian XL, Dong SL et al (2015) Effects of four different additives on non-specific immunity and expression of intestinal immune gene (Aj-p105, Aj-p50, Aj-rel and Aj-lys) in sea cucumber (Apostichopus japonicus). J Fish China 39:638–647 (in Chinese). http://en.cnki.com.cn/Article_en/CJFDTOTAL-SCKX201505004.htm
Zhang BZ (2019) Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish Immunol 88:359–363. https://doi.org/10.1016/j.fsi.2019.03.006
Zhang JM (2018) Modulation of growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia upon dietary fulvic acid supplementation. Fish Shellfish Immunol 83:158–161. https://doi.org/10.1016/j.fsi.2018.09.012
Zhao YC, Ma HM, Zhang WB et al (2011) Effects of dietary β-glucan on the growth, immune responses and resistance of sea cucumber, Apostichopus japonicus against Vibrio splendidus infection. Aquaculture 315:269–274. https://doi.org/10.1016/j.aquaculture.2011.02.032
Funding
This research was supported by A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the ethics committee of Jiangsu Ocean University of Technology, China. All procedures were conducted in compliance with relevant laws and institutional guidelines.
Conflict of Interest
The authors have declared that no competing interests exist.
Additional information
Handling editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, K., Li, S., Wu, S. et al. Dietary astaxanthin supplementation improves the growth performance, immune response, and immunity-related gene expression of sea cucumber (Apostichopus japonicas). Aquacult Int 32, 1235–1246 (2024). https://doi.org/10.1007/s10499-023-01214-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01214-4