Abstract
Protein diets are required for the normal development of the reproductive system and their inadequacy or deficiency might have hazardous functional complications during maturational and developmental stages. The study was carried out to evaluate the effect of selenium (Se) and zinc (Zn) supplementation on the male and female reproductive organs of rats with postnatal protein malnutrition. Male and female weanling rats were randomly assigned to six groups respectively. The adequate protein diet rats were fed with 16% casein diet while the protein malnourished diet (PMD) rats were fed with 5% casein diet. After the 8th week of feeding, Se (sodium selenite; Na2SeO3) and Zn (zinc sulfate; ZnSO4·7H2O) were supplemented for 3 weeks. The growth curve of body weights, lipid profile, testosterone and progesterone level, Na+-K+-ATPase activity, oxidative stress, and antioxidant status were evaluated. The results showed that PMD reduced the body weights of male and female rats. It also reduced the activities of catalase and glutathione peroxidase in the testes, but reductions in superoxide dismutase and glutathione-S-transferase activities, glutathione, vitamins C and E, testosterone, and progesterone levels were observed in both the testes and ovaries. Furthermore, PMD increased the nitric oxide level in both organs and altered the plasma lipid profiles in both sexes. Se and Zn supplementation, however, restored almost all the alterations observed in all the parameters analyzed. In conclusion, Se and Zn supplementation protects the male and female reproductive organs of rats against postnatal protein malnutrition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein malnutrition (PM) is a nutritional disorder, which poses a global concern, especially among young infants in Africa, Latin America, Asia, and many underdeveloped or developing countries [1]. Proper nutrition is essential for the normal growth and development of the reproductive system and its associated processes. PM has been reported to cause hazardous functional complications during maturational and developmental stages to both male and female reproductive organs. It has been demonstrated that PM changes the testicular structure and causes a permanent effect on their capacity to produce spermatozoa and/or decreases daily sperm production [2]. Moreover, evidence shows that PM reduces the weights of the testis, seminal vesicle, and epididymis [3, 4]. Also, reduction in the level of serum testosterone and impairment in sperm production have been reported in protein-malnourished rats [5]. Furthermore, PM reduces the thickness of the uterine endometrium, and the level of androgen receptors, and increased the estrogen receptor β [6].
It has been reported that oxidative damage from free radicals is accentuated in protein-malnourished animals leading to loss of protein function and altered cellular redox balance resulting in impairment in tissue function [7]. In addition, reactive oxygen species (ROS) has been implicated in male infertility [8] as well as female infertility and impaired ovarian functions [9]. Antioxidants are essential for normal function and the maintenance of redox balance in both testes and ovaries. For example, testes possess high concentrations of antioxidants that protect against oxidative stress and thus play critical roles in the prevention of testicular atrophy and maintenance of spermatogenesis [10]. However, it has been reported that deficiency of Se, Zn, and other micronutrients usually occurs in plasma and cells after exposure to a low-protein diet [11, 12].
Selenium (Se), with atomic number 34, is a trace element required for cellular functions in animals and other organisms. It is covalently incorporated into peptide chains as a component of selenoproteins occurring as selenocysteine [13]. Se, via the functions of seleno-dependent enzymes and selenoproteins, plays an essential role in redox reactions and male reproduction as it is highly expressed in the testes [14]. Se is an essential component of glutathione peroxidase (GPx) selenoprotein P (Sel P), thioredoxin reductase (TXNRD), and selenoprotein V (Sel V) which are highly expressed in the testes [15, 16]. The GPx has been documented as an important antioxidant for spermatogenesis [15]. Moreover, Abedelahi et al in an in vitro study showed that Se reduced ROS levels and increased the total antioxidant capacity and GPx activity resulting in improved follicular development [17]. Furthermore, Se confers protection on ischemia/reperfusion injury in a rat ovary model [18]. Li et al. showed that dietary selenium-deprived chicks exhibited poor development of testis and impaired sex hormone synthesis. It also reduces antioxidant enzyme activity and increased the expressions of mRNA and protein autophagy-related factors [19]. Se enhanced reproductive performance, progesterone levels, and animal health [20]. It improves semen quality and reproductive hormone concentration of Saanen goat kids [21].
Zn, another trace element with atomic number 30, is essential as a cofactor in stabilizing proteins structurally and it is useful in enzymatic catalysis. The antioxidant capacity of Zn has been associated with its ability to induce metallothioneins, confer protection on protein sulfhydryls, and also reduce the formation of hydroxyl radicals through the antagonism of redox-sensitive transition metals [7, 22]. Deficiency of Zn has been linked to insufficient dietary intake, malabsorption, chronic liver and renal diseases, and other sicknesses [23]. Deficiency in Zn in the male reproductive system has been associated with impotence, hypogonadism, or delayed sexual development [22, 24] whereas several pathological conditions like abnormal ovarian development, prolonged gestation period, impaired synthesis and/or secretion of follicle-stimulating hormone and luteinizing hormone (LH), and disruption of the menstrual cycle have been reported for female [25, 26]. Zn supplements have been used for cancer prevention and it regulates primary ovarian tumor growth and metastasis [27] and also for a reduction of prostate size in benign prostate hyperplasia [28]. Zn supplementation can improve testosterone levels and sexual function in postmenopausal women [29].
Although several studies have been reported on male and female reproductive organs, and the effects of Se and Zn supplementation on them, yet there is a paucity of information on the role of Se and Zn on the reproductive organs of rats subjected to postnatal protein malnutrition. We hypothesize that Se and Zn supplementation reverses biochemical alterations arising from postnatal protein malnutrition in male and female reproductive organs. Therefore, the study was carried out to investigate biochemical parameters and the effects of Se and Zn supplementation on the male and female reproductive organs of rats exposed to postnatal protein malnutrition.
Materials and Methods
Animal and Diets
Seventy-eight rats, 39 male and 39 female weanlings Wistar rats (3–4 weeks old), were procured from Animal House, University of Lagos Teaching Hospital (LUTH), Lagos, Nigeria. The rats were housed in polypropylene cages and were allowed to acclimatize for 2 weeks. The rats were fed with commercial rat chow and water during the period and maintained at 12 h of light-dark cycles at room temperature throughout the experiment. The procedures on animal handling were approved by the Animal Ethical Committee of Olabisi Onabanjo University, Ogun State, Nigeria, and were following the principle of NIH Guidelines for Humane Use and Care of Laboratory Animals. The protein malnourished diet (PMD) contained 5% casein while the adequate protein diet (APD) contained 16% casein. The other components of the PMD diets include carbohydrates, vitamins, fats, and minerals mixture; all these are in sufficient amounts with an adequate amount of iodine (32.5 mg per 100 g) as previously reported by Adebayo et al. [7, 30].
Experimental Protocols
Male and female weanling rats were randomly assigned to six groups respectively after acclimatization. The APD rats which serve as the control group contained five rats per group and were fed with 16% casein diet, while the PMD rats contained eight rats per group and were fed with 5% casein diet. After the 8th week of feeding, Se (sodium selenite; Na2SeO3) and Zn (zinc sulfate; ZnSO4·7H2O) were supplemented at a concentration of 0.15 mg−1L and 227 mg−1L respectively in drinking water (for both male and female groups) for 3 weeks as previously reported by Adebayo et al. [7, 31]. Both the APD and PMD rats continue with their respective diets until the 11th week when the experiment was terminated and rats were sacrificed.
The grouping and treatments are as follows:
Group A: APD containing 16% casein diet.
Group B: PMD containing 5% casein diet.
Group C: APD containing 16% casein diet was supplemented with Se (0.15 mg−1L).
Group D: PMD containing 5% casein diet was supplemented with Se (0.15 mg−1L)
Group E: APD containing 16% casein diet was supplemented with Zn (227 mg−1L)
Group F: PMD containing 5% casein diet was supplemented with Zn (227 mg−1L).
The rats were weighed every week to determine the growth curve of body weights and to evaluate the effect of Se and Zn supplementation on the weight by sex.
At the end of the treatments, blood samples from male and female rats were collected via ocular sinus using micro-hematocrit capillary tubes. The blood samples were carefully kept in sterilized anticoagulant-free bottles and centrifuged at 5000 rpm for 5 min using Eppendorf Centrifuge 5415R. The plasma collected was refrigerated and stored at −20 °C to be used later for lipid profile and hormonal assays.
Testes and Ovaries Collection
Male and female rats were sacrificed after diethyl ether anesthesia by decapitation and the testes were excised from males and ovaries were removed from female rats in each of the groups. The testes and ovaries were kept in the freezer at −20 °C until used.
Sample Preparation for Biochemical Estimations
Ten percent (w/v) testis and ovary homogenates were prepared in 0.1 M phosphate buffer, pH 7.4, by using a Potter-Elvehjem-type glass homogenizer. The homogenates were centrifuged at 5000 rpm in a cold centrifuge for 5 min. The supernatants were kept at −20 °C and used for various biochemical assays.
Biochemical Assays
The oxidative markers were assessed to determine the level of oxidative stress in the testes and ovaries of PMD-fed rats and the effects of Se and Zn supplementation on them by analyzing the following parameters.
Estimation of Lipid Peroxidation Level
Malondialdehyde (MDA), a by-product of lipid peroxidation (LPO), was quantified by measuring the amount of MDA formed as described by Varshney and Kale [32]. The method is based on the formation of pink chromophores when malondialdehyde is treated with 2-thiobarbituric acid. Briefly, 0.5 mL of 30% trichloroacetic acid (TCA) was added to a mixture of homogenate (0.4 mL from the supernatant of tissue samples) and 1.6 mL Tris-KCl buffer. This was followed by the addition of 0.5 mL of 0.75% thiobarbituric acid and then incubated in a water bath for 45 min at 80 °C. The mixture, after it was cooled down in ice, was centrifuged at 3000 ×g. The clear supernatant was collected and absorbance was measured against a reference blank of distilled water at 532 nm. The results were expressed as micromoles/milligram of protein using a molar extinction coefficient chromophore of 1.56 × 10−5 M−1 cm−1.
Estimation of Nitric Oxide Level
Nitric oxide (NO) in conjunction with other reactive oxygen species contributes to oxidative stress. NO level was determined as described by Tracey et al. [33]. Fifty microliters of the sample supernatants was mixed with 1.5 mL of 0.1 M phosphate-buffered saline and 0.5 mL of Griess’ reagents and the reaction mixtures were incubated at 25 °C for 15 min. The pink chromophore formed was measured at 540 nm and the results were expressed as micromoles per milligram of protein.
Estimation of Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity in the supernatants of both the testis and ovary was determined as described by Mistra and Fridovich [34] based on the inhibition of autoxidation of epinephrine (pH 10.2) at 30 °C. The assay mixture contained 20 μL of sample and 2.5 mL of 0.05 M carbonate buffer (pH 10.2). After equilibration in the spectrophotometer, 300 μL of freshly prepared solution of 0.3 mM epinephrine was added and mixed by inversion. The increase in absorbance at 480 nm was monitored in a spectrophotometer for 150 s at 30-s intervals. The activity of SOD was expressed as units/milligram of protein.
Estimatimation of Catalase Activity
Catalase (CAT) activity in the tissue supernatants was determined following the procedure of Sinha [35]. The diluted sample was added to the mixture of hydrogen peroxide-phosphate. The principle is based on the formation of chromic acetate when hydrogen peroxide reacts with a dichromate-glacial acetic mixture at 100 °C. Briefly, 1 mL of 0.01 M phosphate buffer (pH 7.0), 0.1 mL of sample, and 0.4 mL of 0.2 M H2O2 were mixed. This was followed by the addition of 2 mL of dichromate-glacial acetic acid reagent (5% potassium dichromate and glacial acetic acid in a ratio of 1:3). The absorbance of the decomposition of hydrogen peroxide when acted upon by CAT and the reduction in the green coloration was measured at 570 nm. The results were expressed as micromoles/minute/milligram of protein using an extinction coefficient of 40 M−1 cm−1.
Estimation of Glutathione Peroxidase Activity
GPx activity was determined as described by Ellman [36]. The reaction mixture contained 200 μL of 0.4 M phosphate buffer (pH 7.0), 100 μL of 10 mM sodium azide, 200 μL of 4 mM reduced glutathione, 100 μL of 0.2 mM hydrogen peroxide, and 200 μL of sample. The mixture was incubated at 37 °C for 10 min and the reaction stopped by adding 400 μL of 10% TCA and was later centrifuged at 5000 rpm. One milliliter of the supernatant was added to 0.5 mL of Ellman’s reagent and 3 mL of 0.2 M phosphate buffer (pH 8.0). The absorbance was read at 412 nm. The results were expressed as μmol/min/mg protein.
Estimation of Glutathione-S-Transferase Activity
Glutathione-S-transferase (GST) activity was determined according to Habig et al [37]. Briefly, the assay mixture containing 0.03 mL of tissue supernatant, 2.79 mL of 0.1 M sodium phosphate buffer (pH 7.4), and 0.15 mL of 20 mM 1-chloro-2, 4,-dinitrobenzene in 95% alcohol was incubated at 37 °C for 5 min and then 0.03 mL of 20 mM glutathione (GSH) was added and mixed by inversion. The mixture was immediately read at 340 nm against a blank containing all the components except the sample for 180 s at 60-s intervals in a spectrophotometer. The results were expressed as micromoles/minute/milligram of protein using a molar extinction coefficient of 9.6 × 103 M−1 cm−1.
Estimation of Reduced Glutathione Level
The level of glutathione (GSH) in the testis and ovary was estimated by the method of Sedlak and Lindsay [38]. The method is based on the development of a relatively stable yellow complex formed from the reaction between Ellman’s reagent and free sulphydryl groups that absorbs maximally at 412 nm. The absorbance at 412 nm is proportional to the level of GSH in the sample. Briefly, 2 mL of 10% TCA was added to 1 mL of the sample and centrifuged at 5000 rpm for 10 min. One milliliter of the supernatant was added to the mixture of 0.5 mL of 0.01 M Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) and 3 mL of 0.2 M phosphate buffer (pH 8.0). The absorbance was read at 412 nm and the results were expressed as nanomoles/milligram of protein using 1.34 × 104 M−1 cm−1 as the molar extinction coefficient.
Estimation of Vitamin C Level
Vit C level was determined as described by Roe and Kuether [39]. To 0.1 mL of tissue supernatant, 0.3 mL of 6% TCA was added, stirred, allowed to stand for 5 min, and then centrifuged. To the supernatant was added 0.15 g of acid-washed norit, vigorously stirred, and filtered. To the filtrate, 0.1 mL of 0.1 M 2,4-dinitrophenylhydrazine was added and incubated at 37 °C for 3 h. The color was produced by adding 0.4 mL of 85% H2SO4 and then incubated for 30 min. The color developed was read at 540 nm and results were expressed as milligrams per 100 g.
Estimation of Vitamin E Level
The level of Vit E in the tissue sample was estimated as described by Baker et al. [40]. Lipid was extracted from the tissues according to Folch et al. [41]. 0.5 mL of lipid extract from the tissue was added to 1.5 mL of absolute ethanol and 2 mL of petroleum ether. The mixture was centrifuged at 3500 rpm for 5 min and the supernatant was evaporated to dryness at 80 °C. To this was added 0.5 mL of 0.2% 2–2′dipyridyl solution and 0.1 mL of 0.5% ferric chloride and kept in the dark for 5 min before 4 mL of butanol was added. The developed color was read at a wavelength of 520 nm and the result was expressed as milligrams per 100 g.
Determination of Na+-K+-ATPase Activity
The activity of Na+-K+-ATPase was determined according to the method of Lardy and Wellman [42] with slight modification. Briefly, 0.5 mL of sample was added to 0.4 mL of reaction medium (65 mM Tri-HCl buffer (pH 7.4), 0.5 mM KCl, 25 mM sucrose), and 0.1 mL of 1 mM ATP. The reaction was incubated in a shaker at 25 °C for 30 min and later stopped by the addition of 1 mL of 10% TCA. The mixture was centrifuged at 3000 rpm for 5 min. Zero-time tubes were deproteinized by the addition of chilled 1 mL of 10% TCA before the addition of ATP. All tubes were chilled after deproteinization and analyses for inorganic phosphate were made by the procedure of Stewart [43]. The supernatant was measured at a wavelength of 660 nm and the results were expressed as nanomoles Pi/minute/milligram of protein.
Hormonal Level Determination
Estimations of Testosterone and Progesterone
Considering the importance of sex hormones, testosterone (the primary male hormone that is required for spermatogenesis and the activities of Sertoli cells) and progesterone (a progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis) levels were estimated in the plasma using the ELISA Assay kits (DiaMetra, Spello (PG), Italy; Cat. No: DCM002-10 and DCM006-8; for testosterone and progesterone respectively). Competitive immunoenzymatic colorimetric procedures for the quantitative determination of total testosterone and progesterone concentrations in the plasma were carried out according to the manufacturer’s manuals. The absorbance was read at 450 nm against the respective blank within 5 min.
Estimation of Lipid Profile
Cholesterol (CHOL), high-density lipoprotein (HDL), triglycerides (TG), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) were estimated in the plasma using Randox kits. Assays were carried out as described in the kits’ manuals. VLDL and LDL were obtained through calculation as described by Njike et al. [44].
Statistical Analyses
The significant difference between the evaluations of the rats subjected to protein restriction compared to the controls with adequate diet was evaluated. All data are expressed as the mean ± standard deviation and were analyzed using one-way analysis of variance (ANOVA). Post hoc multiple comparisons to assess the significant differences between the means across the groups were determined using Duncan multiple range test, and values with p < 0.05 were considered statistically significant. IBM SPSS (Statistical Package for the Social Sciences) software version 23 (Armonk, NY) was used for the analysis.
Results
Effect of Protein Malnourished Diet, Se, and Zn Supplementation on the Growth Curve of Body Weights of Male and Female Rats
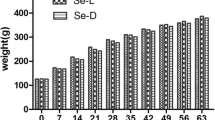
The effect of PMD on the growth curve of body weights of male and female rats is presented in Fig. 1 A and B. At the end of the 11th week of feeding, male and female PMD-fed rats showed significant reductions in their body weights as compared to APD-fed rats. Se and Zn supplementation for 3 weeks did not have significant effects on both PMD- and APD-fed rats.
The growth curve of body weights of protein malnourished diet (PMD)- and adequate protein diet (APD)-fed rats. The body weights of male PMD rats fed with 5% casein diet versus the body weights of male APD rats fed with 16% casein diet (A). The body weights of female PMD rats fed with 5% casein diet versus the body weights of female APD rats fed with 16% casein diet (B). Both males and females were supplemented with Se (sodium selenite; Na2SeO3) and Zn (zinc sulfate; ZnSO4·7H2O) at a concentration of 0.15 mg−1L and 227 mg−1L respectively in drinking water. APD n = 5, PMD n = 8. The level of significance was assessed at p < 0.05
Effect of Se and Zn Supplementation on the Oxidative Markers in the Testes and Ovaries of Protein Malnourished and Adequate Protein Diet–Fed Rats
Oxidant Parameters
Lipid Peroxidation
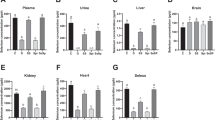
As presented in Fig. 2 A, the level of MDA in the testes of PMD-fed rats did not show any significant effect compared to APD-fed rats whereas a significant reduction was observed in the ovaries when compared to APD-fed rats. Se and Zn supplementation, in APD-fed rats compared to non-supplemented APD-fed rats, significantly reduced the level of MDA in the testes and in the ovaries. Se and Zn supplementation did not affect MDA level in PMD-fed rats when compared to the non-supplemented PMD-fed rats in the testes but a significant increase was observed after Se supplementation in the ovaries.
Oxidative markers and the effects of Se and Zn supplementation in the testes and ovaries of PMD-fed and APD-fed rats. The level of MDA, a by-product of lipid peroxidation (A), assessment of NO level (B), the activity of superoxide dismutase (SOD) (C), and the activity of catalase (CAT) (D) and supplementation with Se and Zn were assessed in the testes and ovaries of PMD- and APD-fed rats. APD n = 5, PM n = 8. *PMD significantly different from APD group; **APD treated significantly different from untreated group; ##PMD treated significantly different from untreated group (p < 0.05)
Nitric Oxide
As shown in Fig. 2 B, there was a significant increase in the level of NO in the testes of PMD-fed rats compared to APD-fed rats. Supplementation with Se and Zn significantly reduced the level of NO in the testes of APD-fed rats but only Se reduces the level of NO in the testes of PMD-fed rats while Zn supplementation increases it. In the ovaries, there was a significant increase in the level of NO in PMD-fed rats compared to the APD-fed rats. Supplementation with Se and Zn significantly reduced the level of NO in both the ovaries of PMD- and APD-fed rats.
Antioxidants Parameters
As shown in Fig. 2 C and D, there were significant reductions in the activities of SOD and CAT in the testes of PMD-fed rats compared to APD-fed rats. Se and Zn supplementation increased the activities of SOD and CAT in the testes of both PMD- and APD-fed rats. Also, the activity of CAT was significantly increased in the ovaries of PMD-fed rats compared to APD-fed rats. Supplementation with Se and Zn significantly increased the ovarian SOD activity in both PMD- and APD-fed rats.
The activities of GPx and GST, as presented in Fig. 3 A and B, were significantly reduced in the testes of PMD-fed rats compared to the APD-fed rats. Se and Zn supplementation significantly increased the activity of GST in the testes of PMD- and APD-fed rats. Se increased the activity of GPx in the testes of PMD- and APD-fed rats and Zn supplementation increased GPx activity only in the testes of APD-fed rats. On the other hand, PMD did not affect GPx activity in the ovaries when compared to the control rats but there was a significant reduction in the activity of GST in the ovaries of PMD-fed rats when compared to APD-fed rats. However, supplementation with Se and Zn significantly increased the activities of both GPx and GST in the ovaries of both PMD- and APD-fed rats. Moreover, there were significant reductions in the level of GSH (Fig. 3C) in both the testes and ovaries of PMD-fed rats when compared to the APD-fed rats. Supplementation with Se and Zn to PMD- and APD-fed rats significantly increased the GSH levels in both tissues.
Antioxidant parameters and the effect of Se and Zn supplementation in the testes and ovaries of PMD and APD rats. The activity of glutathione peroxidase (A), the activity of glutathione-S-transferase (B), the level of reduced glutathione (C), and the level of vitamin C (D), and supplementation with Se and Zn were assessed in the testes and ovaries of PMD and APD rats. APD n = 5, PM n = 8. *PMD significantly different from APD group; **APD treated significantly different from untreated group; ##PMD treated significantly different from untreated group (p < 0.05)
Assessment of vitamins C (Fig. 3D) and E (Fig. 4A) in PMD-fed rats showed significant reductions when compared to the APD-fed rats in both the testes and ovaries. Zn supplementation significantly increased the levels of both vitamins in the testes and ovaries of rats fed PMD and APD. Se increased vitamin C levels in the testes and ovaries of PMD- and APD-fed rats. Se also increased vitamin E levels in the testes of PMD- and APD-fed rats whereas Se only increase vitamin E levels in the ovaries of PMD-fed rats.
Assessment of vitamin E (A), the activity of Na+-K+-ATPase (B) supplemented with Se and Zn in the testes and ovaries of PMD- and APD-fed rats. APD n = 5, PM n = 8. *PMD significantly different from APD group; **APD treated significantly different from untreated group; ##PMD treated significantly different from untreated group (p < 0.05)
Effect of Se and Zn Supplementation on Na+-K+-ATPase Activity in the Testes and Ovaries of Protein Malnourished and Adequate Protein Diet–Fed Rats
As shown in Fig. 4 B, the activity of Na+-K+-ATPase in the testes and ovaries of PMD-fed rats was not statistically different from APD-fed rats. While there was a significant increase in the ovarian Na+-K+-ATPase activity in Se- or Zn-supplemented APD-fed rats as well as a significant increase in Zn-supplemented PMD-fed rats, the activity of testicular Na+-K+-ATPase in Se- or Zn-supplemented rats does not affect PMD- and APD-fed rats.
Effect of Se and Zn Supplementation on the Hormonal Level of Protein Malnourished and Adequate Protein Diet–Fed Rats
Table 1 shows that testosterone and progesterone levels were significantly reduced in PMD-fed rats when compared to APD-fed rats. Supplementation with Se significantly increased testosterone levels in both APD- and PMD-fed rats. Also, Zn supplementation increased the level of testosterone in PMD-fed rats. Furthermore, progesterone level was significantly increased in PMD- and APD-fed rats after Zn supplementation. In the Se-supplemented rats, the progesterone level was increased in the PMD-fed rats.
Effect of Se and Zn Supplementation on Lipid Profile in the Testes and Ovaries of Protein Malnourished and Adequate Protein Diet–Fed Rats
Lipid profiles from the plasma of male and female rats, as presented in Table 2 shows significant reductions in CHOL, HDL, and LDL in the plasma of male PMD-fed rats and significant reductions in HDL, VLDL, and TG in the plasma of female PMD-fed rats when compared to the APD-fed rats. Se supplementation significantly decreased plasma levels of HDL, and LDL in both males and females and CHOL in females but a significant increase in TG was observed in both males and females and VLDL only in male APD-fed rats. Moreover, Se supplementation to PMD-fed rats significantly reduced plasma CHOL, LDL, VLDL, and TG in females and significantly increased plasma HDL levels in females. Also, Zn supplementation in male APD-fed rats significantly decreases CHOL, HDL, VLDL, LDL, and TG while it increases all the parameters in the plasma of female APD-fed rats. Zn supplementation to the PMD-fed rats significantly increased CHOL and HDL in the plasma of both males and females rats and the increase in the levels of VLDL, LDL, and TG was only observed in the female while the LDL and TG levels were reduced in the plasma of male PMD-fed rats.
Discussion
The present study was carried out to evaluate the effect of Se and Zn supplementation on the male and female reproductive organs of rats subjected to postnatal protein malnutrition. Protein is an important nutrient containing essential amino acids that are required for biological functions. Evidence has shown that reproductive maturation and function are influenced by protein malnutrition which is accompanied by changes in testicular structure resulting in a reduction in daily sperm production and/or has a permanent effect on their capacity to produce spermatozoa [2, 45].
Protein Malnourished Diet, Se, and Zn Supplementation on the Body Weights of Male and Female Rats
Body weight has been associated with reproductive potential in animals [46]. The finding from this study showed a significant decrease in the body weights of male and female PMD-fed rats as compared to the APD-fed rats’ counterparts. Reduction in the body weights of PMD-fed rats has previously been reported as a demonstration of the importance of protein for normal development [47]. Moreover, reduction of body weight due to PM has been linked with appetite failure and metabolic disturbances, consequently leading to increased transamination or deamination of amino acids arising from increased endogenous protein degradation [48]. Supplementation with Se and Zn did not have any significant effect on the body weights of both male and female rats fed with either low-protein or adequate-protein diets. There are conflicting reports about the effect of Se and Zn on body weight. Hasani et al. [49] reported that Se improves weight control in obese rats and this was corroborated by another report showing that sodium selenite attenuated increased body weight in high-fat-diet-fed mice [50]. Some have shown that Se in high doses has been associated with weight gain [51]. Similarly, some have reported an increase in body weight and some reduction after Zn supplementation [49]. However, the present study corroborated the earlier report that Se and Zn supplementation does not affect body weight [7]. The reason for these conflicting results may be attributed to dosage, route of administration, duration of exposure, the type and species of animals used, and probably some other mechanisms which unfortunately are not the focus of this present study but may serve as proposed future studies.
Se and Zn Supplementation on Oxidative Markers in the Testes and Ovaries of Protein Malnourished and Adequate-Protein-Diet-Fed Rats
Oxidative stress has been implicated with compromised reproduction and fertility and this includes impaired ovarian functions, deteriorated oocyte quantity, and gynecological disease [52]. In this study, PMD did not influence the testicular level of LPO products whereas a reduction in the ovarian level of LPO products was observed. This result contradicts the normal trends that are usually observed in PMD-fed rats [7, 53, 54] and the reason for this discrepancy could not yet be ascertained. However, NO levels increased in both tissues. NO, produced from l-arginine by NO synthase, is a signaling molecule that is essential for normal cellular function. In this case, NO is acting as a potent oxidant species because it contains an unpaired electron in its highest orbital making it a highly reactive molecule. In the presence of superoxide anion (O2·−), NO is converted to peroxynitrite (ONOO−) which exerts pro-oxidant actions more than NO itself and this can modify lipids, proteins, and DNA [7, 55]. The report has shown that increased NO production has been linked to PM resulting in a higher rate of apoptosis after cytokines or oxidative stress aggregation [56]. Se reduced NO level in the testes and ovaries of both PMD- and APD-fed rats.
Se prevents peroxynitrite formation from NO and controls the level of cellular free radicals via its various selenoproteins. Reduction in NO level by Se has been reported in rat models of ischemia/reperfusion injury in various tissues and a rat ovary model [18, 57]. Se was also reported to protect against NO production and gene expression of inflammatory cytokines in chicken splenic lymphocytes induced by cadmium [58] The antioxidant property of Zn was demonstrated by reducing the level of NO in the ovaries of PMD-fed rats. Zn exerts its protective role through the formation of metallothionein complex thereby protecting against free radical attack. The exact mechanism by which Se and Zn lower NO is not yet fully understood but a study reported that Se attenuates an increase in cytokines expression by inhibiting the binding of nuclear factor kappa B (NF-κB) and thus decrease NO production [59]. Likewise, Zn has been shown to downregulate mRNA and protein expressions of inducible nitric oxide synthase (iNOS) and decreased cytokine-mediated activation of the iNOS promoter which has been attributed to inhibit NF-κB transactivation activity [60].
Additionally, data from the present study show reductions in the activities of SOD, CAT, GPx, and GST in the testicular tissue of PMD-fed rats. A similar observation had earlier been reported for the cortex and cerebellum of PMD-fed rats [7]. Studies have shown that the balance between ROS and antioxidants greatly influences reproductive activities in male and female animals [61, 62]. Supplementation with Se and Zn increased the activities of SOD, GPx, and GST in both tissues of APD- and PMD-fed rats. Zn is a cofactor for SOD activity and it might exert its antioxidant property through the formation of metallothionein which protects against free radical attack. Moreover, Se is incorporated into selenocysteine (SeCys) and then forms part of selenoenzymes which includes glutathione peroxidase (GPx). The protection of GPx against free radical attack is due to the induction of its selenoproteins property and thereby increasing its total catalytic activity [63, 64]. Se and Zn supplementation increased the level of CAT in the testes in both diets but maintained its high activity in the ovaries only in the PMD-fed rats, as observed in the study, protecting the ovaries against free radical attack. PMD reduced the level of GSH in both the testes and the ovaries. The level of cellular GSH is a function of several factors including consumption through the formation of conjugates via glutathione-S-transferase, which may be contributing to the reduction in GST level as observed in this study: de novo synthesis, the extent of oxidation, and reduction of oxidized GSH by glutathione reductase [65]. Se and Zn increased the level of GSH and GST in both the testes and ovaries of APD- and PMD-fed rats. The antioxidant role of Zn has been associated with its competitive effect on iron and copper thereby preventing them from producing toxic hydroxyl radicals. Zn also prevents protein oxidation by binding to the thiol (–SH) group of some proteins [66].
Vitamin C is an essential nutrient with antioxidant properties to protect tissues from oxidative damage [67]. Also, vitamin E acts as an antioxidant and a free radical scavenger by protecting fatty acids mainly phospholipids in the plasma membrane from oxidation by ROS and is considered the core of the antioxidant system [68]. The level of vitamins C and E was reduced in the testes and ovaries of PMD-fed rats. Similar reduction and or deficiency in the level of vitamin C has been reported in critically ill patients despite recommended enteral and parenteral intakes [69]. Supplementation with Se and Zn increased the level of vitamins C and E in both testes and ovaries of APD- and PMD-fed rats. The efficacy of vitamin E, for example, has been reported to be strongly dependent on the action of ascorbic acid and other dietary components such as Se and carotenoids [68, 70, 71]. We may say that a positive correlation exists between Se and Zn supplementation and enhancement of vitamins C and E levels which might be contributing to improving reproductive organ functions by protecting them against free radical attacks.
Se and Zn Supplementation on Na+-K+-ATPase Activity in the Testes and Ovaries of Protein Malnourished and Adequate-Protein-Diet-Fed Rats
Na+/K+-ATPase is an enzyme that regulates ROS and intracellular calcium signaling among several other functions. The present study showed that PMD does not have any significant effect on the activity of Na+/K+-ATPase either in the testes or in the ovaries. A previous report by Calderon Guzman et al. [72] showed that PMD causes alteration in the activity of Na+/K+-ATPase in the brain of rats. The discrepancy might be due to differences in the tissues, animal species, and duration of administration. However, Se and Zn supplementation increases ovarian Na+/K+-ATPase activity. The reason why Se and Zn are not influencing testicular Na+/K+-ATPase activity awaits further investigation.
Se and Zn Supplementation on the Hormonal Level of Protein Malnourished and Adequate-Protein-Diet-Fed Rats
The finding from this study shows that PMD lowers the levels of testosterone and progesterone. Testosterone is an integral component of the hypothalami-pituitary gonadal axis which functions to modulate the release of gonadotropins by the anterior pituitary gland. The reduction in testosterone level observed in this study correlates with the finding of Oliveira and co-workers. They observed that testosterone production was lower in rats subjected to a low-protein diet. Similarly, maternal protein malnutrition has been implicated in lower progesterone levels in rats [73]. Supplementation with Se and Zn increased testosterone and progesterone levels in PMD-fed rats. Several reports have shown the influence of Se on testosterone and progesterone levels. For example, Se supplementation to goat kids and rams showed a marked increase in the level of testosterone and this was attributed to increasing responsiveness of Leydig cells to gonadotropins which are stimulated by luteinizing hormone (LH) receptor mechanisms controlling the storage and release of testosterone [21, 74]. Moreover, Dkhil et al. [75] corroborated that Se nanoparticles elevated testosterone levels in streptozotocin-diabetic rats which have been associated with stimulation of LH and thus positively affects the biosynthesis of testosterone. Furthermore, reports have shown that Se supplementation increased plasma progesterone in pregnant heifers [76] as well as in postpartum dairy cows [77]. In addition, Saccharomyces cerevisiae enriched with Se enhanced progesterone levels in local Iraqi female goats [20]. Zn supplementation modulated and increased the level of testosterone in the blood serum [20, 29]. As reported for Se, Saccharomyces cerevisiae enriched with Zn also enhanced progesterone levels in local Iraqi female goats [20]. Increased progesterone level was also shown in ovariectomized rats supplemented with Zn as compared to its ovariectomized control rats [78].
Se and Zn Supplementation on Lipid Profile in the Testes and Ovaries of Protein Malnourished and Adequate-Protein-Diet-Fed Rats
Malnutrition causes lipid metabolism disorder [79] in both males and females. The increase in TG, as observed in male PMD-fed rats, might be associated with the increase in VLDL and reduction in HDL. Also, the reductions in TG and HDL in female PMD rats were accompanied by increased LDL. The major carrier of triglycerides in the plasma is VLDL. The increase in VLDL may be a major factor in increasing the TG level observed. In addition, the increase in triglyceride levels may also be due to the decrease in the activity of lipoprotein lipase; an enzyme that hydrolyzes triglycerides in chylomicrons. Xue et al. [79] have shown that undernutrition triggered severe lipid metabolism disorder and causes enhanced fatty acid oxidation, ketogenesis, and TG synthesis. Supplementation with Se reduced the levels of CHOL, VLDL, LDL, and TG with an increase in HDL levels in the plasma of female PMD-fed rats. Reductions in these parameters have been reported by Hasani et al. [80] and they concluded that Se supplementation could decrease TG and VLDL levels. A reduction in LDL, as well as an increase in the level of HDL after Se supplementation, was also reported [80, 81]. Conversely, supplementation with Zn increases CHOL in the plasma of male and female PMD-fed rats as well as an increase in VLDL, LDL, and TG in the plasma of female rats only. The reason for the increase cannot be presently explained. However, Zn increased HDL levels in both male and female PMD-fed rats and reduced TG, LDL, and VLDL levels in male PMD-fed rats. An increase in HDL and reduction in TG levels were reported in type 2 diabetes mellitus after Zn supplementation [82]. Moreover, dietary supplementation of Zn oxide and Zn methionine was reported to reduce triglyceride and LDL cholesterol levels in the blood of laying hens [83].
One of the main limitations of the study is that we did not study the combined effects of Se and Zn. It can be speculated that there could be synergy between the action of Se and Zn and the mechanism could involve the antioxidant action of both trace elements. Studies have reported the possibility of potential interaction and mutual influence between Se and Zn and that Zn intake alone may increase Se levels [49, 84]. This study provides further insight into the role of Se and Zn supplementation in the reproductive organs of postnatal protein-malnourished rats. However, an explanation of the synergistic effects of Se and Zn on the male and female reproductive organs of rats exposed to protein-depleted diets awaits further study. Given the antioxidant capacity of Se and Zn, and given that most of the problems derived from protein malnutrition are associated with oxidative stress, it is suggested to promote the use of these trace elements in protein-malnourished populations; since they mediate vital biochemical reactions, acting as cofactors of many enzymes, in addition to acting as centers to stabilize enzymatic and protein structures, related not only to their nutritional status but also to their reproductive potential.
Conclusion
The results of this present study indicate that postnatal protein malnutrition alters the lipid profile, increases the level of oxidants, and reduces the antioxidant status, as well as hormonal levels in the testicles and ovaries. Se and Zn supplementation, however, reversed almost all the alterations observed in the parameters analyzed suggesting that Se and Zn supplementation might protect the reproductive organs of rats against postnatal protein malnutrition.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adebayo OL, Khera A, Sandhir R, Adenuga GA (2016) Reduced expressions of calmodulin genes and protein and reduced ability of calmodulin to activate plasma membrane Ca2+-ATPase in the brain of protein undernourished rats: Modulatory roles of selenium and zinc supplementation. Cell Biochem Funct 34:95–103. https://doi.org/10.1002/cbf.3168
Genovese P, Núñez M, Pombo C, Bielli A (2010) Undernutrition during foetal and post-natal life affects testicular structure and reduces the number of sertoli cells in the adult rat. Reprod Domest Anim 45:233–236. https://doi.org/10.1111/j.1439-0531.2008.01244.x
Elmaz Ö, Cirit Ü, Keser O et al (2007) Effect of two dietary protein levels on testosterone, testicular parameters and semen quality in ram lambs during pubertal development. Med Weter 63:1177–1180
Hanai M, Esashi T (2007) The interactive effect of dietary protein and vitamin levels on the depression of gonadal development in growing male rats kept under disturbed daily rhythm. J Nutr Sci Vitaminol 53:138–144
Karaca F, Dönmez HH, Karsli MA (2003) Effects of protein deficiency on testosterone levels, semen quality and testicular histology in the developing male rat. Scand J Lab Anim Sci 30:7–9
de Morais Oliveira DA, Lupi LA, Silveira HS, de Almeida Chuffa LG (2021) Protein restriction during puberty alters nutritional parameters and affects ovarian and uterine histomorphometry in adulthood in rats. Int J Exp Pathol 102:93–104. https://doi.org/10.1111/iep.12388
Adebayo OL, Adenuga GA, Sandhir R (2014) Postnatal protein malnutrition induces neurochemical alterations leading to behavioral deficits in rats: prevention by selenium or zinc supplementation. Nutr Neurosci 17:268–278. https://doi.org/10.1179/1476830513Y.0000000090
Ziolkowski N, Grover AK (2010) Functional linkage as a direction for studies in oxidative stress: α-adrenergic receptorsThis review is one of a selection of papers published in a Special Issue on Oxidative Stress in Health and Disease. Can J Physiol Pharmacol 88:220–232. https://doi.org/10.1139/Y10-013
Agarwal A, Gupta S, Sikka S (2006) The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol 18:325–332. https://doi.org/10.1097/01.gco.0000193003.58158.4e
Adebayo O, Adenuga G (2012) Oxidative damage on the testes of adult rats by sodium metabisulfite (MBS). Int J Biol Chem Sci 6. https://doi.org/10.4314/ijbcs.v6i2.17
Gautam B, Deb K, Banerjee M et al (2008) Serum zinc and copper level in children with protein energy malnutrition. Mymensingh Med J 17:S12–S15
González-Reimers E, López-Lirola A, Olivera RM et al (2003) Effects of protein deficiency on liver trace elements and antioxidant activity in carbon tetrachloride-induced liver cirrhosis. Biol Trace Elem Res 93:127–140. https://doi.org/10.1385/BTER:93:1-3:127
Jackson MI, Combs GF (2008) Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care 11:718–726. https://doi.org/10.1097/MCO.0b013e3283139674
Hoffmann PR, Hoge SC, Li P-A et al (2007) The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res 35:3963–3973. https://doi.org/10.1093/nar/gkm355
Zhou J-C, Zheng S, Mo J et al (2017) Dietary Selenium deficiency or excess reduces sperm quality and testicular mRNA abundance of nuclear glutathione peroxidase 4 in rats. J Nutr 147:1947–1953. https://doi.org/10.3945/jn.117.252544
Atif F, Yousuf S, Agrawal SK (2008) Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol 600:59–63. https://doi.org/10.1016/j.ejphar.2008.09.029
Abedelahi A, Salehnia M, Allameh AA, Davoodi D (2010) Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod 25:977–985. https://doi.org/10.1093/humrep/deq002
Bozkurt S, Arikan DC, Kurutas EB et al (2012) Selenium has a protective effect on ischemia/reperfusion injury in a rat ovary model: biochemical and histopathologic evaluation. J Pediatr Surg 47:1735–1741. https://doi.org/10.1016/j.jpedsurg.2012.03.053
Li M, Zhang Y, Li S (2020) Effects of selenium deficiency on testis development and autophagy in chicks. Ital J Anim Sci 19:753–761. https://doi.org/10.1080/1828051X.2020.1786739
Shareef MA, Mohammed TR, Alrawi HM (2021) Impact of Saccharomyces cerevisiae enriched with selenium or zinc on reproductive performance, estrogen and progesterone hormone in local Iraqi female goats. IOP Conf Ser: Earth Environ Sci 761:012095. https://doi.org/10.1088/1755-1315/761/1/012095
Mojapelo MM, Lehloenya KC (2019) Effect of selenium supplementation on attainment of puberty in Saanen male goat kids. Theriogenology 138:9–15. https://doi.org/10.1016/j.theriogenology.2019.06.044
Bhowmik D, Bhattacharjee C, Kumar S (2010) A potential medicinal importance of zinc in human health and chronic disease. Int J Res Pharm Biomed Sci 1:5–11
Prasad AS (2003) Zinc deficiency: has been known of for 40 years but ignored by global health organisations. BMJ 326:409–410. https://doi.org/10.1136/bmj.326.7386.409
Murarka S, Mishra V, Joshi P, Kumar S (2015) Role of zinc in reproductive biology—an overview. Aust J Reprod Med & Infert 2:1009
Wang H, Hu Y-F, Hao J-H et al (2015) Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Sci Rep 5:11262. https://doi.org/10.1038/srep11262
Cummings JE, Kovacic JP (2009) The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care 19:215–240. https://doi.org/10.1111/j.1476-4431.2009.00418.x
Zhang R, Zhao G, Shi H et al (2020) Zinc regulates primary ovarian tumor growth and metastasis through the epithelial to mesenchymal transition. Free Radic Biol Med 160:775–783. https://doi.org/10.1016/j.freeradbiomed.2020.09.010
Das K, Buchholz N (2019) Benign prostate hyperplasia and nutrition. Clin Nutr ESPEN 33:5–11. https://doi.org/10.1016/j.clnesp.2019.07.015
Mazaheri Nia L, Iravani M, Abedi P, Cheraghian B (2021) Effect of zinc on testosterone levels and sexual function of postmenopausal women: a randomized controlled trial. J Sex Marital Ther 47:804–813. https://doi.org/10.1080/0092623X.2021.1957732
Adebayo OL, Adenuga GA, Sandhir R (2016) Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci 152. https://doi.org/10.1016/j.lfs.2016.03.008
Adebayo OL, Sandhir R, Adenuga GA (2015) Protective roles of selenium and zinc against postnatal protein-undernutrition-induced alterations in Ca2+-homeostasis leading to cognitive deficits in Wistar rats. Int J Dev Neurosci 43. https://doi.org/10.1016/j.ijdevneu.2015.03.007
Varshney R, Kale RK (1990) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58:733–743. https://doi.org/10.1080/09553009014552121
Tracey WR, Tse J, Carter G (1995) Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther 272:1011–1015
Misra HP, Fridovich I (1972) The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem 247:188–192
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Roe JH, Kuether CA (1943) The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem 147:399–407. https://doi.org/10.1016/S0021-9258(18)72395-8
Baker H, Frank O, De Angells B, Feingold S (1980) Plasma tocopherol in man at various times after ingesting free or acetylaned tocopherol. Nutr Rep Int 21:531–536
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Lardy HA, Wellman H (1953) The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem 201:357–370
Stewart DJ (1974) Sensitive automated methods for phosphate and (Na+ + K+)-ATPase. Anal Biochem 62:349–364. https://doi.org/10.1016/0003-2697(74)90167-5
Njike VY, Ayettey R, Petraro P et al (2015) Walnut ingestion in adults at risk for diabetes: effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res Care 3:e000115. https://doi.org/10.1136/bmjdrc-2015-000115
Hoppe CC, Evans RG, Bertram JF, Moritz KM (2007) Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 292:R1768–R1774. https://doi.org/10.1152/ajpregu.00442.2006
Bharadwaj S, Ginoya S, Tandon P et al (2016) Malnutrition: laboratory markers vs nutritional assessment. Gastroenterology Report gow013. https://doi.org/10.1093/gastro/gow013
Reyes-Castro LA, Rodriguez JS, Rodríguez-González GL et al (2011) Pre- and/or postnatal protein restriction in rats impairs learning and motivation in male offspring. Int J Dev Neurosci 29:177–182. https://doi.org/10.1016/j.ijdevneu.2010.11.002
Ajuogu PK, Al-Aqbi MA, Hart RA et al (2020) The effect of dietary protein intake on factors associated with male infertility: a systematic literature review and meta-analysis of animal clinical trials in rats. Nutr Health 26:53–64. https://doi.org/10.1177/0260106019900731
Hasani M, Saidpour A, Irandoost P et al (2021) Beneficial effects of Se/Zn co-supplementation on body weight and adipose tissue inflammation in high-fat diet-induced obese rats. Food Sci Nutr 9:3414–3425. https://doi.org/10.1002/fsn3.2203
Nido SA, Shituleni SA, Mengistu BM et al (2016) Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res 171:399–409. https://doi.org/10.1007/s12011-015-0552-8
Zeng M-S, Li X, Liu Y et al (2012) A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med 52:1335–1342. https://doi.org/10.1016/j.freeradbiomed.2012.01.017
Ávila J, González-Fernández R, Rotoli D et al (2016) Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reprod Sci 23:1656–1661. https://doi.org/10.1177/1933719116674077
Gavia-García G, González-Martínez H, Miliar-García Á et al (2015) Oxidative damage and antioxidant defense in thymus of malnourished lactating rats. Nutrition 31:1408–1415. https://doi.org/10.1016/j.nut.2015.05.014
Gavia-García G, de los Ángeles Rosas-Trejo M, García-Mendoza E et al (2018) t-BHQ protects against oxidative damage and maintains the antioxidant response in malnourished rats. Dose-Response 16:155932581879630. https://doi.org/10.1177/1559325818796304
Gopalakrishnan B, Nash KM, Velayutham M, Villamena FA (2012) Detection of nitric oxide and superoxide radical anion by electron paramagnetic resonance spectroscopy from cells using spin traps. J Vis Exp. https://doi.org/10.3791/2810
Theys N, Clippe A, Bouckenooghe T et al (2009) Early low protein diet aggravates unbalance between antioxidant enzymes leading to islet dysfunction. PloS One 4:e6110. https://doi.org/10.1371/journal.pone.0006110
Ahmad A, Khan MM, Ishrat T et al (2011) Synergistic effect of selenium and melatonin on neuroprotection in cerebral ischemia in rats. Biol Trace Elem Res 139:81–96. https://doi.org/10.1007/s12011-010-8643-z
Liu S, Xu F, Fu J, Li S (2015) Protective roles of selenium on nitric oxide and the gene expression of inflammatory cytokines induced by cadmium in chicken splenic lymphocytes. Biol Trace Elem Res 168:252–260. https://doi.org/10.1007/s12011-015-0354-z
Hseu Y-C, Wu F-Y, Wu J-J et al (2005) Anti-inflammatory potential of Antrodia Camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int Immunopharmacol 5:1914–1925. https://doi.org/10.1016/j.intimp.2005.06.013
Cortese-Krott MM, Kulakov L, Opländer C et al (2014) Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol 2:945–954. https://doi.org/10.1016/j.redox.2014.06.011
Kavak D, Satici Ö, Kavak V (2021) Comparison of the effects of maternal and postnatal application of protein malnutrition, testes morphology and spermatological parameters in adult rats. Acta Scientific Women’s Health 3:24–35
Wang S, He G, Chen M et al (2017) The role of antioxidant enzymes in the ovaries. Oxid Med Cell Longev 2017:1–14. https://doi.org/10.1155/2017/4371714
Ringuet MT, Hunne B, Lenz M et al (2021) Analysis of bioavailability and induction of glutathione peroxidase by dietary nanoelemental, organic and inorganic selenium. Nutrients 13:1073. https://doi.org/10.3390/nu13041073
Surai PF, Kochish II, Fisinin VI, Velichko OA (2018) Selenium in poultry nutrition: from sodium selenite to organic selenium sources. Poult Sci J 55:79–93. https://doi.org/10.2141/jpsa.0170132
Ji L, Nazarali A, Paterson P (2008) Protein–energy malnutrition increases activation of the transcription factor, nuclear factor κB, in the gerbil hippocampus following global ischemia☆. J Nutr Biochem 19:770–777. https://doi.org/10.1016/j.jnutbio.2007.09.011
Bao B, Prasad AS, Beck FWJ et al (2008) Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl Res 152:67–80. https://doi.org/10.1016/j.trsl.2008.06.001
Shah SA, Yoon GH, Kim H-O, Kim MO (2015) Vitamin C neuroprotection against dose-dependent glutamate-induced neurodegeneration in the postnatal brain. Neurochem Res 40:875–884. https://doi.org/10.1007/s11064-015-1540-2
Surai PF, Kochish II, Romanov MN, Griffin DK (2019) Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E. Poult Sci 98:4030–4041. https://doi.org/10.3382/ps/pez072
Carr AC, Rosengrave PC, Bayer S et al (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care 21:300. https://doi.org/10.1186/s13054-017-1891-y
Zingg J-M (2015) Vitamin E: a role in signal transduction. Annu Rev Nutr 35:135–173. https://doi.org/10.1146/annurev-nutr-071714-034347
Busso D, David A, Penailillo R et al (2021) Intake of vitamin E and C in women of reproductive age: results from the Latin American Study of Nutrition and Health (ELANS). Nutrients 13:1954. https://doi.org/10.3390/nu13061954
Calderón Guzmán D, Barragán Mejía G, Hernández García E, Juárez Olguín H (2006) Effect of nutritional status and ozone exposure on some biomarkers of oxidative stress in rat brain regions. Nutr Cancer 55:195–200. https://doi.org/10.1207/s15327914nc5502_11
Mulay S, Varma DR, Solomon S (1982) Influence of protein deficiency in rats on hormonal status and cytoplasmic glucocorticoid receptors in maternal and fetal tissues. J Endocrinol 95:49–58. https://doi.org/10.1677/joe.0.0950049
Kumar P, Yadav B, Yadav S (2013) Effect of zinc and selenium supplementation on antioxidative status of seminal plasma and testosterone, T 4 and T 3 level in goat blood serum. J Appl Anim Res 41:382–386. https://doi.org/10.1080/09712119.2013.783482
Dkhil M, Zrieq R, Al-Quraishy S, Abdel Moneim A (2016) Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules 21:1517. https://doi.org/10.3390/molecules21111517
Kamada H, Nonaka I, Takenouchi N, Amari M (2014) Effects of selenium supplementation on plasma progesterone concentrations in pregnant heifers. Anim Sci J 85:241–246. https://doi.org/10.1111/asj.12139
Kamada H (2016) Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian Australas J Anim Sci 30:347–354. https://doi.org/10.5713/ajas.16.0372
Sunar F, Baltaci AK, Ergene N, Mogulkoc R (2009) Zinc deficiency and supplementation in ovariectomized rats: their effect on serum estrogen and progesterone levels and their relation to calcium and phosphorus. Pak J Pharm Sci 22:150–154
Xue Y, Guo C, Hu F et al (2019) Maternal undernutrition induces fetal hepatic lipid metabolism disorder and affects the development of fetal liver in a sheep model. FASEB J 33:9990–10004. https://doi.org/10.1096/fj.201900406R
Hasani M, Djalalinia S, Sharifi F et al (2018) Effect of selenium supplementation on lipid profile: a systematic review and meta-analysis. Horm Metab Res 50:715–727. https://doi.org/10.1055/a-0749-6655
Jamilian M, Razavi M, Fakhrie Kashan Z et al (2015) Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol 82:885–891. https://doi.org/10.1111/cen.12699
Asbaghi O, Sadeghian M, Fouladvand F et al (2020) Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 30:1260–1271. https://doi.org/10.1016/j.numecd.2020.03.021
Abd El-Hack ME, Alagawany M, Salah AS et al (2018) Effects of dietary supplementation of zinc oxide and zinc methionine on layer performance, egg quality, and blood serum indices. Biol Trace Elem Res 184:456–462. https://doi.org/10.1007/s12011-017-1190-0
Guo C-H, Chen P-C, Hsu G-S, Wang C-L (2013) Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients 5:1456–1470. https://doi.org/10.3390/nu5041456
Acknowledgements
The authors appreciate the efforts of Mr. S.O. Adenekan for the technical assistance. We also thank the anonymous reviewers for their thoroughness and constructive criticisms.
Author information
Authors and Affiliations
Contributions
Olusegun Lateef Adebayo and Gbenga Adebola Adenuga contributed to the study conception and design. Also, the first draft of the manuscript was written by Olusegun Lateef Adebayo and all authors commented on previous versions of the manuscript. Adedayo Adedeji Obadimu carried out the major laboratory work and Adesewa Omolara Tugbobo-Amisu joined in data collection. Bamidele Sanya Fagbohunka joined in the supervision of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the NIH Guidelines for Humane Use and Care of Laboratory Animals. Approval was granted by the Ethics Committee of Olabisi Onabanjo University, Ogun State, Nigeria.
Consent to Participate
Not applicable
Consent to Publish
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obadimu, A.A., Adebayo, O.L., Tugbobo-Amisu, A.O. et al. Effect of Selenium and Zinc Supplementation on Reproductive Organs Following Postnatal Protein Malnutrition. Biol Trace Elem Res 202, 1126–1139 (2024). https://doi.org/10.1007/s12011-023-03751-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03751-8