Abstract
A 12 week feeding trial was conducted to evaluate the effects of dietary zinc levels on Heteropneustes fossilis. Triplicate groups of fish were fed isoproteic (CP; 400 g/kg) and isocaloric (GE; 17.89 kJ/g) diets increasing levels of zinc (0, 5, 10, 15, 20, 25, 30 mg/kg) achieved by supplementing zinc sulphate heptahydrate to basal diet. Analysed concentrations of zinc in diets were 10.68, 15.83, 21.34, 26.74, 30.61, 34.91 and 41.34 mg/kg. Growth indices increased linearly (P<0.05) up to 26.74 mg/kg Zn. The protein and ash content of whole body also improved significantly up to 26.74 mg/kg Zn. Whole body fat content showed inverse pattern. Haematological parameters also showed an improving trend with the increase in dietary zinc up to 26.74 mg/kg and then levelled off. Activities of antioxidant enzymes were improved with the increase in dietary zinc level up to 26.74 mg/kg followed by no significant change (P>0.05). Serum lysozyme activity also exhibited the similar pattern. Immune response in terms of the activities of lysozyme, alkaline phosphatase and myeloperoxidase was also improved with the increase in dietary zinc levels up to 26.74 mg/kg. Dietary zinc levels affected significantly the whole body as well as vertebrae mineralization. Broken-line regression analysis of weight gain, vertebrae zinc activity, serum superoxide dismutase and protease activity against increasing amounts of dietary zinc revealed that the inclusion of zinc in diet in the range of 26.82–29.84 mg/kg is optimum for growth, haematological indices, antioxidant status, immune response and tissue mineralization in fingerling H. fossilis. The information obtained from present study would be helpful in formulating the zinc-balanced commercial feeds to improve the growth and health status of this important fish, thus contributing to aquaculture production and strengthening the food security.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a dire need of increasing the production of quality protein to feed the continuously increasing global population. Fish is considered as an ideal source of quality protein, lipid and micronutrients eliminating nutrient deficiency diseases. Aquaculture industry is contributing substantially in achieving food and nutritional security. Due to increased demand and stagnation in the supply from capture fishery, global aquaculture production needs to be boosted. In order to enhance the aquaculture production, development of species specific nutritionally-balanced commercial feeds is an important perquisite. For developing such feeds, data on nutrient needs of fish species to be cultured is essential. Most of the research carried in the past focused on species-specific macronutrient requirements such as protein, amino acid and lipid but information on micronutrients like mineral and vitamin which are also important for fish survival, growth, health and reproduction [1, 2] are scanty. Among minerals, zinc (Zn) is a vital micronutrient involved in a several metabolic pathways such as immunity, growth, energy metabolism and protein synthesis [3,4,5,6,7,8,9,10]. It is also a cofactor for various enzymes such as alcohol dehydrogenase, alkaline phosphatase, carbonic anhydrase and DNA polymerase [8, 11, 12]. Its function in antioxidant mechanism and haemoglobin synthesis has also been reported [7, 13,14,15,16]. Reduced growth, high mortality, cataract and dwarfism are the main zinc deficiency signs [4, 6, 8, 11, 12, 15]. Additionally, zinc deficiency has been linked to low IGF-I, growth hormone binding protein mRNA and growth hormone receptor [17,18,19]. Zn also regulates the cellular activities by binding with specific membrane receptors, carriers and channels [20]. Zn is also involved in regulating cell proliferation, differentiation, apoptosis and the gene expression of metallothionein [21]. Furthermore, zinc status is also related to oxidative stress and interacts with various hormones [22, 23]. Excessive zinc levels not only increase the cost of the diet but also load it to the water body [24, 25] and may be toxic for fish [4, 12, 26]. Thus, inclusion of optimum amount of zinc in diet is essential to avoid zinc deficiency and toxicity in fish as well as in environment due to excess.
Aquaculture sector is facing several crucial challenges such as eutrophication and other water quality issue and diseases resulting to significant economic loss [27]. Hence, there is need to develop some strategies that can help to combat with these issues, so that there could be an efficient aquaculture production for the continuously growing population and the demand for quality protein, safe and healthy products for consumption. Minerals, organic substances, are one of the important nutrients. They participate is several crucial life processes such as skeleton formation, transmission of nerve impulse, maintenance of osmotic balance and muscle contraction etc. Among all the minerals, zinc is an essential trace element for all vertebrates including teleost. Zinc has several structural as well as functional roles regulate multiple metalloenzymes as a specific cofactor and catalyst. Deficiency of zinc can lead to several pathological conditions. Thus dietary supplementation of zinc is essential to meet the requirement and avoid zinc deficiency and can participate to combat with the effects of cellular stressors and other pathological factors.
Heteropneustes fossilis is freshwater, air breathing, omnivorous fish, commonly known as singhi. It has high demand in Southeast Asia and India [28,29,30,31,32]. It is hardy and can be cultured at high stocking rate [33, 34]. Consumers prefer it because of its taste and nutritional superiority due to high protein, low fat and high iron content [35]. Though, information on some of its nutrient requirement such as protein [36], vitamins [37], amino acids [38, 39] and minerals [29,30,31, 35] are available but information on its zinc requirement is not available. Hence, in present study effects of dietary zinc on growth, antioxidant status and immune response and optimum requirement of H. fossilis were evaluated.
Materials and Methods
Experimental Diets
Casein-gelatin-based isonitrogenous (400 g/kg CP) and isocaloric (17.89 kJ/g GE) diets containing graded concentrations of zinc (0, 5, 10, 15, 20, 25 and 30 mg/kg) were prepared by supplementing varying levels of zinc sulphate heptahydrate (Loba Chemie, pvt. Ltd.) at the cost of dietary cellulose (0, 21.9, 43.9, 65.9, 87.9, 109.9 and 131.9 mg/kg) to basal diet (Table 1). Diets were made in accordance with the previously established procedure by [35]. The diets were prepared and designated as Zn1, Zn2, Zn3, Zn4, Zn5, Zn6 and Zn7. Analysed values of zinc in these trial diets were 10.68, 15.83, 21.34, 26.74, 30.61, 34.91 and 41.34 mg/kg, respectively.
Experimental Design
Heteropneustes fossilis fingerling was shifted from fish hatchery to the feeding trial facility, disinfected with KMnO4 solution (1:3000), and distributes in indoor tanks. During the acclimation period of 2 weeks, fish were offered H-440 [40] diet (casein-gelatin-based, 40 % crude protein). Acclimatized fish (7.54 ± 0.05 g, 10.5 ± 0.1 cm) were randomly stocked at the rate of 30 fish in triplicate groups in tanks (water volume 55 L) with a water flow of 1–1.5 L/min. During the experiment, water quality parameters were analysed as per APHA [41] and recorded range for water temperature and pH were 26.6–28.5°C and 7.1–7.3, respectively. The values of other parameters such as ammonia nitrogen, oxygen, alkalinity and carbon dioxide were found to range between 0.25–0.30, 5.6–6.4, 66.3–75.4 and 6.3–10.4 mg/L, respectively. The level of zinc in water ranged between 1.81 and 2.43 μg/L.
Feeding Trial and Growth Measurements
Fish were fed semi-moist diets at 8:00 and 16:00 h until they seemed satiated. It was ensured that the feed given was eaten fully. Before each feeding, faeces were removed. Fish were not fed on the day of recording mass weight. All groups of fishes were measured anesthetized in 100 mg/L tricaine methanesulfonate and their mass weights recorded. Proximate composition was done using standard methods [42].
Sample Collection
Fish were killed with a fatal dosage (200 mg/L) of MS-222 at the start and end of the trial. Six sub-samples from a pooled sample of 20 fish were taken and assessed for initial and final body composition. Twelve fish per replicate were collected randomly, killed and kept in a freezer at −20°C for estimating the mineral and proximate composition of whole body. Length and weight of 8 fish per replicate were noted to calculate the condition factor (CF). Blood samples were obtained from above 8 fish of each replicate in heparinized syringe by puncturing caudal vein and pooled. Three sub-samples from each pooled sample were subjected to analysis. Haematological analysis including red blood corpuscles (RBCs), haemoglobin (Hb) and haematocrit (Hct) was done in accordance with the methods used earlier [30]. Upon collection of blood samples, the viscera and liver were dissected out to determine the viscerosomatic index (VSI) and hepatosomatic index (HSI). The intestine and liver from same fish were used for mineral and digestive enzyme analysis. To extract the vertebrae sample from surrounding flesh, the same fish was microwaved for around 5 min to separate the surrounding flesh. Vertebrae were taken out by gently removing the spines, washed, dried at 105°C and defatted using a Soxhlet apparatus and then ground for mineral analysis. Mineral analysis of diets, whole body, serum, liver, and vertebrae samples was done using inductively coupled plasma atomic emission spectroscopy (ICP-AES) technique (SPECTRO Analytical Instruments GmbH, Germany). Superoxide dismutase (SOD) and catalase (CAT) activities in serum were assessed by using the method of Misra and Fridovich [43] and Aebi [44], respectively. Serum glutathione peroxidase (GPx) activity was measured using the method of Rotruck et al. [45]. Malondialdehyde (MDA) content was established as per the method of Utley et al. [46] as adopted by Fatima et al. [47]. The protease activity was assessed by following the method described by Moore and Stein [48]. The lipase activity was determined as per the method adopted by Seligman and Nachlas [49]. Amylase activity was determined by utilizing soluble starch as a substrate [50]. The activity of serum alkaline phosphatase (ALP) was estimated colorimetrically by adopting the method of Apines et al. [51]. Serum myeloperoxidase activity was assessed following the method of Quade and Roth [52], and turbidimetric method of Hultmark et al. [53] as adopted by Wang et al. [54] was used to assess the lysozyme activity.
Growth Assessment
Growth assessment was done by standard formulae used previously [30] which are given below:
Weight gain (WG; %) = Final body weight − Initial body weight/Initial body weight × 100
Feed conversion ratio (FCR) = Dry feed intake (g) / Wet weight gain (g)
Specific growth rate (SGR; %/day) = ln (Final body weight) − ln (Initial body weight)/No. of days ×100
Protein retention efficiency (PRE; %) = Protein gain/Protein fed × 100.
Protein gain (PG; g/fish) = Final body protein (%) × Final body weight (g) − Initial body protein (%) × Initial body weight (g)
Apparent zinc retention (%) = Final body zinc content – Initial body zinc content/Total zinc fed × 100
Hepatosomatic index (HSI; %) = Liver weight (g)/Body weight (g) × 100
Viscerosomatic index (VSI; %) = Viscera weight (g)/Body weight (g) × 100
Condition factor (CF; g/cm3) = Body weight (g)/Body length (cm)3× 100.
Mean corpuscular haemoglobin concentration (MCHC; g/dL) = Hb (g/dL) ×100/ Hct (%)
Mean corpuscular haemoglobin (MCH; pg) =Hb (g/dL) × 10/RBC (×106/mm3)
Mean corpuscular volume (MCV; fL) = Hct (%) × 10/RBC (×106/mm3)
Statistical Analysis
Data were evaluated for normality (Shapiro-Wilk test) and variance homogeneity (Levene’s test) for equality before being subjected to a one-way analysis of variance. Tukey’s test (P<0.05) was performed in comparing the differences among treatment means. Data acquired on several parameters in relation to increasing amounts of dietary zinc were submitted to various statistical models. Due to the highest R2 value obtained in the Broken-line regression model [55] compared to other models, this model was used in determining the optimum dietary zinc requirements of H. fossilis. Origin (version 6.0; Origin Software, San Clemente, CA) was used to statistically analyse the growth data.
Results
Growth Performance
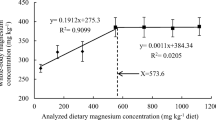
Results pertaining to growth parameters and conversion efficiencies of fingerling H. fossilis fed diets with different levels of zinc are listed in Table 2. Increase in dietary zinc significantly (P<0.05) affected the growth and conversion efficiencies of H. fossilis. Live weight gain (LWG, %), specific growth rate (SGR, %/day), protein retention efficiency (PRE, %), feed conversion ratio (FCR) and protein gain (PG, g/fish) improved with the increasing dietary zinc up to 26.74 mg/kg (Zn4) and remained relatively constant beyond that level. Broken-line regression analysis of LWG and PG against the increasing dietary zinc levels indicated the optimum zinc requirement for fingerling H. fossilis at 26.82 (Fig. 1) and 27.22 (Fig. 2) mg/kg of dry diet, respectively. Fish fed basal diet (Zn1) reflected maximum apparent zinc retention. Significant difference in zinc retention was evident in fish fed diet Zn1 and Zn2 followed by insignificant change (P>0.05) up to diet Zn4 and then significantly declined (P<0.05) to a minimum in fish fed diet containing 41.34 mg/kg zinc (Zn7).
Whole Body Composition and Biometric Indices
Effects different levels of zinc on body composition and biometric indices of H. fossilis are summarized in Table 3. Increase in the zinc significantly affected (P<0.05) whole body protein, fat and ash contents. However, moisture content did not differ significantly (P>0.05). A significant increase in whole body protein and ash was noted in fish fed diets with 10.68 mg/kg (Zn1) to 26.74 mg/kg zinc (Zn4) and then remained unchanged (P>0.05). Fat content was found to decrease (P<0.05) with the increase in dietary zinc concentration up to 26.74 mg/kg (Zn4) and then levelled off. Biometric indices were also significantly affected (P<0.05) by increasing dietary zinc levels. Hepatosomatic index (HSI) and viscerosomatic index (VSI) reflected a decreasing tendency (P<0.05) with incremental dietary zinc levels up to 26.74 mg/kg diet (Zn4) and then stabilized (P>0.05). However, condition factor (CF) improved (P<0.05) with the increase in dietary zinc levels up to 26.74 mg/kg (Zn4) and then steadied.
Haematological Indices
Dietary zinc levels affected the haematological status significantly (P<0.05) in terms of haemoglobin (Hb), red blood cell (RBCs) and haematocrit (Hct) as shown in Table 4. These parameters showed improvement (P<0.05) with the increasing concentration of zinc in the diet from 10.68 mg/kg (Zn1) to 26.74 mg/kg (Zn4) and further inclusion of zinc in the diet (Zn5-Zn7) did not change (P<0.05) the above parameter. MCH and MCV also showed the similar pattern. However, no change (P>0.05) in MCHC was noted.
Digestive Enzyme Activities
Intestinal protease, lipase and amylase activities were found to increase significantly (P<0.05) with the increasing dietary zinc levels up to 26.74 mg/kg (Table 4) indicating the improved intestinal health status. However, higher amounts of zinc did not reflect any significant change (P>0.05) in the activities of these enzymes. Broken-line regression of protease activity against increasing dietary zinc reflected the Zn requirement of fingerling H. fossilis to be 27.33 mg/kg (Fig. 3).
Whole Body and Tissue Mineralization
Significant difference in whole body, vertebrae and liver mineralization (P<0.05) were found in fish receiving diets with increasing levels of zinc (Table 5). The whole body zinc concentration increased (P<0.05) with the increase in dietary zinc up to 26.74 mg/kg diet (Zn4) and then stabilized. However, that in vertebrae increased up to 30.61 mg/kg (Zn5) beyond which it did not change (P>0.05). On the other hand, iron (Fe), copper (Cu) and calcium (Ca) in whole body and vertebrae reduced significantly (P<0.05) in fish fed above diets up to diet containing 30.61 mg/kg zinc compared to diets containing 10.68–26.74 mg/kg zinc. Liver Zn, Cu and Ca concentrations were not affected (P>0.05) by the increment in dietary zinc whereas Fe concentration tend to decline beyond the dietary zinc level of 26.74 mg/kg (Zn4). Regression analysis of vertebral zinc concentration against increasing dietary zinc levels revealed a need of 29.84 mg/kg (Fig. 4).
Antioxidant Enzyme Activities
Results are presented in Table 6. Increased amount of dietary zinc resulted into significant (P<0.05) increase in serum superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities up to 26.74 mg/kg, and further inclusion did not show any significant improvement in the activities of these enzymes. MDA content was found to be influenced by the dietary zinc levels. A significant (P<0.05) and continuous decrease in the MDA level was noted in fish fed diets with graded levels of dietary zinc. Dietary supplementation of zinc at the level of 26.74 mg/kg resulted to minimum MDA content, and no change (P>0.05) in the serum MDA levels was observed on further inclusion of dietary zinc. Broken-line regression analysis of serum SOD activity against increasing dietary zinc levels indicated the requirement at 26.66 mg/kg (Fig. 5).
Immune Parameters
Results pertaining to immune response of H. fossilis after feeding diets supplemented with zinc are presented in Table 6. Lysozyme activity increased (P<0.05) with the increase in dietary zinc concentration from 10.68 to 26.74 mg/kg and then remained unchanged. Myeloperoxidase (MPO) activity was also found to improve with increasing dietary zinc levels. Significantly higher (P<0.05) MPO activity was noted in fish fed diets with 26.74 mg/kg zinc compared to the group fed basal diet containing 10.68 mg/kg zinc. However, no significant change in MPO activity in fish fed higher concentration of zinc compared to those fed 26.74 mg/kg zinc was noted. Dietary zinc significantly affected the activity of alkaline phosphatase (ALP) enzyme. A significant increase (P<0.05) in the activity of enzyme was recorded with increasing dietary zinc concentration from 10.68 to 30.61 mg/kg. However, further inclusion of zinc in the diets at higher levels (34.91 and 41.34 mg/kg) did not show any significant change in the activity of ALP enzyme.
Discussion
The effects of rising concentration of zinc in the diets on growth, conversion efficiencies, haematological parameters, digestive enzymes activity, antioxidant status, mineralization and immune response vary among species due to several factors. During the present study, zinc concentration of rearing water ranging 1.81–2.43 μg/L and zinc-deficient diets containing suboptimal levels of diets was noted to be insufficient to meet the metabolic need of H. fossilis resulting in zinc deficiency signs such as poor growth, conversion efficiencies, antioxidant status, immune response, haematological parameters and reduced zinc concentration in the whole body as well as other tissues. In the present study, zinc supplementation in H. fossilis diet enhanced the growth and improved the above parameters. Role of zinc in controlling the insulin-like growth factor I (IGF-I) [15, 19, 56] and modulating the expression of intestinal genes [57] for the incorporation of minerals might be the reason for its stimulative effects on growth. On subjecting the protein gain, live weight gain, intestinal protease action and vertebral zinc data against incremental concentrations of dietary zinc to broken line regression analysis, the requirement was found to be at 27.22, 26.82, 27.33 and 29.84 mg/kg diet, respectively.
The dietary zinc requirement for H. fossilis (26.82–29.84 mg/kg) obtained in this study is comparable to that stated for other species such as Oreochromis niloticus (30 mg/kg), Clarias batrachus (30 mg/kg), Epinephelus malabaricus (28.9–33.7 mg/kg) and Acipenser baerii (29.24–34.7 mg/kg) [8, 12, 58] but higher than that reported for Pelteobagrus fulvidraco (17–21 mg/kg), Sciaenops ocellatus (20 mg/kg), Oreochromis aureus (20 mg/kg), Ictalurus punctatus (20 mg/kg), Cyprinus carpio (15 mg/kg) and Oncorhynchus mykiss (15 mg/kg) [26, 59,60,61,62] and lower than that of Rachycentron canadum (42.9 mg/kg), Lateolabrax japonicas (103 mg/kg), Cyprinus carpio var. jian (42–49) and Ctenopharyngodon idella (55) [3, 63,64,65]. Similar results of increasing weight gain up to optimum were also stated in a study conducted on common carp [60], grouper [8], Nile tilapia [58, 66], yellow catfish [26], Jian carp [65], channel catfish [61], grass carp [3] and Siberian sturgeon [7]. However, no significant improvement of dietary zinc supplementation on the growth of blue tilapia [62], Atlantic salmon [67] and European sea bass [68] was recorded. In the above fishes, sufficient availability of zinc from basal diet and/or rearing water meeting the requirement may be the reason for no improvement in growth of the fish fed zinc-supplemented diets. However, feeding extremely high levels of zinc (176.7 and 334 mg/kg) resulted in reduced growth in Nile tilapia [15]. This reduction in growth may be because of zinc toxicity.
Many proteases, such as carboxypeptidases A and B, require zinc as a cofactor to function properly [66, 69]. Improvement in PRE with increasing zinc levels in diets may be due to an increase in the activity of carboxypeptidase resulting in an improvement in protein digestion as zinc acts as an essential cofactor for several metalloenzymes which take part in protein metabolism [8, 70]. Several zinc deficiency symptoms reported in fish species include cataract, anorexia, poor development, dermatitis, short trunk, low tissue zinc content and high mortality [12,13,14,15, 71,72,73,74,75]. In the current study, no overt deficient symptoms were identified except for anorexia, poor development, impaired antioxidant capacity, immune response, lower tissue mineralization and low Hb.
The highest apparent zinc retention was recorded in fish fed Zn1diet (basal diet) followed by a significant reduction in the efficiency of retention which was noted in Zn2 diet. However, no significant differences were noted among fish fed Zn2, Zn3 and Zn4 diet suggesting that the zinc retention efficiency in fish fed these diets was almost the same. This may be because of reduced zinc utilization efficiency with the increase in dietary zinc. In grass carp, a similar trend of zinc retention in reaction to increased dietary zinc levels was observed [3]. Increased intestinal absorption was also seen in broilers fed low levels of manganese in diet [76]. Lin et al. [74], Zhang et al. [77] and Zafar and Khan [29] also reported similar patterns of retention in hybrid tilapia, large yellow croaker and stinging catfish fed insufficient levels of manganese, respectively. No significant differences in growth and whole body zinc concentration of H. fossilis fed diets containing higher than optimum level (26.74 mg/kg) were evident indicating that an extra amount of zinc was excreted and not toxic.
Whole body composition is known to reflect the quality of fish as a product and that is why it is regarded as a crucial parameter in nutritional studies. A significant rise in whole body protein with the increase in dietary zinc concentrations up to optimum (26.74 mg/kg) was evident, indicating maximum protein retention efficiency at this dietary level of zinc (Table 2). This could explain the importance of zinc as an essential component of many proteins and its role in stabilization as well as adjustment of protein structure and functioning [78,79,80]. Increasing zinc levels, on the other hand, lowered total body fat. The greater body fat content observed in this study on H. fossilis fed Zn-deficient diets was also noted in yellow catfish [81]. Carnitine palmitoyltransferase (CPT I) is a regulatory enzyme mainly involved in the oxidation of long-chain fatty acids [82]. Decreased hepatic CPT I action was noted in fish fed zinc-deficient diets [81]. Thus, inhibited CPT I activity in fish fed zinc-deficient diets may be due to less lipid utilization and more lipid deposition, while increasing activity of CPT I with increasing dietary zinc levels may result into increased lipolysis and decreased lipid deposition. Increased whole body fat in fish fed diets deficient in zinc was also recorded in the current study. The increase in dietary zinc levels did not affect the whole body moisture in H. fossilis. In hybrid tilapia, a similar tendency for total body hydration was observed [6]. Body ash in H. fossilis fed diets with increasing zinc increases up to Zn4 and then remained static. Several researchers such as Luo et al. [65] in yellow catfish and Moazenzadeh et al. [12] in Siberian sturgeon also noted comparable changes in whole body moisture, protein and fat of fish fed diets containing optimum level of zinc, whereas increasing dietary zinc levels did not exert any significant impact on whole body composition in studies conducted by Tan and Mai [83] on abalone and by Tan et al. [65] on Jian carp.
Biometric indices are generally used to demonstrate the health status of fish. HSI and VSI of H. fossilis decreased with the increase in dietary zinc up to 26.74 mg/kg diet. This decrease up to 26.74 mg/kg (Zn4) may be due to increased lipolysis and reduced deposition. However, no changes in HSI and VSI were reported with the increase of zinc in diet of yellow catfish [26], grass carp [3], Nile tilapia [15] and Siberian sturgeon [7]. Condition factor (CF) is an indicator of the robustness of fish which in H. fossilis increased up to optimum level and stabilized thereafter demonstrating that the fish were in good nutritional and physiological conditions at above dietary zinc level. Contrary to this, reduced CF was recorded in Siberian sturgeon fed higher levels of zinc in diets [7], whereas no major changes in the CF of yellow catfish [26] and grass carp [3] were reported with the increase in dietary zinc.
Tissue mineral concentration, enzyme activities and haematological indices are routinely used parameters in growth and nutritional studies [12,13,14,15, 60,61,62,63,64,65,66,67,68]. In the present study, digestive enzymes, haematological parameters, serum antioxidant enzymes activities, whole body, vertebrae and liver mineralization against increasing levels of dietary zinc were also used as response variables to assess the nutritional and physiological health status of H. fossilis. Digestive enzymes play a crucial role in the digestion of dietary components. Increased activity of intestinal enzymes is associated with enhanced digestive capability and growth performance. The activity level of fish digestive enzymes is regarded as a valuable comparative indication of food usage, digestive capability and growth performance [84, 85]. An increase in digestive enzyme activity, in the current study, with the increasing zinc concentrations in the diets up to optimum (Zn4) resulted in improved digestion and utilization of food and better intestinal health of fish. Several factors influence the activity of digestive enzymes, including life stage, diet, feeding management, and sampling time after feeding [86].
Haematological parameters are used in fish nutrition studies for describing or measuring the effects of diets on growth and physiological processes [6, 7, 15, 66]. In the current study, haemoglobin, RBCs, haematocrit, mean corpuscular haemoglobin and mean corpuscular volume increased with the rise in concentrations of dietary zinc up to 26.74 mg/kg (Zn4). The maximum values of the above parameters achieved at this level demonstrate that the inclusion of zinc at 26.74 mg/kg diet was adequate to optimize haematological parameters and prevent anaemic conditions. Haemoglobin production was reported to increase with increasing dietary zinc levels in this and other studies [7, 66, 87, 88]. Zinc plays a vital role in haemoglobin production through activation of enzyme D-aminolevulinic acid dehydrogenase, crucial for the production of porphobilinogen from two molecules of D-aminolevulinic acid [7]. Moreover, it is one of the important elements which affect the synthesis of superoxide dismutase and carbonic anhydrase in red blood cells [7, 15, 89]. Being an integral component of the antioxidant enzyme superoxide dismutase, zinc is crucial for erythrocyte membrane maintenance by protecting sulfhydryl groups from the harmful effects of superoxide free radicals [90].
Total body, spine and liver mineral levels have often been used to evaluate utilizable mineral intake in several studies [12, 15, 26, 51, 91]. Dietary zinc significantly affected mineralization in liver, vertebrae and whole body of H. fossilis. Whole body zinc concentration considerably enhanced with the increment in zinc up to 26.74 mg/kg diet (Zn4), and subsequently, at higher levels, no significant change was observed. Similar result was reported in P. fulvidraco [26, 81], C. idella [3] and Oryzias melastigma [5]. However, zinc concentration in vertebrae improved up to 30.61 mg/kg (Zn5) followed by no change. In Nile tilapia, a parallel increasing tendency was noted up to a specific threshold, followed by no significant difference in vertebral zinc concentration with increasing dietary zinc levels [15, 66]. Zinc stabilization in the whole body or vertebrae of fish receiving diets containing greater than optimal may be because of strong regulation of mineral homeostasis. The level of zinc in the liver of fish consumed diets containing increasing doses of zinc did not alter significantly. A similar pattern of liver zinc in reaction to rising dietary zinc levels was observed in O. niloticus [66], C. idella [3], E. malabaricus [8] and E. coioides [92]. A small quantity of zinc is retained in the liver and stomach, where it is connected to molecules known as metallothioneins. They promptly deliver zinc to the organism when it is required [92,93,94,95]. Hepatic metallothionein concentration due to its physiological relevance largely remains stable and does not vary with the source and zinc levels [96]. Thus, in the present study, constant liver zinc concentration with respect to an increase in dietary zinc points to the fact that the liver has strong regulation of zinc homeostasis.
Inclusion of dietary zinc beyond the requirement level (Zn4) decreased Fe, Ca and Cu concentrations in whole body and vertebrae which is in accordance with other studies [15, 97,98,99]. Clearwater et al. [100] reported that the inclusion of higher amounts of zinc than optimum hampered the assimilation of other elements such as Fe, Ca and Mg. In liver, the concentration of Fe was found to reduce when H. fossilis were fed more than 26.74 mg/kg zinc. In tilapia, there was a negative connection between zinc in the diet and liver Fe (59). Cousins and Mc Mahon [101] also reported that Zn and Fe compete for binding sites on the divalent cations transporter I. This may be the reason for the lower Fe concentrations in the total body, vertebrae and liver of H. fossilis fed diets with greater than optimal Zn levels. Concentrations of other minerals such as Zn, Cu and Ca in the liver were not significantly affected by increasing dietary zinc levels. No significant change in muscle Ca concentration of Nile tilapia [66] fed diets with rising levels of zinc was noted.
Reactive oxygen species (ROS) are produced as by-products of metabolism. Normally, there is an equilibrium between production and elimination of these oxygen free radicals, but when production is higher than elimination, then it causes oxidative stress. These intermediate species attack cell membranes resulting in lipid peroxidation. Excess ROS generation in the metabolic system causes oxidative stress, which allows infections to infiltrate and infect the host [102]. Such excess ROS generation results into rise in the action of several antioxidant enzymes like superoxide dismutase, catalase and glutathione peroxidase [103, 104] preventing the damaging effects of ROS and reducing lipid peroxidation [12]. Transmutation of superoxide radical into hydrogen peroxide and molecular oxygen is catalysed by superoxide dismutase. Furthermore, the catalase enzyme decomposes intracellular hydrogen peroxide (H2O2) into water and oxygen without creating free radicals. Antioxidant properties and involvement in the defence system are one of the crucial functions of zinc in fish [7, 105], and zinc is known to improve the antioxidant capability of fish by increasing the activities of catalase, glutathione peroxidase, and superoxide dismutase in the serum, liver, intestine, and muscle tissue of fish [12, 15, 106,107,108]. In the present study, increase superoxide dismutase and catalase activities in H. fossilis fed increasing dietary zinc up to optimum (Zn4) is in accordance with the results obtained in rainbow trout [109], yellow catfish [26], hybrid tilapia [6] and juvenile grouper [110]. However, contrary to our results, a decrease in CAT activity with the increase in dietary zinc concentrations was observed in Nile tilapia [15]. These authors speculated that the decrease in serum hydrogen peroxide due to the rise in superoxide dismutase and glutathione peroxidase activities may be the reason for reduced catalase activity with increased zinc concentration in diets.
Lipid peroxidation (LPO) is a damaging process caused by reactive oxygen species. The degree of LPO is measured by assessing the malondialdehyde (MDA) content which is the end product of LPO. The MDA content provides a well-situated index of lipid peroxidation [111] indicating the antioxidant capacity of fish. Serum MDA content was reduced with the increasing zinc levels up to the optimum level (Zn4). Fish receiving the optimum level of zinc in diet reflected lowest serum MDA content. There are several reports indicating the reduced MDA content in fish fed diets with adequate zinc. In juvenile Jian carp, suppressed lipid peroxidation and protein oxidation were observed by Feng et al. [106] when fed the optimum level of zinc. They concluded that optimum zinc improved the ability of Jian carp to scavenge hydroxyl (OH˙) and superoxide (O2-) free radicals which are the most toxic oxygen species involved in oxidative damage. Onderci et al. [112] in laying hens and Kucukbay et al. [113] in rainbow trout also reported lower liver MDA levels when fed optimum dietary zinc. Luo et al. [114] and Jiang et al. [107] also observed a parallel phenomenon of reduction in MDA level in yellow catfish and blunt snout bream, respectively, when fed diet with an optimum level of zinc. A similar phenomenon was observed by Wu et al. [75] in Nile tilapia receiving optimum dietary zinc, however, they also reported a slight increase in MDA content when higher levels (76.37 mg/kg and 89.2 mg/kg) of dietary zinc were fed.
Alkaline phosphatase (ALP) activity showed improvement up to 30.61 mg/kg zinc with increasing dietary zinc levels and then stabilized. ALP activity is a sensitive tool for assessing zinc status [107, 115]. ALP is a homodimeric metalloenzyme having two Zn ions and one Mg ion at each active site. Zinc is a cofactor of ALP which stimulates the osteoblasts for bone formation [116] and enzyme aminoacyl-tRNA synthetase for protein synthesis [117]. Increased vertebrae zinc concentration and serum ALP activity in the present study also point to the role of zinc in bone formation. It has been noted that decreased plasma ALP activity is related to zinc deficit in fish [3, 66]. The reduced ALP activity in H. fossilis fed on basal diet indicates that the amount of zinc was inadequate which was increased with the rise in dietary zinc concentration up to optimum level (Zn4) and then stabilized. A similar pattern of improved ALP activity with increasing dietary zinc levels was also observed in channel catfish [61], Atlantic salmon [25], cobia [63], hybrid tilapia [74], grass carp [3], Nile tilapia [75], blunt snout bream [107] and Siberian sturgeon [12].
The immune system is a critical and essential defence mechanism. The environment has a large number of microorganisms and has a significant impact on the health status of fish. Nonetheless, fishes defend themselves and maintain basic health conditions due to intrinsic and unique defence mechanisms. In fish, innate immunity is faster than adaptive immunity, which takes much longer in mammals and is regarded as a critical defensive mechanism against infectious infections [118].
Potent antibacterial proteins present in secretions and tissues such as the kidney, alimentary tract, spleen, mucus, gills and serum include myeloperoxidase (MPO) and lysozyme [119]. Lysozyme catalyses the breakdown of a glycosidic link in the bacterial cell wall between N-acetylmuramic acid and N-acetylglucosamine. Lysozyme, a common invertebrate immunological enzyme, may kill bacteria by destroying the peptidoglycan layer of bacterial cell walls [120]. Zinc is essential for maintaining a healthy immune system, including both specific and non-specific immunity [121]. Several studies have reported that lysozyme activity can be used as a tool to evaluate the immune status of organisms [122, 123]. In the current study, dietary zinc supplementation boosted lysozyme activity up to optimum, but higher levels of zinc in the diet did not affect lysozyme action in fish. Parallel improvement in the activity of lysozyme activity was noted in some other previously conducted studies on other fish species [124]. Paripatananont and Lovell [125] reported that the amount of Zn in the diet improved the channel catfish resistance to harmful microorganisms by activating immunological responses. Myeloperoxidase (MPO) is a key enzyme secreted by polymorphic nuclear neutrophils that produce deadly oxidants that disrupt nitric oxide-dependent signalling pathways in the vasculature [126]. MPO is a crucial enzyme for many fish species because it boosts macrophages and neutrophil activity in the blood [126,127,128]. During a respiratory burst, MPO uses hydrogen peroxide to make hypochlorous acid [129]. The highest MPO activity detected in H. fossilis fed Zn4 diet signifies its well-developed immune status.
Conclusion
Results of the present study demonstrate the necessity of supplementing zinc in diet. Based on the findings of this study, inclusion of zinc in the range of 26.82–29.84 mg/kg diet is optimum for growth, conversion efficiencies, haematological parameters, antioxidant status, intestinal health, mineralization and immune response of H. fossilis.
Data Availability
All data utilized for this trial and to support the findings are available within the article.
References
Giatsis C, Sipkema D, Smidt H, Heilig H, Benvenuti G, Verreth J, Verdegem M (2015) The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci Rep 5:18206
Aliko V, Qirjo M, Sula E, Morina V, Faggio C (2018) Antioxidant defense system, immune response and erythron profile modulation in gold fish Carassius auratus after acute manganese treatment. Fish Shellfish Immunol 76:101–109
Liang JJ, Yang HJ, Liu YJ, Tian LX, Liang GY (2012) Dietary zinc requirement of juvenile grass carp (Ctenopharyngodon idella) based on growth and mineralization. Aquac Nutr 18:380–387
Lin S, Lin X, Yang Y, Li F, Luo L (2013) Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp (Litopenaeus vannamei). Aquaculture 406–407:79–84
Wang LG, Li EC, Qin JG, Du ZY, Yu N, Kong YQ, Chem LQ (2015) Effect of oxidized fish oil and α-tocopherol on growth, antioxidation status, serum immune enzyme activity and resistance to Aeromonas hydrophila challenge of Chinese mitten crab Eriocheir Sinensis. Aquac Nutr 21:414–424
Li MR, Huang CH (2016) Effect of dietary zinc level on growth, enzyme activity and body trace elements of hybrid tilapia, Oreochromis niloticus× O. aureus, fed soy bean meal-based diets. Aquac Nutr 22:1320–1327
Moazenzadeh K, Islami RH, Zamini A, Soltani M (2017) Dietary zinc requirement of Siberian sturgeon (Acipenser baerii, Brandt 1869) juveniles, based on the growth performance and blood parameters. Int Aquac Res 9:25–35
Houng Yung C, Yu Chun C, Li Chi H, Meng Hsien C (2014) Dietary zinc requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 432:360–364
Lall SP, Kaushik SJ (2021) Nutrition and metabolism of minerals in fish. Animals 11(9):2711
Yarahmadi S, Silva MS, Holme MH, Morken T, Remø S, Araujo P, Lock EJ, Waagbø R, Antony Jesu Prabhu P (2022) Impact of dietary zinc and seawater transfer on zinc status, availability, endogenous loss and osmoregulatory responses in Atlantic salmon smolt fed low fish meal feeds. Aquaculture 549:737804
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp.Washington. National Academies Press, DC, p 376
Moazenzadeh K, Rajabi Islami H, Zamini A, Soltani M (2018) Effects of dietary zinc level on performance, zinc status, tissue composition and enzyme activities of juvenile Siberian sturgeon, Acipenser baerii (Brandt 1869). Aquac Nutr 24:1330–1339
Trevisan R, Flesch S, Mattos JJ, Milani MR, Bainy ACD, Dafre AL (2014) Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp Biochem Physiol C Toxicol Pharmacol 159:22–30
Yu HR, Li LY, Shan LL, Gao J, Ma CY, Li X (2021) Effect of supplemental dietary zinc on the growth, body composition and anti-oxidant enzymes of coho salmon (Oncorhynchus kisutch) alevins. Aquac Rep 20:100744
Huang F, Jiang M, Wen H, Wu F, Liu W, Tian J, Yang C (2015) Dietary zinc requirement of adult Nile tilapia (Oreochromis niloticus) fed semi-purified diets, and effects on tissue mineral composition and antioxidant responses. Aquaculture 439:53–59
Houman KM, Abasali RI, Soltani ZM, Moazenzadeh K, Islami ÁHR, Islami HR, Zamini A, Soltani M (2017) Dietary zinc requirement of Siberian sturgeon (Acipenser baerii, Brandt 1869) juveniles, based on the growth performance and blood parameters. Int Aquac Res 9:25–35
Clegg MS, Keen CL, Donovan SM (1995) Zinc deficiency—induced anorexia influences the distribution of serum insulin-like growth factor—binding proteins in the rat. Metab Clin Exp 44:1495–1501
McNall AD, Etherton TD, Fosmire GJ (1995) The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin-like growth factor I and growth hormone receptor genes. J Nutr 125:874–879
Ekinci D, Ceyhun SB, Aksakal E, Erdoğan O (2011) IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C 153:336–341
Swain PS, Rao SB, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2:134–141
Colvin RA, Bush A, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR (2008) Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Physiol Cell Physiol 294:726–742
Baltaci AK, Mogulkoc R, Baltaci SB (2019) Review: the role of zinc in the endocrine system. Pak J Pharm Sci 32:231–239
Cunha TA, Vermeulen Serpa KM, Grilo EC, Leite Lais L, NetoJ B, Vale SHL (2022) Association between zinc and body composition: an integrative review. J Trace Elem Med Biol 71:126940
Buentello JA, Goff JB, Gatlin DM (2009) Dietary zinc requirement of hybrid striped bass, Morone chrysops × Morone saxatilis, and bioavailability of two chemically different zinc Compounds. J World Aquacult Soc 40:687–694
Maage A, Julshamn K (1993) Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture 117:179–191
Luo Z, Taz XY, Zheng JL, Chen QL, Liu X (2011) Quantitative dietary zinc requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture 319:150–155
Abdel-Latif HMR, Abdel-Tawwab M, Khafaga AF, Dawood MAO (2020) Dietary origanum essential oil improved antioxidative status, immune-related genes, and resistance of common carp (Cyprinus carpio L.) to Aeromonas hydrophila infection. Fish Shellfish Immunol 104:1–7
Farhat Khan MA (2017) Growth, feed conversion and body composition of fingerling stinging catfish Heteropneustes fossilis (Bloch) fed varying levels of dietary l-threonine. Aquac Res 48:2355–2368
Zafar N, Khan MA (2019) Growth, feed utilization, mineralization and antioxidant response of stinging catfish Heteropneustes fossilis fed diets with different levels of manganese. Aquaculture 509:120–128
Zafar N, Khan MA (2020) Effects of dietary iron on growth, haematology, oxidative stress and hepatic ascorbic acid concentration of stinging catfish Heteropneustes fossilis. Aquaculture 516:734642
Zafar N, Khan MA (2021) Effects of dietary magnesium supplementation on growth, feed utilization, nucleic acid ratio and antioxidant status of fingerling Heteropneustes fossilis. Animal Feed Sci Tech 273:114819
Puvaneswari S, Marimuthu K, Karuppasamy R, Haniffa MA (2009) Early embryonic and larval development of Indian catfish, Heteropneustes fossilis. Euras J Bios 3:84–96
Vijayakumar C, Shridhar S, Haniffa MA (1998) Low cost breeding and hatching techniques of catfish (Heteropneustes fossilis) for small scale farmers. Naga 21:15–17
Haniffa MA, Shridhar S (2002) Induced spawning of spotted murrel (Channa punctatus) and catfish (Heteropneustes fossilis) using human chorionic gonadotropin and synthetic hormone (ovaprim). J Vet Arch 72:51–56
Zafar N, Khan MA (2018) Determination of dietary phosphorus requirement of stinging catfish Heteropneustes fossilis based on feed conversion, growth, vertebrae phosphorus, whole body phosphorus, haematology and antioxidant status. Aquac Nutr 24:1577–1586
Siddiqui TQ, Khan MA (2009) Effects of dietary protein levels on growth, feed utilisation protein deposition efficiency and body composition of young Heteropneusteus fossilis (Bloch). Fish Physiol Biochem 35:479–488
Mohamed SJ, Ibrahim A (2001) Quantifying the dietary niacin requirement of the Indian catfish, Heteropneustes fossilis (Bloch) fingerlings. Aquac Res 32:157–162
Farhat Khan MA (2013) Dietary L-lysine requirement of fingerling stinging catfish, Heteropneustes fossilis (Bloch) for optimizing growth, feed conversion, protein and lysine deposition. Aquac Res 44:523–533
Farhat Khan MA (2014) Total sulfur amino acid requirement and cystine replacement value for fingerling stinging catfish, Heteropneustes fossilis (Bloch). Aquaculture 426-427:270–281
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, SanDiego, CA, pp 61–141
APHA (1992) Standard Methods for the Examination of Water and Wastewater. (21st edn). American Public Health Association, American Water Works Association, and Water Pollution Control Federation, Washington, DC
AOAC (2005) Official Methods of Analysis of Association of Official Analytical Chemists (AOAC) International Method 950.46, Method 920.153, Method 985.29, Method 960.39. 18. AOAC, Gaithersburg, MD, USA
Misra H, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biolumin Chemilumin 247:3170
Aebi H (1984) Catalase in vitro. Methods in Enzymol 105:121–126
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Utley HC, Bernheim F, Hachslien PH (1967) Effects of sulfhydryl reagent on peroxidation in microsome. Arch Biochem Biophys 260:521–531
Fatima M, Ahmad I, Sayeed I, Athar M, Raisuddin S (2000) Pollutant-induced over-activation of phagocytes is concomitantly associated with peroxidative damage in fish tissues. Aquat Toxicol 49:243–250
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388
Seligman AM, Nachlas MM (1963) Lipase. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis. Academic Press, London, pp 776–778
Bernfeld P (1955) Amylases-a and b. In: Colowick SP, Kaplan K (eds) Methods in Enzymology, vol 1. Academic Press, New York, pp 149–150
Apines Amar MJS, Satoh S, Kiron V, Watanabe T, Aoki T (2003) Availability of supplemental amino acid chelated trace elements in diets containing tricalcium phosphate and phytate to rainbow trout Oncorhynchus mykiss. Aquaculture 225:431–444
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239–248
Hultmark D, Steiner H, Rasmuson T, Boman HG (1980) Insect immunity, Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 106:7–16
Wang J, Wang WX (2015) Optimal dietary requirements of zinc in marine medaka Oryzias melastigma: Importance of daily net flux. Aquaculture 448:54–62
Robbins KR, Saxton AM, Southern LL (2006) Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci 84:155–165
Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM (1996) Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am J Clin Nutr 63:514–519
Blanchard RK, Moore JB, Green CL, Cousins RJ (2001) Modulation of intestinal gene expression by dietary zinc status: effectiveness of cDNA arrays for expression profiling of a single nutrient deficiency. PNAS 98:13507–13513
Eid AE, Ghonim SI (1994) Dietary zinc requirement of fingerling Oreochromis niloticus. Aquaculture 119:259–264
Ogino C, Yang GY (1978) Requirement of rainbow trout for dietary zinc. Bull Jpn Soc Sci Fish 44:1015–1018
Ogino C, Yang GY (1979) Requirement of carp for dietary zinc. Bull Jpn Soc Sci Fish 45:967–969
Gatlin DM, Wilson RP (1983) Dietary zinc requirement of fingerling channel catfish. J Nutr 113:630–635
McClain WR, Gatlin DM (1988) Dietary zinc requirements of Oreochromis aureus and effects of dietary calcium and phytate on zinc bioavailability. J World Aquacult Soc 19:103–108
Xu Z, Dong X, Liu C (2007) Dietary Zinc requirement of juvenile cobia (Rachycentron canadum). Fisheries Sci 26:138–141
Zhou LB, Zhang W, Wang AL, Ma XL, Zhang HF, Liufu YZ (2009) Effects of dietary zinc on growth, immune response and tissue concentration of juvenile Japanese seabass Lateolabrax japonicus. Oceanologia et Limnologia Sinica/Hai Yang Yu Hu Chao 40(1):42–47
Tan LN, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquacult Nutr 17:338–345
Do Carmo E, Sá MV, Pezzato LE, Ferreira Lima MMB, De Magalhães PP (2004) Optimum zinc supplementation level in Nile tilapia Oreochromis niloticus juveniles diets. Aquaculture 238:385–401
Maage A, Julshamn K, Berge GE (2001) Zinc gluconate and zinc sulphate as dietary zinc sources for Atlantic salmon. Aquac Nutr 7:183–187
Fountoulaki E, Morgane H, Rigos G, Antigoni V, Mente E, Sweetman J (2010) Evaluation of zinc supplementation in European sea bass (Dicentrarchus labrax) juvenile diets. Aquac Res 41:208–216
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Apines Amar MJS, Satoh S, Kiron V, Watanabe T, Aoki T (2003) Availability of supplemental amino acid chelated trace elements in diets containing tricalcium phosphate and phytate to rainbow trout Oncorhynchus mykiss. Aquaculture 225:431–444
Satoh S, Takeuchi T, Watanabe T (1987) Availability to rainbow trout of zinc in white fish meal and of various zinc compounds. Nippon Suisan Gakk 53:595–599
Ketola HG (1979) Influence of dietary zinc on cataracts in rainbow trout (Salmo gairdneri). J Nutr 109:965–969
Yamamoto H, Satoh S, Takeuchi T, Watanabe T (1983) Effects on rainbow trout of deletion of manganese or trace elements from fish meal diet. Nippon Suisan Gakkaishi 49:287–293
Lin YH, Jiang LC, Shiau SY (2008) Dietary zinc requirements of juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Fish Soc Taiwan 35:117–125
Wu YP, Feng L, Jiang WD, Liu Y, Jiang J, Li SH, Tang S, Kuang SY, Zhou XQ (2015) Influence of dietary zinc on muscle composition, flesh quality and muscle antioxidant status of young grass carp (Ctenopharyngodon idella Val.). Aquac Res 46:2360–2373
Bai S, Lu L, Luo X, Liu B (2008) Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poult Sci 87:2596–2604
Zhang HL, Sun RJ, Xu W, Zhou HH, Zhang WB, Mai KS (2016) Dietary manganese requirement of juvenile large yellow croaker Larimichthys crocea (Richardson, 1846). Aquaculture 450:74–79
Beattie JH, Kwun I (2004) Horizons in nutritional science: is zinc deficiency a risk factor for atherosclerosis? Br J Nutr 91:177–181
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Jain RB (2014) Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol Trace Elem Res 159:87–98
Zheng JL, Luo Z, Hu W, Liu CX, Chen QL, Zhu QL, Gong Y (2015) Different effects of dietary Zn deficiency and excess on lipid metabolism in yellow catfish Pelteobagrus fulvidraco. Aquaculture 435:10–17
Kerner J, Hoppel C (2000) Fatty acid import into mitochondria. Biochem Biophys Acta 1486:1–17
Tan BP, Mai KS (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 192:67–84
Ling J, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2010) Effect of dietary iron levels on growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 16:616–624
Qian J, Xiao L, Feng K, Li W, Liao C, Zhang T, Liu J (2022) Effect of dietary protein levels on the growth, enzyme activity, and immunological status of Culter mongolicus fingerlings. PLoS One 17(2):e0263507
García Meilan I, Ordóñez Grande B, Valentín JM, Hernández MD, García B, Fontanillas R, Gallardo MA (2016) Modulation of digestive and absorptive processes with age and/or after a dietary change in gilthead sea bream. Aquaculture 459:54–64
Sobhanirad S, Naserian AA (2012) Effects of high dietary zinc concentration and zinc sources on hematology and biochemistry of blood serum in Holstein dairy cows. Anim Feed Sci Technol 177:242–246
Eze JI, Ayogu LC, Abonyi FO, Eze UU (2015) The beneficial effect of dietary zinc supplementation on anaemia and immunosuppression in Trypanosoma brucei infected rats. Exp Parasitol 154:87–92
Hsieh SI, Castruita M, Malasarn D, Urzica E, Erde J, Page MD, Yamasaki H, Casero D, Pellegrini M, Merchant SS, Loo JA (2013) The proteome of copper, iron, zinc, and manganese micronutrient deficiency in Chlamydomonas reinhardtii. Mol Cell Proteomics 12:65–86
O’Dell BL, Browning JD, Reeves PG (1987) Zinc deficiency increases the osmotic fragility of rat erythrocytes. J Nutr 117:1883–1889
Nie JQ, Dong XH, Tan BP, Chi SY, Yang QH, Liu HY, Shuang Z (2016) Effects of dietary manganese sources and levels on growth performance, relative manganese bioavailability, antioxidant activities and tissue mineral content of juvenile cobia (Rachycentron canadum L). Aquac Res 47:1402–1412
Huang QC, Wang EL, Dong XH, Tan BP, Chi SY, Yang QH, Zhang S, Liu HY, Yang YZ (2018) Investigations on zinc bioavailability of different sources and dietary zinc requirement in juvenile grouper Epinephelus coioides. Aquac Res 49:2763–2773
Bremner I, Beattie JH (1990) Metallothionein and the trace minerals. Annu Rev Nutr 10:63–83
Mvdo CES, Pezzato LE, Mmbf L, Pdem P (2004) Optimum zinc supplementation level in Nile tilapia Oreochromis niloticus juveniles diets. Aquaculture 238:385–401
Patel RN, Singh N, Shukla KK, Gundla VL, Chauhan UK (2005) Synthesis, structure and biomimetic properties of Cu(II)-Cu(II) and Cu(II)- Zn(II) binuclear complexes: possible models for the chemistry of Cu-Zn superoxide dismutase. J Inorg Biochem 99:651–663
Henriques GS, Cozzolino SMF (2001) Determination of metallothionein levels in tissues of young rats fed zinc-enriched diets. Rev Nutr 13:163–169
Knox D, Cowey CB, Adron JW (1982) Effects of dietary copper and copper: zinc ratio on rainbow trout Salmo gairdneri. Aquaculture 27:111–119
Wekell JC, Shearer KD, Gauglitz EJ (1986) Zinc supplementation of trout diets: tissue indicators of body zinc status. Prog Fish Cult 48:205–212
Spry DJ, Hodson PV, Wood CM (1988) Relative contributions of dietary and waterborne zinc in the rainbow trout, Salmo gairdneri. Can J Fish Aquat Sci 45:32–41
Clearwater SJ, Farag AM, Meyer JS (2002) Bioavailability and toxicity of diet borne copper and zinc to fish. Comp Biochem Physiol 132:269–313
Cousins RJ, Mc Mahon RJ (2000) Integrative aspects of zinc transporters. J Nutr 130:1384
Paiva CN, Bozza MT (2014) Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal 20:1000–1037
Wu P, Liu Y, Jiang WD, Jiang J, Zhao J, Zhang YA, Zhou XQ, Feng L (2017) A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: different change patterns of varied antioxidant enzyme genes and Nrf2 signaling factors. PLoS One 12:1–21
Wang J, Xiao J, Zhang J, Chen H, Li D, Li L, Cao J, Xie L, Luo Y (2020) Effects of dietary Cu and Zn on the accumulation, oxidative stress and the expressions of immune-related genes in the livers of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 100:198–207
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130:1447–1454
Feng L, Tan LN, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr 17:875–882
Jiang M, Wu F, Huan F, Wen H, Liu W, Tian J, Yang C, Wang W (2016) Effects of dietary Zn on growth performance, antioxidant responses, and sperm motility of adult blunt snout bream, Megalobrama amblycephala. Aquaculture 464:121–128
Shi B, Xu F, Zhou Q, Regan MK, Betancor MB, Tocher DR, Sun M, Meng F, Jiao L, Jin M (2021) Dietary organic zinc promotes growth, immune response and antioxidant capacity by modulating zinc signaling in juvenile Pacific white shrimp (Litopenaeus vannamei). Aquacult Rep 19:100638
Hidalgo MC, Expósito A, Palma JM, Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193
Huang QC, Wang EL, Dong XH, Tan BP, Chi SY, Yang QH, Zhang S, Liu HY, Yang YZ (2018) Investigations on zinc bioavailability of different sources and dietary zinc requirement in juvenile grouper Epinephelus coioides. Aquac Res 49:2763–2773
Devasena T, Lalitha S, Padma K (2001) Lipid peroxidation, osmotic fragility and antioxidant status in children with acute post-streptococcal glomerulonephritis. Clin Chim Acta 308:155–161
Onderci M, Sahin N, Sahin K, Kilic N (2003) The antioxidant properties of chromium and zinc: in vivo effects on digestibility, lipid peroxidation, antioxidant vitamins and some minerals under a low ambient temperature. Biol Trace Elem Res 92:139–150
Kucukbay Z, Yazlak H, Sahin N, Tuzcu M, Cakmak MN, Gurdogan F, Juturu V, Sahin K (2006) Zinc picolinate supplementation decreases oxidative stress in rainbow trout (Oncorhynchus mykiss). Aquaculture 257:465–469
Luo Z, Taz XY, Zheng JL, Chen QL, Liu X (2011) Quantitative dietary zinc requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture 319:150–155
Swinkels JW, Kornegay ET, Zhou W, Lindemann MD Jr, Webb M, Verstegen W (1996) Effectiveness of a zinc amino acid chelate and zinc sulfate in restoring serum and soft tissue zinc concentrations when fed to zinc-depleted pigs. J Anim Sci 74:2420
Yamaguchi M (1998) Role of zinc in bone formation and bone resorption. J Trace Elem Exp Med 11:119–135
Eberle J, Schindmayer S, Erben RG, Stangassinger M, Roth HP (1999) Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol 13:21–26
Dezfuli BS, Bosi G, DePasquale JA, Manera M, Giari L (2016) Fish innate immunity against intestinal helminths. Fish Shellfish Immunol 50:274–287
Hamidoghli A, Won S, Lee S, Lee S, Farris NW, Bai SC (2020) Nutrition and feeding of olive flounder Paralichthys olivaceus: a Review. Rev Fish Sci Aquac 28:340–357
Bayarri M, Oulahal N, Degraeve P, Garshallaoui A (2014) Properties of lysozyme/low methoxyl (LM) pectin complexes for antimicrobial edible food packaging. J Food Eng 131:18–25
Shankar AH, Prasad AS (1998) Zinc and immune function: the bio-logical basis of altered resistance to infection. Am J Clin Nutr 68:447–463
Burge EJ, Madigan DJ, Burnett LE, Burnett KG (2007) Lysozyme gene expression by hemocytes of Pacific white shrimp, Litopenaeus vannamei, after injection with Vibrio. Fish Shellfish Immunol 22L:327–339
Shi B, Xu F, Zhou Q, Regan MK, Betancor MB, Tocher DR, Sun M, Meng F, Jiao L, Jin M (2021) Dietary organic zinc promotes growth, immune response and antioxidant capacity by modulating zinc signaling in juvenile Pacific white shrimp (Litopenaeus vannamei). Aquacult Rep 19:100638
Gharaei A, Khajeh M, Khosravanizadeh A, Mirdar J, Fadai R (2020) Fluctuation of biochemical, immunological, and antioxidant biomarkers in the blood of beluga (Huso huso) under effect of dietary ZnO and chitosan ZnO NPs. Fish Physiol Biochem 46:547–561
Paripatananont T, Lovell RT (1995) Chelated zinc reduces the dietary zinc requirement of channel catfish, Ictalurus punctatus. Aquaculture 133:73–82
Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM (2005) Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci U S A 102:431–436
Grattendick K, Stuart R, Roberts E, Lincoln J, Lefkowitz SS, Bollen A (2002) Alveolar macrophage activation by myeloperoxidase: a model for exacerbation of lung inflammation. Am J Resp Cell Mol 26:716–722
Castro R, Piazzon MC, Noya M, Leiro JM, Lamas J (2008) Isolation and molecular cloning of a fish myeloperoxidase. Mol Immunol 45:428–437
Dalmo RA, Ingebrightsen K, Bogwald J (1997) Non-specific defense mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J Fish Dis 20:241–273
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Acknowledgements
The authors are grateful to the Chairperson, Department of Zoology, Aligarh Muslim University, Aligarh, India for providing necessary laboratory facilities. This work was financially supported by University Grants Commission (grant no F. 53-2/2/2013(CU)).
Author information
Authors and Affiliations
Contributions
Noorin Zafar: Feeding trial; analyses; writing, original draft; funding acquisition
Mukhtar A Khan: Conceptualization, supervision; writing — editing
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zafar, N., Khan, M.A. Effects of Dietary Zinc on Growth, Haematological Indices, Digestive Enzyme Activity, Tissue Mineralization, Antioxidant and Immune Status of Fingerling Heteropneustes fossilis. Biol Trace Elem Res 202, 1249–1263 (2024). https://doi.org/10.1007/s12011-023-03749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03749-2