Abstract
This study was conducted to evaluate the effect of nano copper (nano Cu) and nano zinc (nano Zn) supplementation on the biomarkers of immunity and antioxidant and health status attributes in young dairy calves. Twenty-four young cattle calves were randomly assigned into four groups (6 calves per group) on a body weight and age basis for a period of 120 days. The feeding regimen was the same in all the groups except that these were supplemented with 0.0 mg nano Cu and nano Zn (control), 10 mg nano Cu (nanoCu10), 32 mg nano Zn (nanoZn32), and a combination of nano Cu and nano Zn (nanoCu10 + nanoZn32) per kg dry matter (DM) basis in four respective groups. Supplementation of nano Cu along with nano Zn improves immune response which was evidenced from higher immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA), total immunoglobulin (TIg), and Zn sulphate turbidity (ZST) units and lower plasma concentrations of tumour necrosis factor-α (TNF-α) and cortisol in the nanoCu10 + nanoZn32 group. There was no effect of treatment on the plasma concentrations of immunoglobulin E (IgE) and interferon-gamma (IFN-γ). Antioxidant status was also better in the nanoCu10 + nanoZn32 group as evidenced by lower concentrations of malondialdehyde (MDA) and higher activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), ceruloplasmin (Cp), and total antioxidant status (TAS). However, treatment did not exert any effect on catalase (CAT) activity. Although the nano Cu or nano Zn supplementation, either alone or in combination, did not exert any effect on growth performance or body condition score (BCS), the frequency of diarrhoea and incidence of diarrhoea were lower, while faecal consistency score (FCS) and attitude score were better in the nanoCu10 + nanoZn32 groups. In the control group, one calf was found affected with joint illness and two calves were found affected with navel illness. During the experimental period, none of the calves in all four groups were found to be affected by pneumonia. The findings of this study revealed that dietary supplementation of nano Cu in combination with nano Zn improved the health status of young dairy calves by improving immunity and antioxidant status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minerals fulfil several important functions for the maintenance of animal growth and reproduction as well as health status [1, 2]. A number of trace elements have been shown to be important for adequate functioning of the immune system, among which copper (Cu) and zinc (Zn) play a major role. Cu can effectively maintain the stability of the internal environment and is closely related to growth, health status, haematopoiesis, metabolism, and reproduction [3]. Cu is part of the active sites of many enzymes, including superoxide dismutase (SOD), ceruloplasmin, cytochrome oxidase, L-lysine oxidase, ascorbate oxidase, tyrosinase, and dopamine beta-hydroxylase [4]. Among the principal enzymes, ceruloplasmin (Cp) and Cu–Zn-superoxide dismutase (SOD) are the major Cu-containing antioxidant enzymes. Ceruloplasmin (Cp) may function as an antioxidant in two different ways: by binding to Cu and Cp prevents free Cu ions from catalysing oxidative damage. Involvement of Cu in antioxidant defense protects cell membranes from damage caused by free radicals or oxidative stress. Cu actively participates in immune processes and, by contributing to the transformation of arachidonic acid and prostaglandin synthesis, it plays a vital role in reducing the severity of inflammatory processes. By taking part in the oxidation of membrane thiol groups to disulphides, it stabilizes the permeability of cell membranes [5]. Both deficiency and excessive of Cu have been reported to reduce several aspects of immune response in animal models, including neutrophil numbers and its phagocytic activity, lymphocyte proliferation, and antigen-specific antibody production [6]. Cu added at a higher level than normal requirements has a growth promoting effect because Cu inhibits intestinal harmful microbes, so it has the function to stimulate growth and improve feed efficiency [7].
Similar to Cu, Zn also influences various biological functions and is a cofactor for more than 300 metallo-enzymes [8]. Zn is essential for the body’s proper physiological functions like growth [9], health status, reproduction [10], DNA synthesis, cell division and gene expression [11], wound healing [12], ossification [13], augmenting the immune system of the body [14], lymphocyte replication and proliferation, and protection of cell membranes from bacterial endotoxins and antibody production [15]. There is an increase in the immunoglobulin level in blood serum as well as colostrum by supplementing organic Zn [16]. A significantly higher total IgG concentration was observed in the Zn-supplemented calves than in the control calves [17]. Zn may play a key role in the suppression of free radicals and in the inhibition of NADPH-dependent lipid peroxidation, as well as in the prevention of lipid peroxidation via inhibiting glutathione depletion [18]. Along with Cu, Zn is the primary cytosolic superoxide detoxification enzyme in eukaryotes. It has been assumed that SOD has a central role in the defense against oxidative stress. Zn has been considered an effective anti-inflammatory and anti-diarrheal agent [19]. Optimum dietary Zn supplementation is essential for the growth of the animals, and lower levels of Zn in the diet are associated with a negative effect on growth performance and feed intake [9]. Steers that were deficient in Zn had a decrease in fractional protein degradation and protein accretion when compared to control steers with an adequate Zn status. As a result, decreases in ending body weight, average daily gain (ADG), and gain to feed (G: F) efficiency were observed [17]. Oral Zn supplementation has been used in the prevention and treatment of diarrhoea in infants and children, but also in animals [20], because it improves immune function, reduces the number of pathogenic bacteria, and increases the relative abundance of beneficial gastrointestinal microbes [21].
Nano minerals have unlimited potential as mineral feed supplements in animals, even at very lower doses than the conventional organic and inorganic sources [22]. The use of nanominerals, such as nano Cu or nano Zn, however, may increase the animal production parameters, their healthiness, and the quality of products obtained from them. In addition, the objectives of using nanoparticles in animal feed are to reduce the number of harmful bacteria and stimulate the growth of beneficial bacteria, which may improve the growth performance of animals [23]. So far, numerous studies have been carried out regarding the use of nanominerals in ruminant nutrition. In the past, numerous studies in farm animals have been conducted with organic and inorganic sources of supplementation. Furthermore, studies with nano Cu and nano Zn are restricted until separate use of these minerals. None of the studies has been conducted to see the effect of nano Cu and nano Zn in combination. Studies on the effect of nano Cu and nano Zn on the diarrheal occurrence and other health status attributes in young dairy calves are also lacking. Considering these facts, this study was designed to study the effects of either nano Cu or nano Zn alone or in combination on the biomarkers of immunity and antioxidant status and health conditions. We hypothesized that supplementation of Cu and Zn nanoparticles would improve the health status of young dairy calves by reducing the incidence of diarrhoea and improving the immune response and antioxidant activity.
Materials and Methods
Animals, Diets, and Experimental Design
A total of 24 young dairy calves were randomly assigned to four dietary treatments on a body weight (27.52 ± 3.43 kg) and age (25.33 ± 8 days) basis for a period of 120 days. The experimental calves either received a basal diet devoid of supplemental nano Cu and nano Zn (control group) or were supplemented with 10 ppm of nano Cu (nanoCu10) as cupric oxide nanopowder (CuO, molecular weight 79.54, minimum assay purity 99%, Sisco Research Laboratories Pvt. Ltd. India), 32 ppm of nano Zn (nanoZn32) as zinc oxide nanopowder type 1 (ZnO, molecular weight 81.38, minimum assay purity 99.9%, Sisco Research Laboratories Pvt. Ltd. India), or a combination of nano Cu and nano Zn, i.e. 10 ppm nano Cu + 32 ppm nano Zn (nanoCu10 + nanoZn32). The nutrient requirements of calves were met by feeding milk, calf starter, available green fodder, and wheat straw NRC [24]. Milk and calf starter were offered at the rates of 10% and 1% of the body weight, respectively. Berseem fodder and wheat straw were available ad libitum. Calves were housed in a well-ventilated shed having the proper arrangement for feeding and watering. Deworming of all the experimental animals was done before the start of the experiment by the oral administration of Fentas bolus (Intas Pharmaceuticals Pvt. Ltd., India) at a dose level of 10 mg/kg body weight. The nutrient composition of feedstuffs and milk fed during the experimental period is presented in Table 1.
Observation Recorded and Analytical Procedures
The body weight of the experimental calves was recorded at the start of experiment and then at a fortnightly interval by using a computerized weighing machine (Leotronic Scales Pvt. Ltd., India). Calves were weighed for 2 consecutive days in the morning at 06:00 h before offering feeds, fodders, and water. The average of consecutive 2 days was considered BW for that fortnight and was considered for average daily gain (ADG). Samples of feeds and fodders offered and leftover residue were dried in a hot air oven at 60 °C until a constant weight was achieved and then ground to pass through a 1-mm sieve in a Wiley mill. The samples were analysed for DM (method 973.18c), CP (method 4.2.08), ether extract (EE; method 920.85), and total ash (TA; method 923.03) [25]. Neutral detergent fibre (NDF) and acid detergent fibre (ADF) were determined according to the procedures described by Van Soest et al. [26]. Mineral content in feeds and fodders and milk was determined by using ICP-OES (5800 ICP-OES, Agilent, CA, USA).
Peripheral blood samples were collected before feeding and watering of heifers at 07:00 h in heparinised vacuutainer tubes (BD Franklin, USA) at 0, 30, 60, 90, and 120 days post-nano Cu and nano Zn supplementation. Collected blood samples were centrifuged at 3000 rpm for 30 min to remove the plasma from packed erythrocytes. Samples of plasma were stored at − 20 °C until further analysis. IgG, IgM, IgA, IgE, INF-γ, TNF-α, cortisol, SOD, CAT, Cp, and GSH-Px were estimated in plasma by using bovine specific ELISA Test Kits (Bioassay Technologies, China). The minimum detectable levels was 1.03 µg/mL for IgG, 0.054 µg/mL for IgM, 0.045 µg/mL for IgA, 14.96 ng/mL for IgE, 2.35 pg/mL for IFN-γ, 5.56 ng/L for TNF-α, 0.20 ng/mL for cortisol, 0.26 ng/Ml for SOD, 0.28 ng/mL for CAT, 0.24 IU/L for Cp, and 0.31 ng/mL for GSH-Px. TAS is measured as the ferric reducing antioxidant power (FRAP) assay procedure described by Benzie and Strain [27]. The ZST unit was estimated by the zinc turbidity method [28]. The MDA was estimated by the method of Shafiq-Ur-Rehman [29]. TIg was determined by the addition of IgG, IgM, IgA, and IgE.
Diarrhoea frequency, time until resolution of diarrhoea, incidence of diarrhoea, faecal consistency score (FCS), attitude score, no calves affected with pneumonia, no calves affected with joint ill, and no calves affected with navel ill and calf mortality attributes were used to access the health status of experimental calves. The incidence of diarrhoea in each group was calculated using the following formula:
FCS was given according to Pazoki et al. [30] as (1) firm and well-formed, (2) soft and pudding-like, (3) runny and pancake batter, and (4) liquid and splatters. A calf with an attitude score of 1 was bright, alert, and readily stood with stimulation; a calf with a score of 2 was quiet, alert, and stood only with moderate stimulation; a calf with a score of 3 exhibited a dull mentation and remained recumbent in response to stimulation. Apart from these health attributes, the faeces of diarrheal calves were also observed for causative bacteria by using Mac Conkey Lactose agar (MLA). Biochemical characterization of E. coli was done by using different biochemical tests such as oxidase test, catalase test, indole production, Voges–Proskauer (VP) test, methyl-red test, and citrate test.
Statistical Analysis
The generated data was analysed by using theMIXED procedure of SPSS (Version 20.0, Inc., Chicago, IL) [31], using repeated measures. The model used was as follows:
where, Yijk is the dependent variable, μ is the overall mean of the population, Ti is the mean effect of the treatment (0, 10 ppm Cu, 32 ppm Zn, and 10 ppm Cu + 32 ppm Zn), Dj is the mean effect of period of blood sampling (0, 30, 60, 90, and 120 days post-treatment), (T × D)ij is the effect of the interaction between the effect of treatment and the period of blood sampling, and eijk is the unexplained residual element assumed to be independent and normally distributed. The effects of treatment, period, and treatment by period interaction were considered fixed and calf as a random effect. If the analysis revealed a significant effect, the differences between treatment, period, and treatment by period interaction were then determined by Duncan’s post hoc test at p ≤ 0.05. Results are presented as least squares means and pooled standard errors of the means (SEMs).
Results
Biomarkers of Immunity
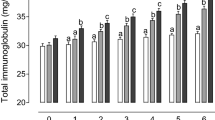
In this study, plasma IgG, IgM, IgA, IgE, TIg, INF-γ, TNF-α, cortisol, ZST units, and WBCs count were used as the biomarkers of immunity. The plasma concentrations of these biomarkers in different groups are depicted in Table 2. Treatment showed a significant (p < 0.05) effect on plasma IgG concentration, and IgG levels were reported the highest in the combination group (Fig. 1). As similar to the trend of IgG, the nanoCu10 + nanoZn32 group showed a greater (p < 0.05) IgM (Fig. 2) and IgA (Fig. 3) concentration in comparison to the calves in other groups. The feeding of a diet supplemented with either nano Cu or nano Zn alone or in combination did not exert any significant effect on the plasma IgE concentrations. TIg showed a significant (p < 0.05) effect of nano Cu and nano Zn supplementation, with the highest values being observed in the nanoCu10 + nanoZn32 group. There was no effect of treatment on plasma IFN-γ concentration. However, TNF-α concentrations were observed significantly (p < 0.05) higher in the calves fed on the diet supplemented with both nano Cu as well as nano Zn. The experimental calves in nanoCu10 + nanoZn32 group showed a significant (p < 0.05) lower plasma cortisol (Fig. 4) and higher ZST units, whereas no effect of either Cu or Zn alone or in combination was observed on WBC counts. There were no effects of period and treatment × period interaction on studied immunity biomarkers.
Biomarkers of Antioxidant Status
The plasma MDA, SOD, CAT, GSH-Px, Cp, and TAS concentrations were used as the biomarkers of antioxidant status (Table 2). Treatment showed a significant (p < 0.05) effect on the plasma concentration of MDA, SOD, GSH-Px, Cp, and TAS. The effect on plasma MDA concentration began on day 60 of the experiment, and lowest concentration of MDA was observed in the nanoCu10 + nanoZn32 groups in comparison to other groups (Fig. 5). However, the activity of SOD (Fig. 6) and GSH-Px (Fig. 7) was observed to be greater (p < 0.05) in the calves of the combination group where they are fed on the diet supplemented with both nano Cu and nano Zn. The treatment effect on SOD activity began on the day 90 of the experiment and continued till the end of the study. The feeding of a diet supplemented with 10 ppm of nano Cu, or 32 ppm of nano Zn or their combination did not exert any significant effect on plasma CAT activity. Treatment showed a significant (p < 0.05) effect on plasma Cp concentration, with higher Cp concentrations which were observed in nano Cu-supplemented groups, i.e. nanoCu10 and nanoCu10 + nanoZn32 groups than in other groups (Fig. 8). Better (p < 0.05) TAS activity was observed in nanoCu10 + nanoZn32 group (Fig. 9). There was no significant effect of period and treatment × period interaction on the biomarkers of antioxidant status.
Growth Performance and Health Status
Table 3 shows the findings regarding the effect of nano Cu and nano Zn supplementation on the growth performance and health status attributes. Although the mean ADG in treatment groups was numerically high but statistical analysis of the data revealed a non-significant effect of treatment. The number of calves affected by diarrhoea was lower in the nano Zn and combination groups than in the nano Cu and control groups. However, the frequency of diarrhoea, time until resolution of diarrhoea, and incidence of diarrhoea were lower (p < 0.05) in the nano Zn and nanoCu10 + nanoZn32 group. The FCS and attitude score were also better (p < 0.05) in the nano Zn and combination groups. One calf was found affected with joint ill and two calves were found affected with navel ill in control group. No cases of calf mortality or pneumonia were reported in all four groups. Calves affected by diarrhoea were also analysed for the causative agent. After Gram’s staining and bio-chemical examination, the following IMViC patterns were presented: + , + , -, -. This IMViC pattern is suggestive of E. coli.
Discussion
Biomarkers of Immunity
In the present study, the experimental calves fed on the diet supplemented with the combination of nano Cu and nano Zn had better immunity which was evidenced from higher plasma concentrations of IgG, IgM, IgA, and TIg and lower plasma TNF-α and cortisol concentrations in nanoCu10 + nanoZn32 group. The concentrations of circulating immunoglobulins, especially IgG, IgM, and IgA, are important indicators of immune function. No work has been conducted to see the combined effect of nano Cu and nano Zn on immune response in animals. Therefore, the findings of the present study have been discussed with those of the others who used any source of Cu and Zn separately or in combination. Kushwaha et al. [32] observed a significant (p < 0.05) improvement in immune response in nano Cu-supplemented Sahiwal heifers. He indicated that nano Cu does not have pro-inflammatory properties and does not interact with humoral responses in growing heifers. Pineda et al. [33] found the same results that the expression of immune-related genes (TNF-α) was not affected, indicating the absence of the pro-inflammatory property of nano CuO. Gonzales-Eguia et al. [34] showed significant improvements in the IgG and γ-globulin levels of the nano Cu group of piglets. Ognik et al. [35] also reported increased immune defense of chickens by supplementing their diets with nano-Cu. Compared with the control group, the dietary supplementation with 100 mg/kg of nano-Cu significantly increased the concentrations of IgA, IgG, IgM, and lysozyme in the serum of broilers [36]. It could be explained by the fact that the immunoglobulin’s enhancement may be the result of the activation of phagocytes, which indicates an improved immune status of broilers after nano Cu treatment [35]. Another study reported that nano Cu-loaded chitosan improved immune status, enhanced protein synthesis, and was beneficial to the caecal microbiota of broiler chickens [36].
Zn plays a role in molecular and membrane stability. One of the first lines of defense the body has against an immunological attack is the skin. The most direct connection between Zn and immune function is its role in cell replication and proliferation, which is of great importance for maintaining the normal activity and integrity of immune cells and systems (Wang et al.) [37]. Sharish et al. [38] reported that plasma total immunoglobulin concentration was found higher in the nano Zn-supplemented group than the control group at 30, 60, and 90 days, and total immunoglobulin concentration was found higher in the inorganic Zn-supplemented group than the control group. He concluded that nano Zn supplementation at 25 and 50 ppm has better immunogenic effects and thus may replace inorganic Zn sources at a lower level of Zn, i.e. 25 ppm. Nagalakshmi et al. [39] demonstrated that administration of a low level of organic Zn improves growth performance and the immune response of calves. Consistent with these findings, they found that ZnO supplementation increased serum IgG and IgM concentrations above those of the control by 3.85 and 2.86 mg/mL, respectively, compared with Zn-methionine supplementation, indicating that the administration of a low level of ZnO is superior to Zn-methionine with respect to the immune function of dairy calves. Serum IgG, IgM, and IgA can protect the extra vascular compartment against pathogenic viruses and microorganisms [40], so the increase in the levels of serum IgG and IgM indicates that weaned piglets fed on diets supplemented with nano ZnO may undergo improvements in immune function. Feng et al. [41] reported an improvement in IgA, IgM, and IgG levels with the dietary replacement of 120 mg/kg of inorganic Zn with 90 or 120 mg/kg of organic Zn. In the study of Chang et al. [42], total serum IgG and IgM concentrations in the ZnO group were significantly higher than in the control group. Similarly, Nagalakshmi et al. [43] and Wang et al. [37] observed better immune responses with organic Zn supplementation compared to inorganic Zn supplementation in lambs and dairy cows, respectively. Engle et al. [44] implied that when cattle are in a Zn deficient state, cell-mediated immune responses are decreased, making calves more susceptible to infectious disease.
Biomarkers of Antioxidant Status
Antioxidant status is known to be a significant predictor of disease and mortality in infants, especially premature infants. In present study, plasma MDA level was used as biomarker of oxidative stress, whereas SOD, CAT, GSH-Px, Cp, and TAS were used as the biomarkers of antioxidant status. Similar to the immune response, the calves supplemented with a combination of nano Cu and nano Zn showed better antioxidant status than calves in control, nano Cu, or nano Zn alone groups. Better antioxidant status in the nanoCu10 + nanoZn32 group is evidenced by lower MDA concentrations and higher SOD, GSH-Px, Cp, and TAS activity in this group. Pineda et al. [45] found that the nano Cu injection reduced lipid oxidation, which could be associated with the lower O2 consumption in broiler chicks. SOD is one of the main antioxidants (Cu–Zn linked metallo-enzymes), which can remove excess free radicals in the body and reduce the degree of nucleic acid damage [46]; Zhao et al. [47]. There was a significant (p < 0.05) improvement in antioxidant status in nano Cu-supplemented groups in Sahiwal heifers, and nano Cu supplementation improves mRNA expression of SOD and CAT genes [32]. Shen et al. [48] showed that when compared with the Cu-deprived goats, serum SOD, GSH-Px, CAT, and total antioxidant capacity in the nano Cu and CuSO4 groups were significantly higher, while serum MDA content was significantly lower. Likewise, Vaswani et al. [49] found that antioxidant activity (TAS) was higher in heifers receiving Cu-supplemented diets. Dezfoulian et al. [50] also reported that Cu source had a significant effect on Cp concentration (p < 0.05) in lambs. Total antioxidant capacity, SOD, and GSH-Px were more in the birds fed diet inclusion of 60 and 90 mg nano CuO than other treatments and lowest MDA level was observed [51]. The antioxidant mechanisms of the blood, reflected by elevated catalase and plasma FRAP, became more intensive during nano-Cu supplementation in wistar rats [52]. The replacement of inorganic Cu with nano Cu differentially modulated the redox status of selected tissues, i.e. enhanced SOD activity in small intestinal tissue and decreased total glutathione levels in the bursa of fabricius of turkeys [53]. The nano-Cu supplementation in the rabbit significantly increased the activity of the SOD enzyme compared with the control group, but catalase activity was unaffected [54]. On the contrary, Dezfoulian et al. [50] observed that Cu supplementation (regardless of source and level) had no significant effect on SOD activity in lambs.
The antioxidant effect of Zn may be mediated through direct action of the Zn ion, its structural role in antioxidant proteins, and modulation of metallothionein induction. The direct antioxidant activity of Zn ions is associated with their binding to thiol groups, thus protecting them from oxidation [55, 56]. Sharish et al. [38] reported that plasma SOD concentration was found higher in the nano Zn-supplemented group than in the control group and inorganic Zn-supplemented group at 30, 60, and 90 days, and TAS concentration increased within all groups over the time, and TAS concentration was higher in all treatment groups than the control group. Wang et al. [57] reported that the serum SOD levels increased and MDA levels decreased with the inclusion of ZnO and 0.4–0.6 mg/kg nano-ZnO in their diets in weaning piglets. These results are similar to the findings of Zhao et al. [12], who observed that dietary supplementation with 0.06 and 0.1 g/kg nano ZnO improved serum Cu–Zn-SOD activity but decreased serum MDA levels in broilers on days 28 and 35. Bakhshizade et al. [58] noticed in cows that the SOD concentration was higher in the nano Zn and Zn-Glycine-supplemented groups than in the inorganic Zn-supplemented group. Zn as Zn oxide nanoparticles in Japanese quails and broiler chickens improved the total antioxidant capacity and reduced the MDA concentrations compared to controls [12, 59]. The activity of GSH-Px increased in high Zn and coated nano ZnO fed pigs compared with the control group. Pigs fed on coated nano ZnO had a higher activity of serum SOD (p < 0.05) compared with the control and high Zn groups [60]. Furthermore, the antioxidant capacity of growing pigs is fundamental for maintaining the normal metabolic state to protect a pig’s health; we hypothesized that the effects of dietary nano ZnO could promote growth by indirectly regulating the antioxidant capacity of pigs. High doses of ZnO (3000 mg/kg) supplementation reduced the serum MDA concentration and increased the SOD activity in piglets [61]. His findings also show that pigs fed a high dose of ZnO had an improved antioxidant capacity by increasing GSH-Px activity. Meanwhile, a low dose of coated nano ZnO could increase the activities of SOD and GSH-Px in the serum. Previous studies have shown that Zn has an antioxidant function [17], and supplementation with Zn methionine reportedly decreases the concentration of MDA but increases that of MT and T-AOC in the serum of ruminants [62].
Growth Performance and Health Status
Dietary supplementation of either nano Cu or nano Zn alone or in combination did not exert any impact on the growth performance of the experimental calves. Kushwaha et al. [32] found no effects of 10 ppm inorganic Cu, 5.0 and 10.0 ppm nano Cu on the growth performance in growing Sahiwal heifers, which was similar to the findings of our study. Vaswani et al. [49] reported a similar observation that supplementing 8.0 mg Cu/kg DM either in the form of Cu-proteinate, Cu-propionate, or Cu sulphate did not affect ADG in growing heifers. Kim et al. [63] also found a similar effect on growth performance in nano Cu-supplemented pigs compared with inorganic and organic Cu. The results of the present study are similar to the observations of Dezfoulian et al. [50] who reported that there was no significant effect of Cu supplementation on ADG in lambs. Waghmare et al. [64] observed that supplementation of Cu as CuSO4 and Cu-methionine did not alter ADG and feed: gain ratio in kids. However, in contrast to the findings of the present study, some studies compared the inorganic forms of Cu with nano Cu and the latter showed an improvement in the growth performance of piglets [34, 65]. Zhang et al. [66] reported that supplementation of the basal diet with 10 mg Cu/kg DM in the basal diet enhanced growth performance in Cashmere goats. Chang et al. [65] reported that dietary supplementation with 25 mg/kg body weight nano Cu improves the performance of weaning piglets.
No significant difference in body weight observed on supplementation of different levels and different sources of Zn has been observed in previous research, though overall body weight increases as the age of experimental animal advances. Zn supplementation above NRC (2001) recommended requirements did not consistently affect growth rate in cattle [67]. The results of the present study are similar to the observations of Zaboli et al. [68], who reported that ADG in goat kids was not affected due to the supplementation of Zn from different sources at different levels. However, contrary to the findings of present study, Chang et al. [42] showed that supplementation with Zn-methionine but not ZnO significantly increased the ADG of new-born calves in the first 2 weeks after birth. Anil et al. [69] observed significantly higher body weight gain and ADG in the 20 ppm nano Zn-supplemented calves group, followed by 10 ppm nano Zn, 5 ppm nano Zn-supplemented groups, and the 25 ppm ZnSO4 group. Hongfu et al. [70] observed a significant increase in ADG in the nano-Zn oxide (200, 400, and 600 mg/kg)-supplemented group and higher ZnO (3000 mg/kg)-supplemented piglet groups compared to the no Zn-supplemented group. The discrepancy in growth performance in the findings of different studies may be a consequence of different sources and levels of Cu and Zn used, differing ages of animals used in the study, differences in study period, different genetics in various breeds, etc.
In the present study, the frequency of diarrhoea, incidence of diarrhoea, FCS, attitude score, pneumonia occurrence, joint ill and navel ill, and mortality were used to assess the health status of the experimental calves. The number of calves affected by diarrhoea, frequency of diarrhoea, time until resolution of diarrhoea, and incidence of diarrhoea was lower in the nanoZn32 and nanoCu10 + nanoZn32 groups. FCS and attitude scores were also better in the nano Zn and combination groups. One calf from the control group was found affected with joint ill and two calves from the control group were found affected with navel ill. No any case of calf mortality and pneumonia were reported in all four groups. Calves having diarrhoea were found affected by E. coli. The anti-diarrheal function of Cu and Zn may be associated with their role in immunity [19, 71]. The mechanisms of the anti-diarrheal effect of Zn are thought to involve the regulation of intestinal fluid transport and mucosal integrity, the promotion of immunity, and the modulation of oxidative stress [72, 73]. Wang et al. [74] reported that the incidence of diarrhoea in control calves fluctuated between 20 and 34.29% during the first 2 weeks of life. However, supplementation with ZnO or Zn-Met helped to reduce the incidence of diarrhoea in neonatal dairy calves during their early lives, and no diarrhoea was found in calves in the ZnO group during the first 3 d after birth, which is consistent with previous findings [74, 75]. In addition, supplementation with Zn reduced the incidence of diarrhoea, which is consistent with the results obtained by Feldmann et al. [20], who showed that Zn-Met-treated calves had a 14.7% lower risk of diarrhoea than placebo-treated calves. Hu et al. [76] reported that 0.3 g/kg Zn as nano ZnO inclusion in the diet decreased the incidence of diarrhoea in early weaned piglets (5.7 kg), exhibiting a similar effect to 3.0 g/kg Zn as ZnO administration in a 14-day experiment. Dietary ZnO at therapeutic concentrations of 2000 to 4000 mg/kg could effectively prevent and treat post-weaning diarrhoea [77]. Limited information is available on the role of Cu in controlling calf diarrhoea. Within individual calves, the onset of diarrhoea, incidence of leg abnormalities, and anaemia were correlated with the onset of Cu deficiency and are indicative of relatively severe deficiency [78].
Conclusions
The findings of the present study revealed that supplementation of nano Cu along with nano Zn improves immune response as well as antioxidant status compared to a diet supplemented with either nano Cu or nano Zn alone. Nano Cu and nano Zn supplementation did not influence growth performance. However, supplementation of nano Cu in combination with nano Zn showed a lower frequency of diarrhoea, time until resolution of diarrhoea, and incidence of diarrhoea. FCS and attitude scores were also better in the nano Zn and combination groups. Taking into account the beneficial effects of nano Cu or nano Zn on immunity and antioxidant status and health status attributes, the use of nano Cu in combination with nano Zn should be considered in young dairy calves.
Data Availability
The authors declare that the data supporting the findings of this study are available within the manuscript.
Abbreviations
- BCS:
-
Body condition score
- CAT:
-
Catalase
- Cp:
-
Ceruloplasmin
- FCS:
-
Faecal consistency score
- GSH-Px:
-
Glutathione peroxidase
- IgA:
-
Immunoglobulin A
- IgE:
-
Immunoglobulin E
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- IFN-γ:
-
Interferon gamma
- MDA:
-
Malondialdehyde
- Nano-Cu:
-
Nano copper
- Nano-Zn:
-
Nano zinc
- SOD:
-
Superoxide dismutase
- TAS:
-
Total antioxidant status
- TIg:
-
Total immunoglobulin
- TNF-α:
-
Tumour necrosis factor-α
- ZST:
-
Zn sulphate turbidity
References
Close WH (1998) The role of organic trace mineral proteinates in pig nutrition. In: Biotechnology in the Feed Industry: Proc Alltech’s 14th Ann Sym, pp 469–483
Suttle NF (2010) Mineral nutrition of livestock, 4th edn. CABI, Wallingford, UK
Ognik K, Krauze M (2016) The potential for using enzymatic assays to assess the health ofturkeys. World’s Poult Sci J 72:535–550
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxico 189:147–163
Kim BE, Nevitt T, Thiele DJ (2008) Mechanisms for copper acquisition, distribution and regulation. Nature Chem Bio 4:176–185
Pocino M, Baute L, Malave I (1991) Influence of the oral administration of excess copper on the immune response. Fundam appltoxicol 16:249–256
Adu OA, Egbunike GN (2010) Enhancing growing rabbits performance with diets supplemented with copper. Adv Bio Res 4(1):18–22
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zn and human health, an update. Arch Toxicol 86(4):521–534
Case CL, Carlson MS (2002) Effect of feeding organic and inorganic sources of additional Zn on growth performance and Zn balance in nursery pigs. J Ani Sci 80:1917–1941
Uchida K, Mandebvu P, Ballard CS, Sniffen CJ, Carter MP (2001) Effect of feeding a combination of Zn, Mn and Cu amino acid complexes, and cobalt glucoheptonate on performance of early lactation high producing dairy cows. Anim Feed Sci Technol 93:193–203
Prasad AS (1991) Discovery of human Zn deficiency and studies in an experimental human model. Americ J Clini Nutri 53:403–412
Zhao CY, Tan SX, Xiao XY, Qiu XS, Pan JQ, Tang ZX (2014) Effects of dietary Zn oxide nanoparticles on growth performance and anti-oxidative status in broilers. Biol Trace Elem Res 160:361–367
Roughead ZK, Kunkel ME (1991) Effect of diet on bone matrix constituents. J Amer Coll of Nutr 10:242–246
Parashuramulu S, Nagalakshmi D, Srinivasa Rao D, Kishan Kumar M, Swain PS (2015) Effect of zinc supplementation on antioxidant status and immune response in buffalo calves. Ani Nutr F Tech 15(2):179–188
Nockels CF (1994) Micronutrients and the immune response. In: Montana Nutrition Conference Proc. Bozeman, Montana, p 3.1
Kinal S, Korniewicz A, Jamroz D, Zieminski R, Slupczynska M (2005) Dietary effects of zinc, copper and manganese chelates and sulphates on dairy cows. J F Agri Envir 3:168–172
Dresler S, Illek J, Zeman L (2016) Effects of organic zinc supplementation in weaned calves. Acta Vet Brno 85:48–53
Prasad AS, Bettger WJ, Dell BL (1997) The role of zinc in brain and nerve functions. Plenum Press, New York, pp 95–111
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimm Rev 14:277–285
Feldmann HR, Williams DR, Champagne JD, Lehenbauer TW, Aly SS (2019) Effectiveness of zinc supplementation on diarrhea and average daily gain in pre weaned dairy calves: a double-blind, block-randomized, placebo-controlled clinical trial. PLoS ONE 14:e0219321
Sales J (2013) Effects of pharmacological concentrations of dietary zinc oxide on growth of post weaning pigs: a meta-analysis. Biol Trace Elem Res 152:343–349
Rajendran D (2013) Application of nano minerals in animal production system. Res J Biotech 8(3):1–3
Hill EK, Li J (2017) Current and future prospects for nanotechnology in animal production. J Ani Sci Biotech 8:26
NRC (2001) Nutrient requirements of dairy cattle, 7th revised. National Academy Press, Washington, DC
AOAC (2005) Official methods of analysis. Association of Official Analytical Chemists, Washington DC
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Benzie IFF, Strain JJ (1999) Ferric reducing/ antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth Enzymol 299:15–27
Mc Ewan AD, Fisher EW, Salman IE, Penhale WJ (1970) A turbidity test for the estimation of immune globulin levels in neonatal calf serum. Clin Chem Acta 27:155163
Shafiq-ur-Rehman, (1984) Lead-induced lipid peroxidation in brain. Toxicol Lett 2:333
Pazoki A, Ghorbani GR, Kargar S, Sadeghi-Sefidmazgi A, Drackley JK, Ghaffari MH (2017) Growth performance, nutrient digestibility, ruminal fermentation, and rumen development of calves during transition from liquid to solid feed: effects of physical form of starter feed and forage provision. Anim Feed Sci Technol 234:173–185
SPSS (2011) Statistics version 20.0. IBM SPSS Inc, USA
Kushwaha R, Kumar V, Kumar M, Vaswani S, Kumar A (2021) Effects of inorganic and nano copper supplementation on growth performance, nutrient utilization andmineral availability in growing Sahiwal heifers. Indian J Anim Nutri 38(2):278–285
Pineda L, Sawosz E, Vadalasetty KP, Chwalibog A (2013) Effect of copper nanoparticles on metabolic rate and development of chicken embryos. Anim Feed Sci Technol 186:125–129
Gonzales-Eguia A, Fu CM, Lu FY, Lien TF (2009) Effects of nanocopper on copper availability and nutrients digestibility, growth performance and serum traits of piglets. Lives Sci 126:122–129
Ognik K, Sembratowicz I, Cholewinska E, Jankowski J, Kozlowski K, Juskiewicz J, Zdunczyk Z (2018) The effect of administration of copper nanoparticles to chickens in their drinking water on the immune and antioxidant status of the blood. Anim Sci J 89:579–588
Wang C, Wang MQ, Ye SS, Tao WJ, Du YJ (2011) Effects of copper-loaded chitosan nanoparticles on growth and immunity in broilers. Poult Sci 90:2223–2228
Wang RL, Liang JG, Lu L, Zhang LY, Li SF, Luo XG (2013) Effect of zinc source on performance, zinc status, immune response, and rumen fermentation of lactating cows. Biol Trace Elem Res 152(1):16–24
Kumar S, Kumar V, Kumar M, Vaswani S, Kushwaha R, Kumar A, Prakash A (2021) Comparing efficiency of nano zinc on performance, nutrient utilization, immune and antioxidant status in Hariyana cattle. Proc Nat Acad Sci 91(3):707–713
Nagalakshmi D, Sridhar K, Satyanarayana M, ParashuRamulu S, Narwade VS, Vikra L (2018) Effect of replacing inorganic zinc with a lower level of organic zinc (zinc propionate) on performance, biochemical constituents, antioxidant, immune and mineral status in buffalo calves. Indian J Anim Res 52:1292–1297
Li P, Yin YL, Li D, Kim SW, Wu GY (2007) Amino acid and immune function. British J Nutr 98:237–252
Feng J, Ma WQ, Niu NH, Wu XM, Wang Y (2010) Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol Trace Elem Res 133:203–211
Chang MN, Wei JY, Hao LY, Ma FT, Li HY, Zhao SG et al (2020) Effects of different types of zinc supplement on the growth, incidence of diarrhea, immune function, and rectal microbiota of newborn dairy calves. J Dairy Sci 103:6100–6113
Nagalakshmi D, Dhanalakshmi K, Himabindu D (2009) Effect of dose and source of supplemental zinc on immune response and oxidative enzymes in lambs. Vet Res Comm 33(7):631–644
Engle TE, Nockels CF, Kimberling CV, Weaber DL, Johnson AB (1997) Zinc repletion with organic or inorganic forms of zinc and protein turnover in marginally zinc deficient calves. J Anim Sci 75:3074–3081
Pineda L, Sawosz E, Vadalasetty KP, Chwalibog A (2013) Effects of copper nanoparticles on metabolic rate and development of layer embryos. In: Oltjen JW, Kebreab E, Lapierre H (eds) Energy and protein metabolism and nutrition in sustainable animal production. Wageningen Academic Publishers, Wageningen, pp 417–418
Saban C (2019) Effect of dietary vitamin E, selenium and their combination on concentration of selenium, MDA, and antioxidant enzyme activities in some tissues of laying hens. Pak J Zoo 51:1155–1161
Zhao K, Chi YK, Shen XY (2020) Studies on edema pathema in Hequ horse in the Qinghai Tibet plateau. Biol Trace Elem Res 198(1):142–148. https://doi.org/10.1007/s12011-02002043-02049
Shen X, Song C, Wu T (2020) Effects of nano-copper on antioxidant function in copper deprived Guizhou black goats. Biol Trace Elem Res 199(6):2201–2207. https://doi.org/10.1007/s12011-020-02342
Vaswani S, Kumar V, Roy D, Kumar M, Kushwaha R (2018) Effect of different sources of copper supplementation on performance, nutrient utilization, blood biochemicals and plasma mineral status of growing Hariana heifers. Indian J Anim Sci 88(7):812–818
Dezfoulian AH, Aliarabi H, Tabatabaei MM, Zamani P, Alipour D, Bahari A, Fadayifar A (2012) Influence of different levels and sources of copper supplementation on performance, some blood parameters, nutrient digestibility and mineral balance in lambs. Livestock Sci 147:9–19
Nassiri M, Ahmadi F (2015) Effects of copper oxide nanoparticles on the growth performance, antioxidant enzymes activity and gut morphology of broiler chickens. Int J Agri Biosyst Eng 9:1–11
Majewski M, Ognik K, Juskiewicz J (2019) Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in Wistar rats. Pharmacol Rep 71(3):509–516
Jankowski J, Otowski K, Kozlowski K, Pietrzak P, Ferenc K, Ognik K, Juskiewicz J, Sawosz E, Zdunczyk Z (2020) Effect of different levels of copper nanoparticles and copper sulfate on morphometric indices, antioxidant status and mineral digestibility in the small intestine of turkeys. Annals Anim Sci 20(3):975–990
Refaie AM, Ghazal MN, Easa FM, Barakat SA, GE Y (2015) Nano-copper as a new growth promoter in the diet of growing New Zealand white rabbits. Egypt J Rabbit Sci 25:39–57
Oteiza PI (2012) Zinc and the modulation of redox homeostasis. Free Rad Bio Med 53(9):1748–1759
Korkmaz-Icöz S, Atmanli A, Radovits T et al (2016) Administration of zinc complex of acetyl alicylic acid after the onset of myocardial injury protects the heart by upregulation of antioxidant enzymes. J Physio Sci 66(2):113–125
Wang Y, Tang JW, Ma WQ, Feng J (2010) Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol Trace Elem Res 133:325–334
Bakhshizadeh S, Aghjehgheshlagh FM, Taghizadeh A (2019) Effect of zinc source on milk yield, milk composition and plasma concentration of metabolites in dairycows. South African J Anim Sci 49(5):882–889
Atakisi O, Atakisi E, Kart A (2009) Effects of dietary zinc and L-arginine supplementation on total antioxidants capacity, lipid peroxidation, nitric oxide, egg weight, and bloodbiochemical values in Japanese quails. Biol Trace Elem Res 132(13):136–143
Prasad AS (2008) Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol 43:370–377
Zhu C, Chen Z, Wang L, Wu X, Chen Z, Zhang W, Liang R, Jiang R (2017) Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejuna gene expression in weaned piglets. Biol Trace Elem Res 175:331–338
Wei JY, Ma FT, Hao LY, Shan Q, Sun P (2019) Effect of differing amounts of zinc oxide supplementation on the antioxidant status and zinc metabolism in newborn dairy calves. Livestock Sci 230:103819
Kim M, Hosseindoust A, Choi Y, Lee J, Kim K, Kim T, Cho H, Kang W, Chae B (2020) Effects of hot-melt extruded nano-copper as an alternative for the pharmacological dose of copper sulfate in weanling pigs. Biol Trace Elem Res doi:org/. https://doi.org/10.1007/s12011-020-02426-y
Waghmare S, Dass RS, Garg AK, Mohanta RK, Dhayagude RS (2014) Effect of copper methionine supplementation on growth rate and nutrient utilization in male goat kids. Indian J Anim Nutri 31:44–48
Chang Z, Zhang H, Dong H, Mehmood K, Ijaz M, Ahmad HI, Naeem MA, Wu Q, Nabi F, Zhu H (2018) Effect of CuSO4 and nano copper on serum antioxidant capacity in weaned piglets. J Bio Regulators Homeo Agents 32(2):99–104
Zhang W, Wang R, Kleemann DO, Lu D, Zhu X, Zhang C, Jia Z (2008) Effects of dietary copper on nutrient digestibility, growth performance and plasma copper status in cashmere goats. Small Rumin Res 74:188–193
Spears JW (1995) Overview of mineral nutrition in cattle. In: 13th Annual Florida Ruminant Nutrition Symposium. pp 113–126
Zaboli K, Aliarabi H, Bahari AA, Abbas AKR (2013) Role of dietary nanozinc oxide on growth performance and blood levels of mineral: a study on in Iranian Angora (Markhoz) goat kids. J Pharma Health Sci 2(1):19–26
Anil TSV, Seshaiah VC, Ashalatha P, Sudhakar K (2019) Effect of dietary nano zinc oxide supplementation on growth performance in crossbred calves. Int J Curr Microbio App Sci 8(12):1852–1856
Hongfu Z, Yu B (2008) Effects of nano-ZnO on growth performance and diarrhea rate in weaning piglets. China Feed 1:008
Pei X, Xiao Z, Liu L, Wang G, Tao W, Wang M, Zou J, Leng D (2019) Effects of diet aryzinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J Sci Food Agri 99:1366–1374
Berni Canani R, Buccigrossi V, Passariello A (2011) Mechanisms of action of zinc in acute diarrhea. Curr Opin Gastroenter 27:8–12
Wei JY, Ma FT, Hao LY, Shan Q, Sun P (2019) Effect of differing amounts of zinc oxide supplementation on the antioxidant status and zinc metabolism in newborn dairy calves. Lives Sci 230:103819
Wang J, Zeng YX, Wang SX, Liu H, Zhang DY, Zhang W, Wang Y (2018) Swine-derived probiotic Lactobacillus plantarum inhibits growth and adhesion of enterotoxigenic Escherichia coli and mediates host defense. Front Microbio 9:1364
Sun YB, Xia T, Wu H, Zhang WJ, Zhu YH, Xue JX, He DT, Zhang LY (2019) Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim Feed Sci Tech 258:114312
Hu CH, Xiao J, Song J, Luan ZS (2013) Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Anim Feed Sci Tech 181:65–71
Shelton NW, Tokach MD, Nelssen JL, Goodband RD, Dritz SS, Derouchey JM (2011) Effects of copper sulfate, tri-basic copper chloride, and zinc oxide on weanling pig performance. J Anim Sci 89:2440–2451
Suttle NF, Angus KW (1976) Experimental copper deficiency in the calf. J Compa Path 86:595
Acknowledgements
The authors would like to express their sincere thanks to the staff of Livestock Farm Complex (LFC), DUVASU, Mathura, for diligent animal care and feeding. The authors also express their appreciation to Dr. Brijesh Yadav (Associate Professor, Department of Veterinary Physiology, DUVASU, Mathura, India) for their help and support during biochemical analysis.
Author information
Authors and Affiliations
Contributions
Pooja Pandey carried out animal trial, sample analysis, and wrote the first draft of the manuscript. Muneendra Kumar conceptualized and designed this study, conducted data analysis, and wrote the final draft of the manuscript. Vinod Kumar designed this study and reviewed and edited the final manuscript. Raju Kushwaha, Shalini Vaswani, and Avinash Kumar reviewed and edited the final manuscript. Yajuvendra Singh raised the experimental animals, and Pankaj Kumar Shukla designed this study and edited the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
Animal care procedures were approved (approval number IAEC/21/15) and conducted under the established standard of the Institutional Animal Ethics Committee (IAEC), constituted as per article number 13 of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) rules laid down by the Government of India.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, P., Kumar, M., Kumar, V. et al. The Dietary Supplementation of Copper and Zinc Nanoparticles Improves Health Condition of Young Dairy Calves by Reducing the Incidence of Diarrhoea and Boosting Immune Function and Antioxidant Activity. Biol Trace Elem Res 201, 3791–3803 (2023). https://doi.org/10.1007/s12011-022-03481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03481-3