Abstract
Two experiments were conducted to examine the effect of zinc (Zn) source on the performance, Zn status, immune response, and rumen fermentation of lactating cows to find the most available Zn source for dairy production. In Experiment 1, a total of 30 multiparous Holstein cows were randomly allocated by body weight and milk yield to one of five treatments in a completely randomized design. Cows were fed a total mixed ration (TMR) with no Zn addition (containing 37.60 mg Zn/kg TMR by analysis), and the basal TMR supplemented with 40 mg Zn/kg TMR from either Zn sulfate or one of three organic Zn chelates with weak (Zn-AA W), moderate (Zn-Pro M), or strong (Zn-Pro S) chelation strengths, respectively for 55 days. In Experiment 2, the in vitro rumen fermentation method was used in a completely randomized design involving a 4 × 3 factorial arrangement of treatments. The four Zn sources were the same as those used in Experiment 1, and the three supplemental Zn levels in the rumen fluid were 0, 10, and 20 μg/mL, respectively. The feed intake, milk composition, and somatic cell count (SCC) were unaffected (P > 0.05) by treatments. However, the milk yield was increased (P < 0.05) by addition of Zn from both the Zn-AA W and Zn-Pro S. Plasma Zn level at the end of the experiment was increased (P < 0.05) by addition of Zn from all three organic sources. Serum antibody titers on day 21 after vaccination with foot and mouth disease (FMD) vaccine were increased (P < 0.05) by both supplemental Zn-AA W and Zn-Pro S. The organic Zn sources with different chelation strengths supplemented at the added Zn level of 10 μg/mL were more effective (P < 0.05) in improving the rumen fermentation than Zn sulfate, with the most effective being Zn-AA W. In conclusion, Zn source had no influence on the feed intake, milk composition, and SCC; however, both the Zn-AA W and Zn-Pro S were more effective than Zn-Pro M and Zn sulfate in enhancing the rumen fermentation, Zn status, and humoral immune response as well as improving milk yield of lactating cows. The improved milk production might be attributed to the improved rumen fermentation, Zn status, and immune function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential trace element for animals, and it is involved in many physiological functions in the body through numerous metalloenzyme systems [1]. The use of organic Zn sources in the feed industry has been receiving increasing attention in recent years. Numerous studies have been conducted to investigate the efficacies or relative bioavailabilities of organic Zn sources in comparison to inorganic Zn in ruminants [2–18]. Kellogg et al. [11] summarized 12 trials on the efficacies of Zn–methionine (Met) complex in dairy cows and reported that Zn–Met could improve the lactation performance and milk components as compared to the inorganic Zn. Cope et al. [17] also found that Zn supplementation in an organic form of chelated Zn increased milk yield of dairy cows compared with Zn addition as Zn oxide. However, the results of unchanged milk production were obtained in dairy cows fed on the diets supplemented with Zn–Met in comparison with Zn sulfate [18]. Zn is also crucial for maintenance of integrity and the barrier function of skin, and it is involved in the immune system through complex ways. Several studies have been done to assess the effect of organic Zn source on somatic cell count (SCC) and udder health in dairy cows [11, 17, 19–21]; however, the results have been inconsistent. Researches in either lambs [22] or beef cattle [3, 14] indicated that organic Zn sources of Zn proteinate, Zn–Met, or Zn propionate were more effective in improving immunity of these animals as compared to their inorganic forms. However, no immune responses were observed upon supplemental Zn from different forms in other studies of calves, steers, or heifers [5, 8, 15]. Conflicting results in plasma Zn [5–10, 12–16, 23] and alkaline phosphatase (ALP) activity [2, 9, 12, 13, 22] were also reported in previous studies. Such discrepancies might be due to the differences in animal species, dietary Zn levels, and characteristics of tested organic Zn sources. It has been widely accepted that the chelation strength is the most important factor for evaluating the characteristics of organic mineral sources. Researches either in ruminants or in chickens demonstrated that the organic Zn sources significantly differed in their chelation strengths [6, 24, 25]. Relative bioavailability of Zn, evaluated in lambs supplemented with high concentrations of Zn, appeared to differ among different organic Zn sources, with only one of three organic Zn sources evaluated being more bioavailable than reagent-grade Zn sulfate [6]. Another study in steers also found that Zn-Gly was more bioavailable than Zn sulfate or Zn–Met [12]. A series of experiments in chickens from our lab demonstrated that not all organic Zn sources were more effective for chicks than its inorganic form, and their efficacies were closely related to their chelation strengths [25–27]. To our knowledge, no studies have been carried out yet to investigate the relative efficacies of organic Zn sources with different chelation strengths in influencing lactation performance, Zn metabolism, and immune response of lactating cows compared to its inorganic Zn sulfate.
The Zn source may also affect rumen microbes and their degradation activities for feed fiber and protein in ruminants [12]. If organic Zn sources remain complexed or chelated in the ruminal environment, they may affect this rumen function differently from inorganic Zn. It has been reported that steers supplemented with Zn proteinate had higher ruminal soluble Zn concentrations than those supplemented with Zn oxide [8]. Also, in steers, Zn–Met supplementation resulted in the higher ruminal soluble Zn concentration and lower ruminal volatile fatty acid proportion in comparison with Zn sulfate and Zn-Gly [12]. Recent in vitro experiment from our lab indicated that the organic Zn sources with different chelation strengths were stable in the rumen, and the bypass percentages of Zn from the organic Zn sources with strong, moderate, and weak different complex strengths were 99.6 %, 92.2 % and 94.9 %, respectively [24]. It is assumed that the ruminal fermentation might be differently affected by the inorganic Zn source and the organic Zn sources with different chelation strengths. However, no information on this aspect has been available yet so far. Therefore, the present study was conducted to evaluate the effect of organic Zn sources with different chelation strengths in comparison to inorganic Zn sulfate source on the performance, Zn status, and immune response as well as rumen fermentation of lactating cows.
Materials and Methods
Two experiments were conducted, and a completely randomized design was adopted in the present study. The first experiment (Experiment 1) was an in vivo cow feeding trial, and the second (Experiment 2) was an in vitro rumen fermentation trial. All three organic Zn sources used in both experiments had the same characteristics as those from the previous studies [24, 25]. These Zn sources included Zn amino acid chelate with a weak chelation strength (Zn-AA W, Q f = 6.55, containing 119.3 g of Zn per kilogram; Zinpro Corp., Eden Prairie, MN, USA), Zn-proteinate chelate with a moderate chelation strength (Zn-Pro M, Q f = 30.7, containing 132.7 g of Zn per kilogram; Fenyahua Bioengineering Co., Changzhi, China), and Zn proteinate chelate with a very strong chelation strength (Zn-Pro S, Q f = 944.0, containing 186.1 g of Zn per kilogram; Alltech Inc., Nicholasville, KY, USA). Both Zn-Pro M and Zn-Pro S are chelated Zn products between Zn, amino acids, and small peptides. Reagent-grade Zn sulfate (ZnSO4·7H20) was used as the inorganic Zn source. Three organic Zn sources were chosen with similar solubility in buffers at pH 2 or 5. Care, handling, and sampling of the animals used in this study were approved by the Office of the Beijing Veterinarian.
Experiment 1: In Vivo Feeding Trial
A total of 30 multiparous Holstein lactating cows with the average body weight of 578.57 ± 7.60 kg and milk yield of 25.60 ± 1.91 kg/day at the same lactation period were selected in this experiment and randomly allocated by body weight and milk yield to one of five treatments with six replicate pens of one cow each. All cows were housed in individual pens equipped with automatic waterers and fed a total mixed ration (TMR) based on corn silage but no Zn addition for 15 days to accommodate the experimental feeding system and also to deplete the body Zn storage of cows and, thus, enhance their sensitivity to Zn addition. After a 15-day adjustment and Zn depletion period, cows were then fed the above Zn unsupplemented basal TMR (the control) or the basal TMR supplemented with 40 mg Zn/kg TMR from either Zn sulfate or one of three organic Zn sources (Zn-AA W, Zn-Pro M, and Zn-Pro S), respectively, for 55 days. Animals were vaccinated with type O-Asia I bivalent inactivated vaccines (Division of Biological Products, Lanzhou Veterinary Research Institute, Lanzhou, Chinese Academy of Agricultural Sciences, Lanzhou, China) against foot and mouth disease (FMD) through neck intramuscular injection (3 mL/head) on day 6 of the feeding trial. The cows had been vaccinated for FMD 6 months before the start of the experiment as recommended by the Ministry of Agriculture of China because FMD has been ranked as one of the most important infectious diseases today in China by Chinese Veterinary Officials.
The ingredients and composition of the basal TMR were shown in Table 1. It was formulated to meet all nutrient requirements recommended by National Research Council (NRC) [28] for lactating cows based on the body weight of 580 kg, milk yield of 25 kg, and milk fat of 3.5 % with the exception of Zn (containing 37.60 mg Zn/kg TMR by an analysis). Zn in experimental diets was added as either Zn sulfate or one of three organic Zn sources to the premix using finely ground limestone as a carrier and mixed with the concentrate twice a day before feeding. Cows were offered the TMR diets daily at 0700 and 1700 h in two equal portions. All animals had free access to water containing undetectable concentrations of Zn.
Feeds and refusals were weighed and sampled weekly for calculation of the amount consumed and stored at room temperature for subsequent analysis. Cows were milked twice a day and milk yield was recorded automatically at each milking. Samples were taken from each udder half for analyses of composition and SCC on days 18, 36, or 54. Blood samples were collected from each cow before morning feeding via jugular venipureture into heparinized and nonheparinized vacuum tubes on day 27 or 55 of the experiment and then centrifuged at 11,000 ×g for 15 min at 4 °C to harvest plasma and serum for analyses of ALP activity and the antibody titer against FMD, respectively.
Experiment 2: In Vitro Rumen Fermentation Trial
A 4 × 3 factorial arrangement of treatments was used in this experiment. Four Zn sources were the same as in Experiment 1, and three supplemental Zn levels in the rumen fluid were 0, 10, and 20 μg/mL, respectively. The basal diet in Experiment 1 was used as solid rumen fermentation digesta. Because four Zn sources shared the same basal diet control with no supplemental Zn, there were a total of nine treatments in this experiment. There were three replicate tubes of one fermentation vessel each for each treatment.
In vitro rumen fermentation technique as described by Zhao and Feng [29] was adopted. The fermentation appratus was equipped with test tubes as fermentation vessels with a capacity of 100 mL for each vessel. The constant temperature bath oscillator was used to maintain the fermentation temperature. Whole ruminal contents (approximately 300 mL) were collected from three ruminally fistulated Holstein lactating cows fed the basal TMR for 7 days. The fluid from all three cows was mixed, placed in preheated vacuum containers, transported to the laboratory, and strained through four layers of cheese cloth. The artificial buffer saliva was prepared as described by Baumgardt et al. [30]. The filtrated rumen fluid was mixed with the artificial buffer saliva as a ratio of 1:1 to form the mixed fluid and then to be placed into a glass bottle providing carbon dioxide continuously and maintained at 39 °C by the circulating water both. The basal diet was ground to pass a 1-mm screen and preserved at 4 °C as solid rumen digesta. The fermentation vessels were filled with 50 mL of mixed fluid, 0.5 g of solid rumen digesta, 1 mL of Met [31], 1 mL of urea supplement, and 1 mL of Zn solution from one of four Zn sources. The Zn concentrations in Zn solutions added as one of four Zn sources were 0, 530, or 1,060 μg/mL that provided 0, 10, or 20 μg/mL of Zn in the rumen fermentation fluid, respectively. All fermentation vessels were placed into the constant temperature bath oscillator after the ruminal contents were thoroughly stirred and continually fermented for 2 days. Carbon dioxide was bubbled into each tube initially to establish an anaerobic environment.
After a 24-h period of fermentation, the fermentation vessels were chilled by iced water and the ruminal contents were filtrated with the weighed nylon bags and then the filtrated rumen fluid was placed into two 50-mL vacuum tubes. One part was used to measure pH, NH3-N, and the total volatile fatty acids (TVFA), and another part was centrifuged at 11,000 ×g for 15 min at 10 °C to obtain the supernatant and excreta. The supernatant was used to determine Zn concentration, whereas the excreta was used to determine the contents of Zn, bacteria, and bacteria protein. The unfiltrated ruminal contents in nylon bags were dried to analyze dry matter (DM) content.
Sample Analyses
Feed samples and the unfiltrated ruminal contents in nylon bags were dried to a constant weight at 65 °C in a forced-air drying oven, ground through a 1-mm stainless steel screen, and analyzed for DM, crude protein (CP), crude fiber, ether extract, ash, Ca, and P according to Association of Official Analytical Chemists (AOAC) [32]. Zn concentrations in diets, water, plasma, and the filtrated ruminal fluid (supernatant and excreta) were determined by inductively coupled plasma emission spectroscopy (Model IRIS Intrepid II, Thermo Jarrell Ash Corporation, Waltham, MA, USA) as described by Huang et al. [25]. Unhomogenized milk samples were analyzed for contents of total solids (TS), fat, CP, and lactose using a near-infrared spectrometer (Technicon InfraAlyzer 450, Bran and Luebbe SL, Nordersted, Germany) by the method of Albanell et al. [33]. The milk SCC was determined using an automatic cell counter (Fossomatic 250, Foss-Electric, Hillerod, Denmark), serum ALP activity by a kinetic assay (ALP 20, Sigma Diagnostics Inc., St. Louis, MO, USA), and serum antibody titer by the liquid phase blocking enzyme linked immunosorbent assay (ELISA) using commercially available assay kits (Type O and Asia I antibody assay kits against FMD, Division of Biological Products, Lanzhou Veterinary Research Institute, Lanzhou, China). The pH in filtrated ruminal fluid was determined immediately using a portable pH meter within 4 to 5 min of sampling, the NH3-N concentration by colorimetric method, and TVFA by gas liquid chromatography (Model 3380, Varian, Walnut Creek, CA, USA) using a Nikol fused silica column (15 m, 0.53 mm i.d., and 0.50 μm film thickness; Supelco, Bellefonte, PA, USA). The microbial fractions were separated by differential centrifugation and bacterial counts were determined as described by Frumholtz et al. [34] and expressed as colony forming units (log10 cfu) per gram. The bacterial N was determined using the method of AOAC [32].
Statistical Analyses
The data from body weight, feed intake, and milk production of lactating cows in Experiment 1 were analyzed by one-way analysis of variance using the General Linear Models (GLM) procedure of statistical analysis system (SAS) [35], and the model included the effect of treatment. Statistical analyses of milk composition and SCC, plasma, and serum parameters were subjected to two-way analysis of variance using the GLM procedure of the SAS, and the model included the main effects of treatment, sampling time point (days) and their interaction. Pen (individual cow) served as the experimental unit. Differences among means were tested by the least significant difference (LSD) method, and the significance was declared at P < = 0.05. The interactions (P < 0.05) between treatment and the sampling time point (days) were observed for plasma Zn and antibody titer; therefore, the effect of treatment on these indices was presented by the sampling time point (days). However, there were no treatment × sampling time point (days) interactions (P > 0.05) observed for milk composition, SCC, and serum ALP activity; therefore, these data were pooled across sampling time points and the main effects of treatments were reported. All data in Experiment 2 were analyzed by two-way analysis of variance using the GLM procedure of the SAS, and the model included the main effects of Zn source, added Zn level, and their interaction. The replicate tube served as the experimental unit.
Results
Feed Intake, Milk Yield, and Quality of Lactating Cows (Experiment 1)
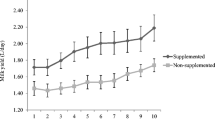
Data were listed in Table 2. Dietary treatment affected (P = 0.009) daily milk yield but did not affect (P > 0.05) daily feed intake, milk contents of fat, lactose, protein and TS, and milk SCC. Compared to the control, the milk yield was increased (P < 0.05) by supplementation of Zn from either Zn-Pro S or Zn-AA W but was not affected (P > 0.05) by supplementation of Zn from either Zn-Pro M or Zn sulfate. Among Zn sources, the cows fed the TMR supplemented with Zn-Pro S had a higher (P < 0.05) milk yield than those fed the TMR supplemented with Zn-Pro M or Zn sulfate, and the cows fed on the Zn-AA W TMR had a higher (P < 0.05) milk yield than those fed on the Zn-Pro M TMR. However, no differences (P > 0.05) were observed between the Zn-Pro S and Zn-AA W treatments or the Zn-Pro M and Zn sulfate treatments.
Plasma or Serum Parameters of Lactating Cows (Experiment 1)
Data were listed in Table 3. Dietary treatment did not affect (P > 0.05) serum ALP activity. Plasma Zn concentration (P = 0.760) on day 27 did not differ among treatments but reached the significant (P < 0.015) level on day 55. Compared to the control, plasma Zn concentration on day 55 was increased (P < 0.05) by supplementation of Zn from any of the three organic Zn sources, but was not affected (P > 0.05) by supplementation of Zn from the Zn sulfate. Among Zn sources, the cows fed on the Zn-Pro S TMR had a higher (P < 0.05) plasma Zn concentration on day 55 than those fed on the Zn-Pro M or Zn sulfate TMR, but there were no differences (P > 0.05) among Zn-Pro M, Zn-AA W, and Zn sulfate treatments or between Zn-Pro S and Zn-AA W treatments.
Immune Function (Experiment 1)
Data were given in Table 3. Dietary treatment affected (P = 0.003) the serum antibody titer on day 27 against the FMD vaccine but did not affect (P = 0.993) it on day 55. Compared to the control, the serum antibody titer on day 27 was increased (P < 0.05) by supplementation of Zn from either Zn-Pro S or Zn-AA W, but was not affected (P > 0.05) by supplementation of Zn from either Zn-Pro M or Zn sulfate. Among Zn sources, the cows fed on the Zn-Pro S or Zn-AA W TMR had a higher (P < 0.05) serum antibody titer than those fed on the Zn-Pro M or Zn sulfate TMR, and no difference (P > 0.05) was found between Zn-Pro S and Zn-AA W treatments or between Zn-Pro M and Zn sulfate treatments.
In Vitro Rumen Fermentation Parameters (Experiment 2)
Data were given in Table 4. Zn source, level, and their interaction did not affect (P = 0.940) the NH3-N concentration in the filtrated rumen fluid. All other rumen fermentation indices were influenced (P < 0.001) by both Zn source and level. An interaction between Zn source and level did not affect (P > 0.05) the pH and the soluble Zn concentration in the filtrated rumen fluid but affected (P < 0.001) all other indices. There were no differences (P > 0.05) in the rumen pH among Zn-Pro S, Zn-Pro M, and Zn sulfate sources, but the rumen pH values of these three Zn source treatments were all higher (P < 0.05) than that of the Zn-AA W treatment. Supplemental Zn decreased (P < 0.001) the rumen pH, but no difference (P > 0.05) was observed between the two supplemental Zn levels. There was a higher (P < 0.05) rumen soluble Zn concentration for the Zn-AA W treatment than for all other Zn source treatments or for the Zn-Pro S treatment than for the Zn sulfate treatment. However, no difference (P > 0.05) was found between Zn-Pro S and Zn-Pro M sources or between Zn-Pro M and Zn sulfate sources. The rumen soluble Zn linearly increased (P < 0.05) as the supplemental Zn increased.
As the rumen supplemental Zn increased from 0 to 10 μg/mL, both the rumen DM degradability and TVFA did not increase (P > 0.05) for Zn sulfate source but increased (P < 0.05) for all organic Zn sources, and both the rumen bacteria and bacterial N concentrations increased (P < 0.05) for all Zn sources. However, as supplemental Zn increased from 10 to 20 μg/mL, all of the above four indices decreased (P < 0.05) for all Zn sources, indicating that the supplemental Zn level of 20 μg/mL regardless of Zn source was obviously excessive for the in vitro fermented rumen microbes. The three organic Zn sources were more effective (P < 0.05) than the inorganic Zn sulfate in improving the in vitro rumen DM degradability, TVFA, and bacteria concentrations, but there were no differences (P > 0.05) in the rumen TVFA among the three organic Zn sources. The Zn-AA W was more effective (P < 0.05) than both the Zn-Pro M and Zn-Pro S sources in improving the DM degradability and bacteria concentration, and the Zn-Pro M was more effective (P < 0.05) than the Zn-Pro S in improving the DM degradability, but no difference (P > 0.05) was observed in bacteria concentration between the Zn-Pro M and Zn-Pro S sources. At the supplemental Zn level of 10 μg/mL, both the Zn-Pro M and Zn-Pro S sources were more effective (P < 0.05) than both the Zn-AA W and inorganic Zn sulfate sources, and the Zn-Pro M was more effective (P < 0.05) than the Zn-Pro S in increasing the rumen bacterial N content, but no difference (P > 0.05) was observed between the Zn-AA W and inorganic Zn sulfate sources. The rumen bacterial Zn content increased significantly and linearly (P < 0.05) regardless of Zn source as the supplemental Zn level increased. At either of the two supplemental Zn levels, the bacterial Zn contents were higher (P < 0.05) in the inorganic Zn sulfate treatment than in all other organic Zn treatments, in the Zn-Pro M treatment than in both the Zn-AA W and Zn-Pro S treatments, or in the Zn-Pro S treatment than in the Zn-AA W treatment.
Discussion
Feed Intake, Milk Yield, and Quality of Lactating Cows
The Zn supplementation, regardless of source, had no effect on the feed intake of lactating cows in the present study, which was in agreement with the results of earlier studies in dairy cows [17, 18], in lambs [16], or in beef steers [7–9, 14, 15]. However, supplementations of Zn from inorganic source or the organic sources with different chelation strengths had different impacts on the milk yield. Both the Zn-Pro S and Zn-AA W, instead of Zn-Pro M and Zn sulfate, increased the milk yield and had similarly higher efficacies than the Zn-Pro M, suggesting that the chelation strength of organic Zn sources might be one factor affecting their efficacies in milk production. Similarly, different results have been reported regarding the effect of organic Zn source on the performance in comparison to inorganic Zn sources in dairy cows or dairy goats [10, 11, 17–19, 36]. In addition, Cao et al. [6] reported that one of three organic Zn sources was more available to lambs than the inorganic Zn sulfate. In steers, Spears et al. [12] also found that the Zn-Gly was more available than either Zn sulfate or Zn–Met. A recent study [25] from our lab demonstrated that not all organic Zn sources were more effective for chicks than its inorganic form, and their efficacies were closely related to their chelation strengths. Therefore, it was thought that the disparities among different Zn sources in this study and different reports might be due to the differences in the chelation strengths of organic Zn sources.
The results of unaffected milk composition in the present study were consistent with the previous reports in dairy goats [10] or in dairy cows [11, 17, 18, 37], indicating that the milk composition was not sensitive in response to dietary supplementation of Zn from either the inorganic Zn source or the organic sources with different chelation strengths. A number of studies have shown that Zn supplementation could reduce milk SCC [10, 17, 20, 21]. However, the conflicting results have been reported on the effect of Zn supplementation from different forms on milk SCC [3, 11, 17, 19]. In the present experiment, Zn supplementation, regardless of Zn source, had no influence on milk SCC. The disparities among reports might have resulted from the different initial levels of milk SCC as described by Cope et al. [17] who concluded that beneficial effects of inorganic or organic Zn on SCC were dependent on its initial levels. Studies on the effect of the organic Zn sources with different chelation strengths on milk yield and quality of lactating cows have not been reported before.
Plasma or Serum Parameters of Lactating Cows
The present result indicated that the three organic Zn sources were more effective than the inorganic Zn sulfate, and the Zn-Pro S was the most effective in increasing plasma Zn concentration at the end of the experiment. Conflicting results of Zn source on plasma Zn have been reported in previous studies in lambs [16], in calves [13], in beef steers [7, 8, 12, 15], or in dairy cows [17]. Many factors might contribute to the discrepancies among the above reports, such as chemical characteristics of organic Zn sources used, animal species, dietary Zn levels, duration of the trial, and factors affecting Zn absorption in the gut. Limited information has been available regarding absorptions and utilizations of Zn from different Zn sources in ruminants [2, 38]. However, the differences in rumen bypass and the small intestinal concentrations of Zn among the Zn sources [24] were just in good agreement with the changing trends of the plasma Zn concentration in the present study. It is inferred that the rumen bypass of Zn into the intestine to be absorbed or utilized might be the main factor affecting the body Zn status and thus the alteration of immune response and milk production. Therefore, the cows given the Zn-AA W or Zn-Pro S TMR showed the improved Zn status, immune response, and milk production as compared to the cows fed on the Zn-AA M TMR.
The ALP is a Zn-containing metalloenzyme, and serum ALP activity has been used as an indicator of animal Zn status. However, the affected serum ALP activity was not observed in the current study. Similar results were also reported in several earlier studies [2, 9, 12, 13]. It is speculated that the serum ALP activity might not be the sensitive index for assessment of Zn status, and more reliable measurements, such as tissue Zn [12, 13], metallothionein (MT) concentration, and gene expressions [6, 9, 25, 39], need to be detected to evaluate Zn status of animals supplemented with different forms of Zn.
Immune Function of Lactating Cows
As Zn is needed to maintain the normal activity and integrity of lymphocytes and immune system, its supplementation to diets could improve immune functions. The results from the present study indicated that the Zn addition as either the Zn-Pro S or the Zn-Pro W significantly improved the serum antibody titer on day 27 compared to the control, Zn-Pro M, or Zn sulfate. Similarly, the different impacts on immune function among Zn sources were also observed in earlier studies [3, 5, 8, 14, 15, 22]. Therefore, as mentioned above, the chelation strength of organic Zn sources might be one of the key factors affecting their efficacies in immune response. Comparisons of the results of Zn status and humoral immune response with those of milk yield suggested that the improved Zn status and immune function in lactating cows fed the basal TMR supplemented with either the Zn-AA W or Zn-Pro S might be the reasons for the increased milk yield.
In Vitro Rumen Fermentation Parameters
The ruminal pH depends upon the TVFA and NH3-N degraded from fermented substrate in the rumen. Due to the inconsistent change patterns between ruminal pH and TVFA along with unaffected NH3-N among Zn sources, it is inferred that the ruminal pH might be affected by some other factors, such as pKa of Zn sources that act as acids at neutral pH. Though the ruminal pH was affected by Zn source or level, these data were still within the normal range of 6 to 7, indicating that the in vitro rumen fermentation was normal. Rumen TVFA is related to the type of diet and mainly, results from the degradation of fiber and other carbohydrates. Therefore, the present results showed the similarly improved DM degradability and TVFA production at Zn addition as either of the three organic Zn sources compared to the control or Zn sulfate. However, Spears et al. [12] found that the TVFA concentration was lowered in steers fed the diets supplemented with Zn–Met or Zn-Gly compared to the control or Zn sulfate. The NH3-N production in the rumen is also connected with the type of diet and comes from the degradation of feed protein or nonprotein N. The unaffected NH3-N concentrations among treatments reflected the balance among NH3-N degraded from dietary protein, biosynthesis of bacterial protein, and removal via absorption and passage to the omasum.
Rumen bacteria require N for growing and thus degrading carbohydrate. The primary forms of N that are transported into bacterial cells are ammonia, free amino acids, and some short peptides produced mainly from degradation of feed protein. Therefore, to some extent, bacteria yield and bacterial protein measurements might reflect the greater microbial efficiency in N incorporation and fermentative activity. As like DM degradability and TVFA production, the bacteria and bacterial N were higher at Zn addition as either of the three organic Zn sources compared to the control or Zn sulfate, suggesting that the improved DM degradation and TVFA production might have resulted from the increased bacteria growth or reproduction, and thus better rumen fermentation. Among the three organic Zn sources, the Zn-AA W appeared to be the most effective in improving DM degradability and bacteria reproduction.
As expected, as more Zn was soluble in the rumen environment, it was then taken up less by rumen microorganisms. Both the Zn-AA W and Zn-Pro S in the present study had more ruminal soluble Zn or less bacterial Zn compared to the Zn-Pro M or Zn sulfate, suggesting that the Zn-AA W and Zn-Pro S might have interacted to a lesser degree than Zn-Pro M or Zn sulfate in the rumen to form insoluble chelates. Similarly, earlier studies reported that steers receiving Zn proteinate [8] or Zn polysaccharide complex [40] had higher ruminal soluble Zn concentrations than those receiving Zn oxide. Also, in steers, Zn–Met supplementation resulted in higher ruminal soluble Zn concentrations in comparison with Zn sulfate or Zn-Gly [12]. The more soluble Zn-AA W and Zn-Pro S in the rumen might explain why Zn-AA W and Zn-Pro S were more effective than Zn-Pro M or Zn sulfate in enhancing rumen fermentation.
Despite of limited research on the effect of Zn source on rumen fermentation, the above results from the in vitro rumen fermentation in the current study clearly indicated that the inorganic Zn sulfate or the three organic sources with different chelation strengths did impact the rumen fermentation differently. In general, the three organic Zn sources with different chelation strengths were more effective than the inorganic Zn sulfate, and among the organic Zn sources, both the Zn-AA W and Zn-Pro S, especially the Zn-AA W, were more effective than the Zn-Pro M in enhancing rumen fermentation through DM degradation, TVFA production, bacterial growth, and protein biosynthesis. These results could partially explain why the lactating cows fed the TMR supplemented with either the Zn-AA W or Zn-Pro S enhanced the immune function, and thus increased milk production, which has not been reported before.
Conclusions
Zn source did not influence the feed intake, milk composition, and SCC of lactating cows. However, both the Zn-AA W and Zn-Pro S were more effective than the Zn-Pro M and Zn sulfate in enhancing the rumen fermentation, Zn status, and humoral immune response as well as improving milk yield. The improved milk production might be attributed to the improved rumen fermentation, Zn status, and immune function. There were similar efficacies between the Zn-AA W and Zn-Pro S or between the Zn-Pro M and Zn sulfate in dairy cows.
References
Gaither LA, Eide DJ (2001) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270
Spears JW (1989) Zinc methionine for ruminants: relative bioavailability of zinc in lambs and effects on growth and performance of growing heifers. J Anim Sci 67:835–843
Spears JW, Harvey RW, Brown TT Jr (1991) Effects of zinc methionine and zinc oxide on performance, blood characteristics, and antibody titer response to viral vaccination in stressed feeder calves. J Am Vet Med Assoc 199:1731–1733
Rojas LX, McDowell LR, Martin FG, Wilkinson NS, Johnson AB, Njeru CA (1996) Relative bioavailability of zinc methionine and two inorganic zinc sources fed to cattle. J Trace Elem Med Biol 10:205–209
Kincaid RL, Chew BP, Cronrath JD (1997) Zinc oxide and amino acid as sources of dietary zinc for calves: effects on uptake and immunity. J Dairy Sci 80:1380–1388
Cao J, Henry PR, Guo R, Holwerda RA, Toth JP, Littell RC, Miles RD, Ammerman CB (2000) Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J Anim Sci 78:2039–2054
Malcolm-Callis KJ, Duff GC, Gunter SA, Kegley EB, Vermeire DA (2000) Effects of supplemental zinc concentration and source on performance, carcass characteristics, and serum values in finishing beef steers. J Anim Sci 78:2801–2808
Spears JW, Kegley EB (2002) Effect of zinc source (zinc oxide vs. proteinate) and level performance, carcass characteristics, and immune response of growing and finishing steers. J Anim Sci 80:2747–2752
Kessler J, Morel I, Dufey FA, Stern A, Geyes H (2003) Effect of organic zinc sources on performance, zinc status, and carcass, meat, and claw quality in fattening bulls. Livest Prod Sci 81:171–181
Salama AA, Caja G, Albanell E, Such X, Casals R, Plaixats J (2003) Effects of dietary supplements of zinc–methionine on milk production, udder health and zinc metabolism in dairy goats. J Dairy Res 70:9–17
Kellogg DW, Tomlinson DJ, Socha MT, Johnson AB (2004) Effects of zinc methionine complex on milk production and somatic cell count of dairy cows: twelve-trial summary. Prof Anim Sci 20:295–301
Spears JW, Schlegel P, Seal MC, Lloyd KE (2004) Bioavailability of zinc from zinc sulfate and different organic zinc sources and their effects on ruminal volatile fatty acid proportions. Livest Prod Sci 90:211–217
Wright CL, Spears JW (2004) Effect of zinc source and dietary level on zinc metabolism in Holstein calves. J Dairy Sci 87:1085–1091
Mandal GP, Dass RS, Isore DP, Garg AK, Ram GC (2007) Effect of zinc supplementation from two sources on growth, nutrient utilization and immune response in male crossbred cattle (Bos indicus × Bos taurus) bulls. Anim Feed Sci Technol 138:1–12
Nunnery GA, Vasconcelos JT, Parsons CH, Salyer GB, Defoor PJ, Valdez FR, Galyean ML (2007) Effects of source of supplemental zinc on performance and humoral immunity in beef heifers. J Anim Sci 85:2304–2313
Garg AK, Mudgal V, Dass RS (2008) Effect of organic zinc supplementation on growth, nutrient utilization and mineral profile in lambs. Anim Feed Sci Technol 144:82–96
Cope CM, Mackenzie AM, Wilde D, Sinclair LA (2009) Effect of level and form of dietary zinc on dairy cow performance and health. J Dairy Sci 92:2128–2135
Sobhanirad S, Carlson D, Kashani RB (2010) Effect of zinc methionine or zinc sulfate supplementation on milk production and composition of milk in lactating dairy cows. Biol Trace Elem Res 136:48–54
Whitaker DA, Eayres HF, Aitchison K, Kelly JM (1997) No Effect of a dietary zinc proteinate on clinical mastitis, infection rate, recovery rate and somatic cell count in dairy cows. Vet J 153:197–204
Pechová A, Pavlata L, Lokajová E (2006) Zinc supplementation and somatic cell count in milk of dairy cows. Acta Vet Brno 75:355–361
Gaafar HMA, Basiuoni MI, Ali MFE, Shitta AA, Shamas ASE (2010) Effect of zinc methionine supplementation on somatic cell count in milk and mastitis in Friesian cows. Archiva Zootechnica 13:36–46
Nagalakshmi D, Dhanalakshmi K, Himabindu D (2009) Effects of dose and source of supplemental zinc on immune response and oxidative enzymes in lambs. Vet Res Commun 33:631–644
Pechová A, Misurova L, Pavlata L, Dvorak E (2009) The influence of supplementation of different forms of zinc in goats on the zinc concentration in blood plasma and milk. Biol Trace Elem Res 132:112–121
Liang JG, Lin L, Luo XG, Diao QY, Liu B (2008) Physical and chemical characteristics of supplemental organic zinc sources and their stabilities in vitro fermentation rumen. Acta Vet Zootech Sin 39:1355–1366
Huang YL, Lu L, Li SF, Luo XG, Liu B (2009) Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J Anim Sci 87:2038–2046
Yu Y, Lu L, Luo XG, Liu B (2008) Kinetics of zinc absorption by in situ ligated intestinal loops of broilers involved in zinc transporters. Poult Sci 87:1146–1155
Yu Y, Lu L, Wang RL, Xi L, Luo XG, Liu B (2010) Effects of zinc source and phytate on zinc absorption by in situ ligated intestinal loops of broilers. Poult Sci 89:2157–2165
NRC (2001) Nutrient requirements of dairy cattle, 7th edn. National Academy Press, Washington
Zhao GY, Feng YL (1996) The effects of water temperature on ruminal fermentation using in vitro rumin fermentation method. Chin J Anim Sci 32:8–10
Baumgardt BR, Taylor MW, Cason JL (1962) Evaluation of forages in the laboratory. II. Simplified artificial rumen procedure for obtaining repeatable estimates of forage nutritive value. J Dairy Sci 45:62–68
Lundquist RG, Stern MD, Otterby DE, Linn JG (1985) Influence of methionine hydroxy analog and DL-methionine on rumen protozoa and volatile fatty acids. J Dairy Sci 68:3055–3058
AOAC (2000) Official methods of analysis, 21st edn. Association of Official Analytical Chemists, Arlington
Albanell E, Ca'ceres P, Caja G, Molina E, Gargouri A (1999) Determination of fat, protein, and total solids in bovine milk by near-infrared spectroscopy. J Assoc Off Anal Chem 82:753–758
Frumholtz PP, Newbold CJ, Wallace RJ (1989) Influence of Aspergillus oryzae fermentation extract on the fermentation of a basal ration in the rumen simulation technique (Rusitec). J Agric Sci 113:169–172
SAS (1998) Statistical analysis system. SAS user's guide: statistics. SAS Inst Inc, Cary
Campbell MH, Miller JK, Schrick FN (1999) Effect of additional cobalt, copper, manganese, and zinc on reproduction and milk yield of lactating dairy cows receiving bovine somatotropin. J Dairy Sci 82:1019–1025
Nocek JE, Socha MT, Tomlinson DJ (2006) The effect of trace mineral fortification level and source on performance of dairy cattle. J Dairy Sci 89:2679–2693
Spears JW (1996) Organic trace minerals in ruminant nutrition. Anim Feed Sci Technol 58:151–163
Cao J, Henry PR, Davis SR, Cousins RJ, Miles RD, Littell RC, Ammerman CB (2002) Relative bioavailability of organic zinc sources based on tissue zinc and metallothionein in chicks fed conventional dietary zinc concentrations. Anim Feed Sci Technol 101:161–170
Kennedy DW, Craig WM, Southern LL (1993) Ruminal distribution of zinc in steers fed a polysaccharide–zinc complex or zinc oxide. J Anim Sci 71:1281–1287
Acknowledgments
This study was supported by the special foundation of the Chinese Academy of Agricultural Sciences (CAAS) for the first-place distinguished scientists (Beijing, People's Republic of China), the Research Program of the State Key Laboratory of Animal Nutrition (project no. 2004DA125184G1108; Beijing, People's Republic of China), and the Program of the National Natural Science Foundation of China (project no. 30871798; Beijing, People's Republic of China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, R.L., Liang, J.G., Lu, L. et al. Effect of Zinc Source on Performance, Zinc Status, Immune Response, and Rumen Fermentation of Lactating Cows. Biol Trace Elem Res 152, 16–24 (2013). https://doi.org/10.1007/s12011-012-9585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9585-4