Abstract
An experiment of 150 days was conducted on 42 male Nellore lambs (28.3 ± 0.64 kg) to determine the effect of zinc (Zn) supplementation (0,15, 30 and 45 ppm) in diet from inorganic (ZnSO4) and organic (Zn proteinate) sources on immune response and antioxidant enzyme activities by allotting them randomly to 7 groups in completely randomized design. The basal diet (BD) contained 29.28 ppm Zn. The humoral immune response assessed at 75 d against B. abortus was higher (P<0.01) with 15 or 30 ppm Zn supplementation from organic source. The dose and source had no effect on titres against chicken RBC antigen. The cell mediated immune response assessed as delayed type hypersensitivity (DTH) response against phytohaemagglutinin-P and in vitro lymphocyte proliferative response against concanavalin A at 150 d was higher (P<0.05) at 15 ppm Zn supplementation compared to BD fed lambs. Supplementation of 45 ppm Zn had no positive effect on immune response. The DTH response and antibody titres against B.abortus were higher (P< 0.05) on Zn proteinate compared to ZnSO4 at 15 ppm Zn supplementation. The lipid peroxidase activity was lower (P < 0.01), while the RBC superoxide dismutase and catalase activities were higher (P < 0.01) in lambs at 15 ppm Zn supplementation compared to BD diet fed lambs, assessed at 75 d of feeding. Serum globulin concentration and alkaline phosphatase (ALP) activity (75 d of experiment) was higher in Zn supplemented lambs. The ALP activity increased (P < 0.01) with increase in Zn supplementation and being higher when supplementation was from Zn proteinate compared to ZnSO4. The study indicated that 15 ppm zinc supplementation was required for obtaining higher immune response in lambs when fed a basal diet containing 29.28 ppm Zn and supplementation as Zn proteinate had higher antioxidant enzyme activities and immune response compared to ZnSO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The livestock is exposed to many nutritional (micronutrient deficiency, iron overload, mycotoxins, pesticides and heavy metals) and environmental (temperature, humidity, hyperoxia, pollutants) stressors causing over production of free radicals (reactive oxygen species; ROS) in the body. These free radicals cause damage to immune cells and their receptors, compromising with their function (Wu and Meydani 1998). Zinc (Zn) deficiency reduces immune responses (Chesters 1997) and impairs the antioxidant defense system (Miller et al. 1993). Zinc has a major role in immune responsiveness (Keen and Gershwin 1990) and a dietary deficiency of Zn has been associated with increased morbidity and mortality (Kincaid et al. 1997). Generally, as animal production intensifies, the requirement for nutrients involved in combating stress might also increase more than the recommendations. The effect of Zn on immune response in pigs and poultry is well documented. In ruminants very little research has been carried out to examine the relationship between dietary zinc and immune function (Spears 2000) and its role in combating oxidative stress. Lambs fed on a semi-purified diet severely deficient in Zn showed a decreased blastogenic response to phytohaemagglutinin P, with reduced lymphocytes count (Droke and Spears 1993), but Droke et al. (1998) observed no affect on cellular and humoral immune responses in lambs fed 25 ppm supplemental Zn following adrenocorticotrophin administration. The organic complexes of minerals are reported to be more bioavailable and hence beneficial to animals. But the effects of organic sources of Zn on immune response have been conflicting. Supplementation of Zn (75 ppm) either as Zn polysaccharide to beef heifers or Zn methionine to feeder calves (Spears et al. 1991) increased the humoral immune response to ovalbumin and bovine herpes virus 1, respectively (Salyer et al. 2004). On the other hand no differences between organic and inorganic sources of Zn was observed by Nunnery et al. (2007) and Galyean et al. (1995) on humoral immunity in beef heifers and morbidity in weaner calves, respectively.
Due to the variation in results regarding the effects of concentration and source of Zn in diet on immune response and its role in antioxidant defense, the present study was undertaken to investigate the effect of Zn at different supplemental levels and from two different sources on immune response and activity of antioxidant enzymes in lambs.
Materials and methods
Animal and feeding management
Forty two Nellore Brown lambs (28.26 ± 0.635 kg) aged about 9–10 months were dewormed before study and randomly allotted to 7 groups (n = 6) in a completely randomized design. A basal diet (BD) was prepared using the locally available feed ingredients (Table 1) to meet the nutrient requirements (ICAR 1998) of lambs except Zn. The estimated Zn content in the BD was 29.28 ppm. Both organic and inorganic sources of Zn were added to the BD at 15, 30 and 45 ppm.
The feed grade zinc sulphate (ZnSO4.7H2O) (Venvet chemicals Pvt. Ltd, India) was used as inorganic source of Zn containing 22% of Zn. The organic source of Zn was in the form of Zn proteinate with 10% Zn (Alltech Inc. Nicholasville, USA). According to the Zn concentration in these sources the appropriate amounts were added to the BD to supply the zinc at 15, 30 and 45 ppm. The animals of group-I were offered BD, group II (15-I) and III (15-O) were offered BD supplemented with 15 ppm Zn from inorganic and organic source, respectively; group IV (30-I) and V (30-O) were fed BD supplemented with 30 ppm Zn from Zn sulphate and Zn proteinate; group VI (45-I) and VII (45-O) were offered BD supplemented with 45 ppm Zn from inorganic and organic source, respectively. Lambs were fed the respective diets at 3.5 per cent of their body weight and with 5 per cent extra allowance throughout 150 days of experimental feeding. The feed intake was calculated at every fortnightly interval according to body weight changes.
Animals were housed indoor with individual feeding and watering facility, fed 3 times a day and fresh drinking water was made available at all times, dewormed at regular intervals and all the lambs were vaccinated against Peste-de-petis and enteroxaemia.
Immunity
Humoral immune response
The effect of the dietary treatments on the humoral immunity of lambs was assessed using heat killed Brucella abortus S99 and chicken RBC as immunogens. After 75 d of experimental feeding, all lambs were sensitized with heat killed Brucella abortus S99 and 20 per cent chicken RBC (CRBC) suspension, administered intramuscularly and a booster dose of antigen was given after 15 days. Prior to administration of antigen, lambs were screened for Brucellosis with Rose Bengal Plate Test (RBPT) (Alton et al. 1975).
Serum was collected from the sensitized lambs on 0, 7, 14, 21, 28 and 35th day of post sensitization to assess humoral immune response. The antibody against Brucella abortus antigen was measured by Indirect enzyme-linked immunosorbant assay (ELISA). The immune response against CRBC was measured by direct haemagglutination assay (DHA) (Wegmann and Smithies 1966).
ELISA was performed as per the procedure described by Perlman and Engvall (1971). The protein concentration of autoclaved B. abortus S99 antigen was determined by Lowry’s method (Lowry et al. 1951). The dose of the above antigen was determined by checkerboard analysis. Accordingly, the protein concentration of antigen was fixed at 40 μg/ml and serum samples were diluted to 1:400. The optical density and hence the seroreactivity was measured at 492 nm in ELISA reader.
Cell Mediated Immune Response (CMI)
In -vivo delayed type hypersensitivity (DTH) reaction
After 150 days of experimental feeding, individual animals from each dietary group were used for DTH test. Media for mitogenic solution contained 10 mg phytohemagglutinin- P (PHA-P) (LE 6- 105617, Genei, Bangalore, India) in 10 ml phosphate buffer for skin test to evaluate CMI response at 0, 24, 48, 72 and 96 h post injection as per the procedure described by Quist et al. (1997)
In- vitro lymphocyte proliferation assay (LPA)
At 150 d of experimental feeding, LPA was carried out to assess the CMI response as per the procedure described by Bounous et al. (1992). The peripheral blood mononuclear cells (PBMC) were separated by using Histopaque-1077 (Sigma, Mumbai, India) by centrifuging at 3000 rpm for 15 min. The PBMC were suspended in RPMI-1640 (AL 028A, Himedia, India) media and washed repeatedly for 3 times. By Trypan blue extrusion technique (Mosmann 1983); the line cell concentration was adjusted to 1 x 107 cells/ml of RPMI 1640 medium. The 0.1 ml of cells was layered in each well, for each sample four wells were assigned viz., two for control [without concanavalin A (Con A)] and two for stimulation (with Con A). The Con A was added at the rate of 0.1 ml per well. The plates were incubated in CO2 incubator at 37°C for 92 h. After incubation the plates were removed and MTT (3-[4,5-dimethyl thiozal-2-yl]-2,5-dimethyl tetrazolium bromide) was added at the rate of 10 μg/well and incubated again for 4 h after that the reaction was stopped by using 0.1 N HCl-Isopropanal (4.0%) and optical density (OD) was measured in ELISA reader at 570 nm. The results were expressed as stimulation index (SI).
Enzyme assays
The alkaline phosphatase activity in serum and the oxidative enzymes viz., catalase, lipid peroxidase and superoxide dismutase were estimated in haemolysate at 75 d of experiment.
Alkaline phosphatase
Serum alkaline phosphatase (ALP) activity was estimated by two points method as described by Bergmeyer (1974)
Preparation of haemolysate
The blood was collected at 75 days of experiment, in a clean sterilized vial containing ethylene diamine tetra acetate @ 1 mg/ml and centrifuged at 1500 rpm for 10 minutes and the red blood cells (RBC) were washed with phosphate buffer saline (PBS) for three times then 1 ml of RBC were added with 4 ml of distilled water to prepare 5% erythrocyte lysate.
Lipid peroxidase
The lipid peroxidase (LPx) activity was estimated as per the method of Placer et al. (1966). Malonyl dialdehyde (MDA), a secondary product of lipid peroxidation reacts with 2-thiobarbituric acid (TBA) to form a trimethine colored substance (pink chromogen), which was extracted into butan-1-ol. The color intensity was measured at 548 nm and lipid peroxides level in the erythrocytes was expressed in nmol MDA/ml haemolysate.
RBC Catalase
RBC haemolysate 2 ml (1:500 dilution) was taken into cuvette and 1 ml of hydrogen peroxide solution was added to it, and optical density was recorded at 240 nm against the blank at 15 second interval and expressed as micromole per minute per ml of haemolysate (Bergmeyer 1983).
RBC Superoxide dismutase (RBC SOD)
To 50 μl of the haemolysate, 100 mM of Tris-hydrochloric acid buffer (pH 8.2), 6 mM ethylene diamine tetra acetate and 0.6 mM pyrogallol were added. An increase in absorbance was recorded at 420 nm for 3 minutes by spectrophotometer according to the method of McCord and Fridovich (1969). One unit of enzyme activity is 50 per cent inhibition of the rate of autooxidation of pyrogallol as determined by change in absorbance/30 sec at 420 nm. The activity of RBC SOD was expressed as units per mg haemoglobin (Hb). The Hb in haemolysate was estimated colorimetrically by using Drabkin’s solution according to the procedure described by Cannan (1958).
Serum proteins
The serum was analysed for protein by Biuret method (Hiller et al. 1927) and serum globulin was analysed by first precipitation of albumin by ammonium sulphate and then determination of globulin in supernatant by Biuret method.
Statistical analysis
Data was analyzed by the GLM procedure of SPSS 12.0 with each lamb as replicate for a completely randomized design with Zn level and source as the two factors of variation. The differences between the mean values were compared using Duncan’s multiple range test (Duncan 1955).
Results
The crude protein and crude fibre contents in the diets ranged from 11.26 to 12.86% and 35.40 to 35.90%, respectively. The sorghum straw, maize, soyabean meal, red gram husk contained 32.66, 31.31, 77.84 and 40.88 ppm Zn. The Zn content in the BD was 29.28 ppm. The Zn was supplemented to the BD at 15, 30 and 45 ppm and the amount of Zn in the diets (44.34, 59.55, 74.30 ppm from inorganic and 45.12, 60.46 and 75.79 ppm from organic source, respectively) was similar to the calculated values.
Humoral immunity
All lambs were seronegative for Brucellosis prior to administration of Brucella antigen. The antibody titres against B. abortus antigen was higher (P < 0.01) in Zn supplemented lambs, irrespective of dose and source at 14th and 21st d of post sensitization compared to unsupplemented lambs (Table 2). At 7th d post sensitization the antibody titres were higher (P < 0.01) in lambs fed 30 ppm Zn from inorganic source compared to other groups. On 14 d, the titres were higher (P < 0.01) in lambs on 15 ppm Zn from organic source compared to BD fed lambs. The peak titres were observed on 21st d post sensitization and the titres were 0.96 on BD and ranged from 1.03 to 1.24 in Zn supplemented lambs. The titres were higher (P < 0.01) at 15 and 30 ppm Zn supplemented from organic source. Supplementing 45 ppm Zn had no positive effect on antibody titres against B. abortus. The titres on 28 and 35th d was comparable among all the diets. The haemagglutination (HA) titres against CRBC were comparable among unsupplemented and Zn supplemented lambs on 7, 14, 21, 28 and 35 d post sensitization (Table 2). The dose and source of Zn in diet did not influence the antibody response against CRBC. The peak titres were observed on 7th d of post inoculation (6.17–8.17) and there after reduced gradually.
Cell mediated immunity
The effect of dose and source of supplemental Zn at various levels and from different sources on CMI response measured as DTH response against PHA-P and in vitro lymphocyte proliferation response against Con A is presented in Table 3. The DTH response was maximum 24 h after PHA-P injection and later gradually decreased at 48, 72 and 96 h in all experimental lambs. Supplementation of 15 ppm Zn enhanced (P < 0.01) the DTH response compared to BD fed lambs. Higher (P < 0.01) DTH response was observed in lambs fed organic compared to inorganic Zn at 15 ppm. The Zn supplementation at higher levels (30 ppm or 45 ppm) had no effect on DTH response. At 72 and 96 h of post inoculation, the DTH response was higher (P < 0.05) in lambs fed 15 ppm Zn as Zn proteinate compared to Zn sulphate. Similar trend was observed for in vitro lymphocyte proliferation response, the proliferative response was higher (P < 0.05) in 15ppm Zn supplemented lambs from inorganic (74.04) or organic (67.74) source compared to those fed BD lambs (42.13). The lymphocyte response was similar in lambs on 30 or 45 ppm Zn supplementation compared to BD fed lambs.
Enzyme activities
Alkaline phosphatase
Activity of serum alkaline phosphatase (ALP) was 138.3 IU/L in BD lambs and increased (P < 0.01) gradually by Zn supplementation (Table 4). The ALP activity was highest (P < 0.01) in lambs fed 45 ppm followed by 30 ppm Zn from organic sources. As the concentration of Zn in diet increased, the ALP activity in serum increased (P < 0.01). At all the doses of Zn supplementation, the ALP activity was higher (P < 0.01) in lambs on organic compared to inorganic sources of zinc.
Oxidative enzymes
The effect of Zn supplementation on activities of oxidative enzyme is presented in Table 4. The lipid peroxidase (LPx) activity was 7.84 nmol MDA/ml in BD lambs and decreased (P < 0.01) to 5.62 and 6.84 nmol when BD was supplemented with 15 ppm Zn from inorganic and organic source, respectively. But at higher levels of Zn (30 and 45 ppm) the enzyme activity was similar to that of BD lambs. The RBC catalase activity was higher (P < 0.01) in lambs fed 15 ppm Zn from organic source or with 30 ppm Zn from inorganic source compared to BD fed lambs. The lambs fed 45 ppm Zn had no effect on RBC catalase activity. The RBC SOD activity was higher (P < 0.01) in lambs fed 15 ppm Zn compared to those fed BD or 30 and 45 ppm Zn supplemented ones. In comparison to BD fed lambs, the lambs fed 30 ppm had higher (P < 0.01) SOD activity, but the activity at 45 ppm supplementation was comparable to unsupplemented ones. At 15 and 30 ppm of Zn supplementation, RBC SOD was higher (P < 0.01) from organic source.
Serum proteins
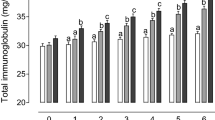
The data pertaining to total protein and globulin in serum of lambs on various Zn containing diets assessed at 75 d of feeding are detailed in Table 5. The serum total protein in lambs was not affected by either dose or source of Zn in diet. The serum albumin concentration was not affected by the dietary treatments except those fed 30 ppm Zn as inorganic form. The average concentration of globulin in BD fed lambs was 3.27 g/dl and was higher (P < 0.05) in Zn supplemented groups (3.34–3.61 g/dl). The globulin concentration was higher (P < 0.01) in 30 ppm Zn supplemented lambs from ZnSO4 and at 45 ppm Zn from either organic or inorganic source, compared to BD fed lambs.
Discussion
Zinc deficiency is known to cause immunosuppression (Chesters 1997). Zinc is a cofactor for more than 300 enzymes, involved in cell proliferation, DNA replication and signal transduction (Coleman 1992). Zinc deficiency causes reduced peripheral T cell counts, oxidative burst and T cell dependent antibody production (Goswami et al. 2005). Lambs fed on a semi-purified diet severely deficient in zinc (3.7 mg Zn/kg) showed a reduced blastogenic response to PHA with lower percentage of lymphocytes in lambs (Droke and Spears 1993). The NRC (1985) recommended minimum requirement of zinc in lambs to be 20–33 mg/kg DM for growth and normal maintenance of pregnancy. The NRC requirement does not consider the amounts required for optimum immune response and that required for combating stress, which may be higher than the recommendation. The main aim of the experiment was to arrive at the optimum level of zinc supplementation for enhanced immunity and its role as an antioxidant in sheep. The BD in the present study contained 29.28 ppm Zn with in the range of NRC recommendation. Three levels i.e., 15, 30 and 45 ppm of Zn supplementation from inorganic and organic sources were tested for immune response and oxidative defense.
Lipid peroxidation is the best marker of oxidative stress (Saygili et al. 2003), its concentration increases during stress condition. Vaccination induces a substantial stress (Surai 2006). Administration of Brucella and chicken RBC antigens at 75 d of experiment must have caused stress to the animals. Zinc prevents lipid peroxidation (Kroncke et al. 1994) and acts as an antioxidant by protecting cells from the damaging effects of oxygen radicals generated during stress and immune activation. The LPx activity, an oxidative enzyme was higher (P < 0.01) in BD fed lambs and lower activity of this enzyme was observed in lambs at 15 ppm Zn supplementation (Table 4). Superoxide radical is the main free radical produced during oxidative stress, superoxide dismutase (SOD) is considered as the main element of 1st level of antioxidant defense in cell (Surai 1999) and this enzyme produces hydrogen peroxide during its scanvenging action. The peroxides formed by the SOD action is detoxified by glutathione peroxidase or catalase which reduces hydrogen peroxide to water. Catalase also plays an important role in the acquisition of tolerance to oxidative stress by protecting NADPH by inactivation of H2O2 (Mates and Sanchez-Jimenez 1999). Thus the enzymatic antioxidants like RBC SOD and catalase play a vital role in scavenging oxidative radicals (Niki 1996). The lambs on 15 ppm Zn supplementation had higher (P < 0.01) catalase and SOD activity compared to BD fed lambs. Feeding of 45 ppm Zn could not enhance the antioxidant enzyme activities and reduced the lipid peroxidase activity (Table 4). This indicated that 15 ppm of Zn supplementation was sufficient to maintain the oxidant-antioxidant balance in lambs to combat the stress conditions and protect the immune cells from peroxidation. The higher levels of Zn supplementation were not effective in reducing the oxidative stress.
Zinc supplementation to BD (29.28 ppm) significantly (P < 0.01) improved the antibody titres against B. abortus. Maximum antibody titres were observed in lambs fed 30 ppm Zn from inorganic source at 7 d post inoculation. But on 14th and 21st day post sensitization, the 15 ppm Zn from Zn proteinate resulted in highest antibody titres. This indicated that addition of 15 ppm zinc to a diet containing 29.28 ppm zinc had positive effect on humoral immune response (Table 2). Supplementation of 15 ppm Zn from organic source increased the antibody titres. Spears et al. (1991) reported greater antibody titres in newly received steers vaccinated against Bovine herpes virus- I with 25 ppm Zn supplementation (51 ppm Zn) compared to controls (no Zn supplementation; 26 ppm Zn), with greatest response observed in steers fed Zn methionine compared to Zn oxide. Spears and Kegley (2002) observed that Zn level (25 ppm) and source (oxide and proteinate) added to a basal diet (33 ppm) did not affect titres against infectious bovine rhinotracheitis (IBR) titres on 14 or 28 d after vaccination against IBR. Reduced antibody titres against Parainfluenza-3 (PI3) vaccine was observed in grazing sheep fed on 144 ppm supplemental Zn (Hatfield et al. 2002). The present findings are in corroboration with the previous reports. Zn supplementation at higher levels (30 or 45 ppm Zn) had no positive effects on antibody titres against B. abortus, but at lower level of supplementation (15 ppm) the humoral immune response against B. abortus was higher preferably from organic source.
Antibody response to chicken RBC was not affected by either dose or source of supplemental Zn in the present study (Table 2). Similarly, Droke et al. (1998) and Nunnery et al. (2007) reported lack of effect of source and level of dietary Zn on antibody titres against porcine RBC and ovalbumin in lambs and steers, respectively. Salyer et al. (2004) compared inorganic and organic source of Zn at 75 ppm supplementation and reported that heifers receiving Zn from Zn polysaccharide had greater ovalbumin IgG titres on d14 and 21 than heifers receiving Zn from ZnSO4.
The in vitro lymphocyte proliferative (LP) response against ConA mitogen (suppressor of T lymphocyte proliferation) was affected by Zn supplementation (Table 3). The LP and DTH response were higher in lambs fed 15 ppm supplemental Zn. But Zn supplementation at higher levels (30 or 45 ppm) did not influence the cell mediated immune response. At 15 ppm Zn supplementation the DTH was higher (P < 0.01) in Zn proteinate compared to Zn sulfate. These results are in line to that of Engle et al. (1997), who reported that calves receiving a diet containing 17 ppm Zn had reduced DTH response to phytohaemagglutinin (PHA) than those fed 40 ppm, being higher (P < 0.05) in those fed Zn methionine and Zn lysine than control or Zn sulfate at 24 h post inoculation. Similarly, Zn deficiency (3.7 ppm) reduced lymphocyte response to T cell mitogens in lambs in studies of Droke and Spears (1993). On other hand, Spears and Kegley (2002) observed no affect of Zn level (25 ppm) or source (oxide or proteinate) of Zn supplementation to a basal diet containing 33 ppm Zn on either DTH or lymphocyte blastogenic response to PHA in steers. In lambs too, Zn supplementation (25 ppm) to basal diet (28 mg Zn/kg DM) did not affect the lymphocyte blastogenesis against various mitogens (PHA, poke weed mitogen and lipopolysaccharide) (Droke et al. 1998). Addition of 150 or 300 mg Zn/kg as oxide, lysine or methionine to a control diet containing 65 mg Zn/kg did not affect mitogen induced blastogenesis in young calves (Kincaid et al. 1997).
The results of the present study indicated that both humoral and cell mediated immune response was affected by Zn supplementation. Dietary Zn concentration around 30 ppm as in the basal diet and as per the NRC (1985) recommendation was not sufficient for obtaining optimum immune response in lambs. An additional supplementation of 15 ppm Zn was required to combat the oxidative stress and maintain proper oxidant-antioxidant balance in the body as evident by lower lipid peroxidation activity, higher activities of RBC SOD and catalase in lambs fed diets supplemented with 15 ppm Zn. The higher antioxidant activity of Zn at 15 ppm supplementation could have protected the immune cells and their receptors from oxidative stress and resulting in higher humoral and cell mediated immune response. Chvapil et al. (1972) and Bray and Bettger (1990) reported Zn as antioxidant, protecting cells from the damaging effect of oxygen radicals generated during immune activation. At higher levels of Zn supplementation (30 and 45 ppm), the lambs could not combat the oxidative stress and no effect on immune response was observed. Similarly, the lack of effect on humoral and cell mediated immune response in studies of Spears and Kegley (2002) and Nunnery et al. (2007) in steers, Kincaid et al. (1997) in calves and Droke et al. (1998) in lambs might be due to higher level of Zn in the diets. Hatfield et al. (2002) also observed reduced humoral immune response against PI 3 vaccine in ewes fed 7 times the NRC recommendations of Zn. The reasons of absence of beneficial response of high dietary zinc concentration on immune response and oxidative stress could not be known but possibly the high level of zinc might have shown antagonistic effect on other minerals that positively modulate the immune mechanism of the host.
Zinc supplementation at 15 ppm by organic source (Zn proteinate) had higher antioxidant enzyme activities, antibody titres against B. abortus and skin induration response against PHA-P which indicated Zn proteinate to be better source of Zn than ZnSO4 for disease resistance. Similar to the present observations, Spears et al. (1991) and Salyer et al. (2004) reported higher immune response when fed organically complexed Zn compared to inorganic ones. The ALP activity was higher (P < 0.01) in Zn supplemented diets and the enzyme activity increased with the level of Zn in diet (Table 3). The enzyme activity is an indicator of Zn status in the body. The enzyme activity increased at higher levels of Zn inclusion in the diet (Wan et al. 1993; Kraus et al. 1997). Compared to ZnSO4, the Zn proteinate had higher ALP activity at each level of mineral supplementation. This suggests higher availability of Zn from organic source compared to inorganic source. Similarly, Droke et al. (1998) also observed higher (P < 0.05) ALP activity on 112 d in Zn supplemented lambs (25 ppm) from Zn methionine compared to ZnSO4 and unsupplemented lambs.
Conclusion
The present study indicated that 15 ppm Zn supplementation to a basal diet with 29.28 ppm Zn increased immune response (humoral and cell mediated) and activities of RBC superoxide dismutase and catalase in lambs. No effect of higher levels (30 and 45 ppm) was observed on these variables. The Zn supplementation at 15 ppm in form of Zn proteinate had higher antioxidant enzyme activities, antibody titres, delayed type hypersensitivity response and alkaline phosphatase activity compared to Zn sulfate.
References
Alton GG, Jones LM, Pietz DE 1975 Laboratory techniques in brucellosis, 2nd edn. WHO, Geneva, WHO Monograph Series, 55
Bergmeyer HU (1974) Alkaline phosphastase. In: Methods of enzymatic analysis. Vol. II. Academic Press, Inc; USA.
Bergmeyer HU (1983). Catalase. In: Methods of enzymatic analysis, Vol 2, pp. 165–166 (Weinheim, Verlag Chemie)
Bounous DL, Raymond P, Campognoli RP, Brown J 1992. Comparison of MTT colorimetric assay and tritiated thymidine uptake for lymphocyte proliferation assays using chicken splenocytes. Avian Diseases 36: 1022–1027. doi:10.2307/1591566
Bray TM, Bettger, WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med., 281–191. doi:10.1016/0891-5849(90)90076-U
Cannan RK (1958) Clinical chemistry 4: 246–251 C F Text book of clinical practical Biochemistry, Varley, H 1991 Vol I, 5th edn, (CBS publisher and Distributors, pp 479–480).
Chesters JK 1997 Zinc. In: Handbook of Nutritionally Essential Mineral Elements, pp. 185–230 [BL O’Dell and RA Sunde, editors]. New York: Marcel Dekker Inc.
Chvapil M, Elias SL, Ryan JN, Zukoski CF (1972) Pathophysiology of zinc. In: International review of neurobiology. Supplement 1st ed. New York: Academy Press, 1972: 105–124.
Coleman JE 1992. Zinc proteins enzymes, storage proteins, transcription factors and replication proteins. Annu. Rev. Biochem. 16: 897–946. doi:10.1146/annurev.bi.61.070192.004341
Droke EA, Spears JW 1993. In vitro and in vivo immunological measurements in growing lambs fed diets deficient, marginal or adequate in zinc. J. Nutr. Immunol. 2: 71–90. doi:10.1300/J053v02n01_08
Droke EA, Gengelbach GP, Spears JW 1998 Influence of level and source (inorganic vs organic) of zinc supplementation on immune function in growing lambs. Asian-Australasian J. Anim. Sci. 11: 139–149.
Duncan DB 1955. Multiple ‘F’ test. Biometrics 1: 142.
Engle TE, Nockels CF, Kimberling CV, Weaber DL, Johnson AB 1997 Zinc repletion with organic or inorganic forms of zinc and protein turnover in marginally zinc deficient calves. J. Anim. Sci. 75: 3074–3081
Galyean ML, Malcolm-Callis KJ, Gunter SA, Berrie RA 1995 Effects of zinc source and level and added copper lysine in the receiving diet on performance by growing and finishing steers. Prof. Anim. Sci. 11: 139–148.
Goswami TK, Bhar R, Jadhav SE, Joardar, SN, Ram GC 2005 Role of dietary zinc as a nutritional immunomodulator. Asian-Australasian Journal of Animal Sciences, 183: 439–452.
Hatfield PG, Robinson BL, Minikheim DL, Kott, RM, Roth, NI, Daniels, JT, Swenson, CK 2002 J. Anim. Sci. 80: 1329–1334
Hiller, Mc Intosh, Van Slyke 1927 Calorimetric determination of proteins. J. Clin. Inves. 4: 235–242. doi:10.1172/JCI100121
ICAR 1998 Nutrient requirement of Livestock and Poultry. (Indian Council of Agricultural Research, New Delhi pp6)
Keen CL, Gershwin ME 1990 Zinc deficiency and immune function. Annu. Rev. Nutr. 10: 415–431. doi:10.1146/annurev.nu.10.070190.002215
Kincaid RL, Chew BP, Cronrath JD 1997 Zinc oxide and amino acids as sources of dietary zinc for calves: Effect on untake and immunity. J. Dairy Sci. 80: 1381–1388
Kraus A, Roth H, Krichgessner M 1997 Supplementation with vitamin C, vitamin E, or beta carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J. Nutr. 127: 1290–1296
Kroncke KD, Fehsel K, Schmidt T 1994 Nitric oxide destroys zinc sulfur clusters inducing zinc release from metallothionein and inhibition of the zinc finger-type yeast transcription activator LAC9. Biochem Biophys Res Commun. 200: 1105–1110. doi:10.1006/bbrc.1994.1564
Lowry OH, Rosenbrough JJ, Farr AL and Randall RJ. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193: 265–275
Mates JM, Sanchez-Jimenez F 1999 Antioxidants enzymes and their implications in pathophysiologic processes. Frontiers in Bioscience, 4: D339–D345. doi:10.2741/Mates
McCord JM, Fridovich I 1969 Superoxide dismutase an enzymic function for erythrocuprein (Hemocuprein). Journal of Biological chemistry, 244: 6049–6055
Miller JK, Brzezinska-Slebodzinska E, Madsen FC 1993 Oxidative stress, antioxidants and animal function. J. Dairy Sci., 76: 2812–2823
Mosmann TJ 1983 Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65: 55–63. doi:10.1016/0022-1759(83)90303-4
Niki E (1996) alpha tocopherol. In: Handbook of antioxidants, Edited by Cadenas E and Packer L, Marcel Dekker, New York-London, pp. 3–25.
NRC. 1985 Nutrient requirements of sheep. 6th Ed. National Research Council. National Academy Press. Washington DC.
Nunnery GA, Vasconcelos JT, Parsons CH, Salyer GB, Defoor PJ, Valdez FR, Galyean, ML 2007 Effect of source of supplemental zinc on performance and humoral immunity in beef heifers. J. Anim Sci. 85: 2304–2313. doi:10.2527/jas.2007-0167
Perlman P, Engvall E 1971 Enzyme linked immunosorbent assay (ELISA) quantitative assay for immunoglobulin. Immuno Chemistry, 8: 871–878. doi:10.1016/0019-2791(71)90454-X
Placer ZA, Cushman LL, Johnson B 1966 Estimation of product of lipid peroxidation (malonyl dialdehyde). Analytical Biochemistry 16: 359–364. doi:10.1016/0003-2697(66)90167-9
Quist CF, Howerth EW, Bounous DI, Stallknecht DE 1997 Cell mediated immune response and IL-2 production in white triated deer experimentally infected with haemorrhage disease viruses. Veterinary Immunology and Immunopathology 56: 283–297. doi:10.1016/S0165-2427(96)05747-9
Salyer GB, Galyean ML, Defoor PJ, Nunnery GA, Parsons CH, Rivera, JD 2004 Effects of copper and zinc source on performance and humoral immune response of newly received, lightweight beef heifers. J. Anim. Science 82: 2467–2473
Saygili E I, Konukoglu D, Papila S, Aksay T 2003 Levels of plasma vitamin E, vitamin C, TBARS and cholesterol in male patients with colorectal tumors. Biochemistry (Moscow), 68: No 3, 317. doi:10.1023/A:1023010418230
Spears JW, Harvey RW, Brown TT 1991 Effects of zinc methionine and zinc oxide on performance, blood characteristics, and antibody titre response to viral vaccination in stressed feeder calves. J. Am. Vet. Med. Assoc. 199: 1731–1733
Spears JW 2000 Micronutrients and immune function in cattle. Proceedings of the Nutrition Society 59: 587–594
Spears JW, Kegley EB 2002 Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics and immune response of growing and finishing steers. J. Anim. Sci. 80: 2747–2752
Surai PF 1999 Vitamin E in avian reproduction. Poultry and Avian Biology Review 10: 1–60.
Surai PF (2006) Anitoxidants systems in animal body. In: Selenium in nutrition and health. Nottingham University Press.
Wan DY, Cerklewski FL, Leklem JE 1993 Increase in plasma pyridoxal-5’ phosphate when alkaline phosphatase activity is reduced in moderately zinc-deficient rats. Biol. Trace. Elem. Res. 39: 203–210. doi:10.1007/BF02783190
Wegmann TG, Smithies O 1966 A simple hemagglutination system requiring small amounts of red cells and antibodies transfusion, Philadelphia, 6: 67–73.
Wu DO, Meydani SN (1998) Antioxidants and immune function. In: Antioxidant Status, Diet, Nutrition and Health, Edited by Papas AM, CRC Press, Boca Raton. Pp.371–400.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagalakshmi, D., Dhanalakshmi, K. & Himabindu, D. Effect of dose and source of supplemental zinc on immune response and oxidative enzymes in lambs. Vet Res Commun 33, 631–644 (2009). https://doi.org/10.1007/s11259-009-9212-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-009-9212-9