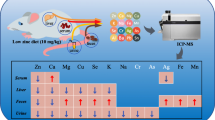
Abstract
Zinc (Zn) is an important trace element in the human body, and Zn deficiency affects the Zn content of major tissues. Marginal Zn deficiency is more common than severe Zn deficiency in humans. The objective of the present study was to compare the content and distribution of Zn and the change in the copper (Cu)–Zn superoxide dismutase (SOD) and metallothionein (MT) levels of soft tissues. Mice were fed with 30 mg/kg (control) or 10 mg/kg (marginally Zn-deficient, MZD) Zn diet for 35 days. We observed that only the Zn contents of serum, bones, and muscles in the control group were higher than those in the MZD group. Autometallography (AMG) was used as a method for staining Zn ions, and the semi-quantitative result indicated that the AMG products of the liver, duodenum, heart, lung, testes, and epididymis in the control group were higher than those in the MZD group. Furthermore, the contents of MT and the activities of Cu–Zn SOD in the testes, brain, duodenum, and liver were higher in the control group than those in the MZD group. However, the AMG products and the activities of Cu–Zn SOD of the kidney in the MZD group were more/higher than those in the control group. These results indicated that a change in the total Zn content of soft tissues may be not obvious and insensitive, and thus, more attention should be given to the distribution and localization of Zn ions. The functional indicators, MT and Cu–Zn SOD, are suitable biomarkers for evaluating zinc nutritional status. The brain, testes, duodenum, and liver are susceptive organs to Zn deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is the second most common trace element in the human body [1]; it plays important role in growth, immunity, eyesight, brain development, and male reproductive capacity [2,3,4,5]. Studies have shown that Zn deficiency can affect various functions. In organisms, Zn is mostly obtained from food. In many animal experiments, the Zn content of standard diets ranged from 25 to 35 mg Zn/kg [6,7,8]. In mice, a diet of 2 mg Zn/kg resulted in renal and myocardial fibrosis [9, 10] and promoted testicular cell apoptosis [5]. Meanwhile, a diet of 3 mg Zn/kg changed behavior, with the procedure being similar to the pathophysiology of depression in rats[11]. Some studies have shown that Zn deficiency is related to preneoplastic lesions in chemically induced hepatocarcinogenesis by establishing a 3.5 mg Zn/kg diet Zn-deficient model [12].
Different indicators have been used to measure the nutritional status of Zn in the body. The Zn contents of organs have been detected in some studies [13, 14]; serum Zn has also been used in considerable research to evaluate the nutritional status of Zn [15]. However, the insensitivity of serum/plasma Zn should be considered [16]. Copper (Cu)–Zn superoxide dismutase (SOD) is a metalloenzyme that is responsible for scavenging harmful reactive oxygen species [17]. When cells are treated with elevated Zn levels, their Cu–Zn SOD activities are markedly increased [18]. Cu–Zn SOD activity is typically used to evaluate oxidative stress in a variety of studies, such as oxidative stress in ischemic brain damage [19]. Cu–Zn SOD is a key biological parameter of Zn status in the body [20]. Huang et al. evaluated body Zn levels by using Cu–Zn SOD activity to explore the effects of Zn supplements [21]. Metallothionein (MT) is a small protein that participates in metal ion-related events, such as metal detoxification, homeostasis, storage, and delivery [22]. Calvo demonstrated that MT expression can be affected by Cu and Zn levels. Moreover, MT tends to bind with cadmium (Cd); hence, it is frequently used as an antidote to Cd poisoning [23]. Previous studies have reported that MT expression decreases with the reduction of Zn level and vice versa [24, 25]. Therefore, Mocchegiani inferred that MT is a useful tool for evaluating Zn supplements [26].
The localization and distribution of Zn in tissues have recently received increasing attention. The common detection method used is autometallography (AMG), a silver amplification technique that is frequently used to detect the distribution of Zn ion. Research has demonstrated that the brown-black band of AMG particles is distributed near the edge area of the nucleus layer in the retina [27]. Wang explored the function of Zn transporter (ZnT)-3 in nerves by detecting the distribution of Zn [28]. The distribution of Zn in mouse ovarian follicles and corpus luteum was also a concern [29]. By using ImageJ software developed at the National Institutes of Health (NIH) in gray scale analysis, the semi-quantitative analysis of Zn content is realized. Therefore, the localization distribution and relative amount of Zn can be determined [30].
However, most studies on Zn deficiency have explored the effects of severe Zn deficiency in animal experiments. Nevertheless, population survey results have shown that the proportions of Zn deficiency and marginal Zn deficiency among the Japanese were 0.4% and 46.0% in men and 0.6% and 38.4% in women. Therefore, increased attention should be given to marginal Zn deficiency [31]. Comprehensive studies on Zn nutritional status in tissues remain lacking. Hence, we designed a study that established a mouse model by feeding mice with marginal Zn concentration to compare the content and distribution of Zn and the change in the Cu–Zn SOD and MT levels of soft tissues, providing a scientific basis for the rational selection evaluation biomarker of Zn nutritional status.
Materials and Methods
Animals
First, 30 SPF ICR male mice aged 4 weeks were purchased from the Experimental Animal Tech Co. of Vital River (Beijing, China) (ID#:110,011,201,107,028,047, Beijing, China). All animal experiments performed in this study followed the ethical standards of the NIH’s Guide for the Care and Use of Laboratory Animals, and the animal study protocol was approved by the Institutional Animal Care and Use Committee of the Animal Experiment Center of Wuhan University (permit no.: WP2020-08,053).
The mice were maintained with a controlled light schedule (12-h light:12-h dark) at animal room temperature (21 ± 2 °C) with constant humidity (30–70%). All the mice had free access to standard commercial food and distilled water (use of laboratory animals’ license no.: SYXK(HB)2019–0013).
Experimental Design
After a normal adaptation period of 7 days, the mice were divided into two groups: the control group and the marginally Zn-deficient (MZD) group, with 15 mice per group. In accordance with the literature [32,33,34], the control group was fed with a standard diet that contained 30 mg/kg Zn, while the MZD group was fed with a low-Zn diet that contained 10 mg/kg Zn for 35 days. Feeds were purchased from Trophic Animal Feed High-Tech Co. (Nantong, China). Body weight was recorded two times a week, and food and water intake were recorded once a week.
Sample Collection
Before 12 h, the mice were sacrificed and dissected, and the feeds of all the animals were removed. Then, 10 mice from each group were anesthetized, weighed, and necropsied. Blood samples were collected from the heart and kept at room temperature for 2 h. After centrifugation for 15 min at 3000 rpm (835 g), the separated serum was stored at –80 °C. The hearts, livers, spleens, lungs, kidneys, brains, testes, epididymides, bones, muscles, and duodena were removed. After weighing, these organs were washed several times with deionized water and then placed in ion-free plastic centrifuge tubes at –80 °C.
Zn Content in Serum and Tissues
Zn concentrations in the serum and tissues were determined via atomic absorption spectrophotometry. First, 150 μL of serum was diluted into 3 mL of 1% nitric acid. For the digestive solution of the tissues, 8 mL of 65% H2O2 and 2 mL of HNO3 were mixed. Then, 100 mg of each tissue was weighted into 10 mL of the mixture. Subsequently, the samples were placed on a thermostatic electric heating plate and heated at 150 ℃ until the liquid became clear. After cooling, the liquid was placed into the cavity of a microwave oven and heated for 0.5 h at 200 ℃. After performing this procedure, clear and transparent solutions were obtained, and the volume of the resulting solution was adjusted to 5 mL by adding 1% HNO3. Blanks were prepared with the same reagents undergoing a similar treatment. Readings were made via flame atomic absorption spectroscopy (FAAS) (AA240FS-GTA120, Varian Inc., Palo Alto, CA, USA). A Zn hollow cathode lamp 213.9 nm (slit at 0.5 nm and current at 10 mA), automatic samplers (for introducing 20 μL of samples or standards), and deuterium lamps were used for background correction. Quality control was ensured by analyzing the reference materials provided by the National Institute of Metrology, China (GBW08620 for Zn measurement) before and after the measurements of routine samples. The values of these reference samples were consistent with the provided ranges.
Distribution of Zn in Tissues
In the experiment, AMG, a technology for detecting Zn ions characteristically, was used to determine the distribution of Zn. Five mice were anesthetized and perfused transcardially with 100 mL of isotonic saline, followed by 100 mL of 2.5% glutaraldehyde in 0.1 M PB. Tissues were removed and immediately immersed into the same fixative at 4 ℃ for 4 h, and then the samples were transferred to NTS (0.1% sodium sulfide and 3% glutaraldehyde in 0.1 M Sørensen phosphate buffer) for 3 days. All the samples were immersed in 30% sucrose at 4 ℃ overnight. After freezing, 10-μm-thick sections were cut and placed on glass slides pretreated with Farmer solution and immersed into AMG developer at 26 ℃ without light. After 1 h, AMG development was stopped by using 5% sodium thiosulfate solution for 10 min. Then, the tissue slices were counterstained with 1% toluidine blue. Finally, the slices were sealed with neutral gum and then analyzed and photographed using light microscope.
Determination of Cu–Zn SOD Activities
Cu–Zn SOD activities in the plasma, hearts, livers, spleens, lungs, kidneys, brains, testes, muscles, and duodenum were measured using a commercially available kit (Nanjing Jiancheng Bioengineering Institute). The tissues were prepared into tissue homogenates, and protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (Beyotime, Jiangsu, China). Plasma Cu–Zn SOD activities were expressed as units per milliliter (U/mL), and testicular Cu–Zn SOD activities were expressed as units per milligram (U/mg) of protein.
Determination of MT Contents
The MT contents of the heart, liver, spleen, lung, kidney, brain, testis, muscle, and duodenum were measured using a mouse MT enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Jining Industrial Co., Ltd., China) in accordance with the manufacturer’s instructions. The tissues were prepared into tissue homogenates, and the protein concentrations in these tissues were measured using a BCA protein assay kit (Beyotime, Jiangsu, China). Finally, MT contents were measured with the MT ELISA kit. MT contents were expressed as nanograms per gram (ng/g) of protein.
Statistical Analysis
SPSS version 26.0 (Chicago, IL, USA) was used for data analysis, and Student’s t test was used for between-group comparisons. If without special instructions, then all the values represent the mean ± standard deviation (SD), and p < 0.05 was considered statistically significant. Image analysis software (ImageJ) was used to analyze the percentage of AMG products in AMG staining silvers.
Results
General Conditions of Mice
The overall conditions of mice in the control and MZD groups were good. No visible abnormal signs of damage were observed throughout the experiment. No significant differences in body weights and food and water intake were noted between the groups (p > 0.05) (Fig. 1A–C). These results suggested that the general conditions of the animals were good, and the animals were unaffected by Zn deficiency.
Analysis of Zn Content Detected via FAAS
As shown in Fig. 2A, the Zn concentration in the serum of the control mice was 1.06 ± 0.07 μg/mL. It was significantly decreased to 0.76 ± 0.04 μg/mL in the mice that were fed with MZD diet for 35 days (p < 0.01).
In the standard Zn diet control group, Zn concentration in the bones was the highest, reaching up to 106.03 μg/g (Fig. 2B). In the major soft tissues, Zn concentration ordered from high to low was as follows: the liver, duodenum, testis, kidney, spleen, heart, lung, brain, muscle, and epididymis. With regard to per gram of muscles consisting of 9.52 μg Zn, Zn concentration was lower than in the liver, duodenum, and the testis by 3.25, 2.48, and 2.21 times, respectively. The Zn contents of the duodenum, testis, kidney, spleen, heart, lung, and brain were nearly the same, and the difference was not statistically significant.
With an MZD diet for 35 days, the Zn level in bones decreased most evidently by 16.71% compared with that of the control group (p < 0.05). Moreover, the Zn level in muscles decreased significantly by 26.15% (p < 0.01 versus control). However, no significant change was detected in the Zn levels of the duodenum, testis, kidney, and other soft tissues.
Localization of Zn Ions by Using AMG Staining
As shown in Fig. 3, the AMG reaction products of Zn appeared as brown-black particles. In accordance with the distribution, number, and size of these particles, the location and concentration of Zn can be estimated approximately.
Zn localization distribution stained using the AMG protocol described in this paper (A–J). A1/A2, liver; B1/B2, duodenum; C1/C2, testis; D1/D2, kidney; E1/E2, spleen; F1/F2, heart; G1/G2, lung; H1/H2, brain; I1/I2, muscle; and J1/J2, epididymis. Arrows indicate AMG staining particles. K denotes a semi-quantity of AMG, the area of AMG products/the area of AMG staining silver. * represents significance at p < 0.05
In control mice, the AMG reaction products accumulated along the hepatic cords toward the central veins in the liver (A1); mostly concentrated on the villi of the small intestine in the duodenum (B2); appeared in the sustentacular cells of the testis (C1); distributed around the kidney spheres in the kidney (D1); accumulated along the myocardium in the heart (F1); and distributed in the upper cortex of the output tubules and the epididymal canals in the epididymis (J1). The staining particles of the lung, muscle, and brain were less than those in the liver and other tissue, and the reaction products of the control were more than the MZD.
The percentage of AMG semi-quantity was computed by the area of the AMG products divided by the area of the total AMG staining silver. The result is presented in Fig. 3K. The AMG products of control mice was significantly higher than that of MZD mice in the liver, duodenum, heart, lung, testis, and epididymis, and no significant reduction was detected in the spleen, brain and muscle, p < 0.05. However, the result in the kidney tissue was the opposite, with more AMG products in the MZD group.
MT Contents of Organs
Figure 4 shows that the MT content of the heart was the highest in mice with standard diet, i.e., up to 1462.78 ng/gprot, followed by those of the muscle, spleen, lung, brain, testis, duodenum, liver, and kidney. The lowest MT content of these tissues was recorded in the kidney, i.e., as low as 253.44 ng/gprot. The MT content of the heart was 5.77 times that of the kidney.
With an MZD diet for 35 days, significant differences can be observed in the heart, liver, spleen, testis, brain, and duodenum. The MT contents of these organs in the MZD group were lower than those in the control group (p < 0.05). Compared with those in the control group, the MT contents of the duodenum, heart, spleen, liver, brain, and testis decreased by 36.93%, 33.08%, 32.61%, 32.14%, 24.16%, and 17.62%, respectively, in the MZD group.
Although statistically significant differences were not observed in the muscle, lung, and kidney between the two groups, the mean value of the MT content of the MZD group was lower than that of the control group. The MT content decreased by 11.08%, 8.55%, and 7.60%, respectively, compared with those in the control group.
Cu–Zn SOD Activities in Organs
As shown in Fig. 5, the activity of Cu–Zn SOD in the testis was the highest in the control mice, reaching up to 263.24 U/mgprot, followed by those in the muscle, brain, duodenum, spleen, heart, liver, lung, and kidney. The lowest activity of Cu–Zn SOD was observed in the kidney, i.e., 43.94 U/mgprot. Cu–Zn SOD activity in the testis was six times higher than that in the kidney.
With an MZD diet for 35 days, significant changes were noted in the testis, brain, duodenum, liver, and kidney (p < 0.05). Among these changes, the Cu–Zn SOD activity of the duodenum was the most significantly decreased by 46.13%, followed by those of the brain by 43.55%, liver by 35.75%, lung by 22.15%, and testis by 13.31%. Although the differences in the Cu–Zn SOD activities between the MZD and control groups were insignificant in the muscle, spleen, heart, and kidney, the mean values of the Cu–Zn SOD activities of these organs in the control group was higher than those in the MZD group. Compared with those in the control group, Cu–Zn SOD activities decreased by 15.72% (muscle), 16.37% (spleen), and 20.67% (heart) in the MZD group. Interestingly, the Cu–Zn SOD activities of the kidney in the MZD group were higher than those in the control group, p < 0.05.
Discussion
Research has indicated that Zn deficiency remains severe and affects human health worldwide [35,36,37], with approximately 20% of the world’s population estimated to have marginal Zn intake [31]. Zn is an important element that affects essential physiological functions. Puberty is a key period for growth and development; hence, focus should be placed on marginal Zn deficiency during puberty. In our study, pubertal mice were selected by feeding with a 10 mg/kg Zn diet to establish a marginal Zn deficiency model. After 35 days of feeding, the mice were dealt with to explore Zn concentration and distribution in tissues and compare the levels of Cu–Zn SOD and MT with the control (standard feed) group.
Zn concentration has been used to reflect Zn status in many studies. As shown by our results, the Zn concentrations of the serum, muscles, and bones of the MZD group were lower than those of the control group. Bones and muscles are Zn storage organs. Zn is carried into the blood after being digested and absorbed, and serum Zn is typically used as an indicator of Zn content in clinical applications [31]. Hence, serum can be considered the starting point after Zn circulates through the body, with bones and muscles as the destinations. In our study, the concentrations of Zn in serum, bones, and muscles decreased conspicuously, but no significant reducibility was observed in the other soft tissues. The results were similar to those in other studies [10, 38]. On this basis, we speculated that the body can mobilize a compensation function with a marginal Zn-deficient diet to ensure the Zn level in the major soft tissues and maintain the function of Zn.
In our study, Zn distribution was detected via AMG staining. The staining particles of the liver had the highest amount, and they accumulated along the hepatic cords toward the central veins. This result may be explained by the manner in which Zn is transported. Zn is transported by the blood; hence, staining particles are distributed densely in the central veins and sparsely in the capillaries. AMG products in the duodenum are largely distributed in the intestinal villi. This situation may be explained by the absorption mechanism of Zn, which was adjusted by ZnT and ZIP [39]. In this study, the mice obtained Zn through ingestion. Zn is an important element for maintaining the function and structure of the heart and testis. Jing suggested that Zn deficiency promotes fibrosis in the myocardium of mice, leading to histopathological changes in testicular tissue and exerting harmful effects on sperm quality [10, 40]. The AMG results indicated that the staining particles of the heart in the MZD group were less than that in the control group; hence, the heart is sensitive to Zn deficiency. The staining results of the epididymis showed that AMG particles were mostly distributed in the upper cortex of the output tubules. The primary function of the epididymis is promoting the maturation and storage of sperms. Dawid suggested that Zn supplements may protect the male reproductive system from oxidative damage and may have a beneficial effect on sperm viability, which is contacted with the epididymis [41]. The AMG products of the liver, duodenum, heart, testis, and epididymis in the MZD group were less than those in the control group. Two Zn pools are found in a cell: (1) slow-acting (structural Zn), which is associated with protein synthesis or cell membrane; and (2) fast-acting (free Zn), which works as a signal transmit and catalyst [42]. Total Zn concentration in human cells is 200–300 μM, and Zn ion concentrations are within the picomolar range in the cytosol. In accordance with these data, the concentration of structural Zn is considerably higher than that of free Zn [43]. The AMG reaction product is free or loosely bound zinc ions [44]. Hence, the change in the total Zn content of tissues is not obvious and insensitive. More attention should be given to the distribution and localization of Zn ion, which can be presented by AMG.
MT is also an important indicator that reflects Zn status. It is a type of binding protein that can combine with Zn, Cu, and Cd. MT expression is related to Zn accumulation in certain organs [45]. Zn deficiency and toxicity can be against protectively by MT I and MT II [46], and MT expression decreased with a decrease in dietary Zn content in previous studies [25]. Hence, MT can be used as an indicator to reflect the Zn level of a body. In addition, Zn deficiency leads to lower serum Zn content and causes higher incidences of heart malformations. MT1 expression decreases in the placenta [47]. A previous study showed that an increase in heart Zn affects MT expression, inhibiting dietary Cu restriction-induced cardiac hypertrophy [24]. MT has been verified to have functions in Zn homeostasis, and dietary Zn affects organ MT content. Elzbieta’s study suggested that MT concentration is responsive to Zn status in the intestine and liver [48]. Magnesium deficiency enhances Zn uptake to increase hepatic Zn concentration, leading to enhanced MT expression [22]. Zn deficiency decreases kidney MT1 and MT2 expressions in the Zn-deficient mouse mode [9]. Our MT expression analysis showed that the MT contents of the heart, liver, spleen, testes, brain, and duodenum were significantly lower in the MZD group than that in the control group (p < 0.05). Furthermore, the means of MT contents in the other organs were also lower in the MZD group. These results are consistent with those of previous studies. Therefore, as an indicator that reflects Zn content, MT content may be more sensitive than the Zn contents of tissues. In accordance with the result, MT content in the heart was the most sensitive to Zn deficiency and exhibited the highest MT content change among these organs.
Zn plays an important role in the function of Cu–Zn SOD [49]. A previous study showed that maintaining an appropriate Zn level protects Cu–Zn SOD activity [50]; Zn supplement was benefit for improving Cu–Zn SOD [51]. Hence, Zn level is closely related to Cu–Zn SOD activity. Only a few studies have been conducted to explore the relationship between Zn concentration and Cu–Zn SOD activities in different organs. However, some studies have confirmed that Zn supplements increase Zn concentration and Cu–Zn SOD activities in serum [21, 52, 53]. In our study, we found that the Cu–Zn SOD activities of the testis, brain, duodenum, and liver decreased significantly with a reduction in dietary Zn. The means of Cu–Zn SOD activities in the other organs were lower in the MZD group than in the control group. Such result is similar to those of previous studies. However, Gowanlock found that Zn concentration is not related to Cu–Zn SOD activity in Zn-deficient pigs [54]. In our study, the Cu–Zn SOD activities of the kidney increased remarkably in the MZD group compared with that in the control group. This finding is consistent with the consequence of AMG semi-quantity in the kidney. Cu–Zn SOD activities are reflected by Zn ions, and AMG stains Zn ions in the tissues. Therefore, the similar trend of the two indicators in the kidney is rational. This finding may be attributed to the dose of diet Zn content being in the margin of Zn deficiency, leading to no kidney damage and activating Cu–Zn SOD activities.
The above results showed that brain, testis, duodenum, and liver were susceptive organs to Zn deficiency. The potential mechanisms may be as follows. The brain is a is a fine regulatory organ. Zinc ions are required to participate in neural development and regulate intra- and intercellular signals as signal molecules [55]. As to testis, Zn is a crucial element for male reproduction. Zinc ions participate in the synthesis and secretion of sex hormones and affect sperm quality [56]. The duodenum is the main organ to maintain Zn homeostasis by regulating Zn uptake and efflux; when dietary zinc is deficient, the content of zinc in duodenum may be the first affected [57]. And the liver is one of “storage” of Zn. when zinc is deficient, the body will mobilize Zn stored in the liver to maintain important functions of other organs and Zn homeostasis [58].
In conclusion, our study indicates the following: (1) the detection of total Zn content is suitable for detecting Zn status in the serum, muscles, and bones. (2) MT and Cu–Zn SOD are suitable biomarkers for evaluating zinc nutritional status. (3) The brain, testis, duodenum, and liver are susceptive organs to Zn deficiency.
References
Baltaci AK, Yuce K, Mogulkoc R (2018) Zinc metabolism and metallothioneins. Biol Trace Elem Res 1831:22–31. https://doi.org/10.1007/s12011-017-1119-7
Ugarte M, Osborne NN (2014) Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 62:189–200. https://doi.org/10.1039/c3mt00291h
Prasad AS (2013) Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 42:176–190. https://doi.org/10.3945/an.112.003210
Omu AE, Al-Azemi MK, Al-Maghrebi M, Mathew CT, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Memon A (2015) Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian J Urol 311:57–64. https://doi.org/10.4103/0970-1591.139570
Chen Y, Yang J, Wang Y, Yang M, Guo M (2020) Zinc Deficiency promotes testicular cell apoptosis in mice. Biol Trace Elem Res 1951:142–149. https://doi.org/10.1007/s12011-019-01821-4
Nuttall JR, Supasai S, Kha J, Vaeth BM, Mackenzie GG, Adamo AM, Oteiza PI (2015) Gestational marginal zinc deficiency impaired fetal neural progenitor cell proliferation by disrupting the ERK1/2 signaling pathway. J Nutr Biochem 2611:1116–1123. https://doi.org/10.1016/j.jnutbio.2015.05.007
McCormick NH, King J, Krebs N, Soybel DI, Kelleher SL (2015) Redistribution of tissue zinc pools during lactation and dyshomeostasis during marginal zinc deficiency in mice. J Trace Elem Med Biol 29:170–175. https://doi.org/10.1016/j.jtemb.2014.06.002
Jinno N, Nagata M, Takahashi T (2014) Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol Trace Elem Res 1581:65–72. https://doi.org/10.1007/s12011-014-9901-2
Xu R, Chen MY, Liang W, Chen Y, Guo MY (2021) Zinc deficiency aggravation of ROS and inflammatory injury leading to renal fibrosis in mice. Biol Trace Elem Res 1992:622–632. https://doi.org/10.1007/s12011-020-02184-x
Cao JW, Duan SY, Zhang HX, Chen Y, Guo M (2020) Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol Trace Elem Res 1961:145–152. https://doi.org/10.1007/s12011-019-01902-4
Doboszewska U, Sowa-Kucma M, Mlyniec K, Pochwat B, Holuj M, Ostachowicz B, Pilc A, Nowak G, Szewczyk B (2015) Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog Neuropsychopharmacol Biol Psychiatry 56:254–263. https://doi.org/10.1016/j.pnpbp.2014.09.013
Romualdo GR, Goto RL, Henrique Fernandes AA, Cogliati B, Barbisan LF (2016) Dietary zinc deficiency predisposes mice to the development of preneoplastic lesions in chemically-induced hepatocarcinogenesis. Food Chem Toxicol 96:280–289. https://doi.org/10.1016/j.fct.2016.08.020
Zieminska E, Ruszczynska A, Augustyniak J, Toczylowska B, Lazarewicz JW (2021) Zinc and copper brain levels and expression of neurotransmitter receptors in two rat ASD models. Front Mol Neurosci 14:656740. https://doi.org/10.3389/fnmol.2021.656740
Staniek H (2019) The combined effects of Cr(III) propionate complex supplementation and iron excess on copper and zinc status in rats. J Trace Elem Med Biol 53:49–54. https://doi.org/10.1016/j.jtemb.2019.01.011
Kheirouri S, Alizadeh M (2014) Decreased serum and mucosa immunoglobulin A levels in vitamin A and zinc-deficient mice. Cent Eur J Immunol 392:165–169. https://doi.org/10.5114/ceji.2014.43716
Moran VH, Skinner AL, Medina MW, Patel S, Dykes F, Souverein OW, Dullemeijer C, Lowe NM (2012) The relationship between zinc intake and serum/plasma zinc concentration in pregnant and lactating women: a systematic review with dose-response meta-analyses. J Trace Elem Med Biol 262–3:74–79. https://doi.org/10.1016/j.jtemb.2012.04.003
Sirisena D, Gayashani Sandamalika WM, Neranjan Tharuka MD, Madusanka RK, Jeong JB, Lee J (2021) A copper-zinc-superoxide dismutase (CuZnSOD) from redlip mullet, Liza haematocheila: insights to its structural characteristics, immune responses, antioxidant activity, and potent antibacterial properties. Dev Comp Immunol 123:104165. https://doi.org/10.1016/j.dci.2021.104165
Tarhan C, Pekmez M, Karaer S, Arda N, Sarikaya AT (2007) The effect of superoxide dismutase deficiency on zinc toxicity in Schizosaccharomyces pombe. J Basic Microbiol 47:506–512. https://doi.org/10.1002/jobm.200700220
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection 14:1505–1517. https://doi.org/10.1089/ars.2010.3576
Sun JY, Jing MY, Weng XY, Fu LJ, Xu ZR, Zi NT, Wang JF (2004) Effects of dietary zinc levels on the activities of enzymes, weights of organs, and the concentrations of zinc and copper in growing rats. Biol Trace Elem Res 1072:153–165
Huang D, Hu Q, Fang S, Feng J (2016) Dosage effect of zinc glycine chelate on zinc metabolism and gene expression of zinc transporter in intestinal segments on rat. Biol Trace Elem Res 1712:363–370. https://doi.org/10.1007/s12011-015-0535-9
Kotani M, Kim KH, Ishizaki N, Funaba M, Matsui T (2013) Magnesium and calcium deficiencies additively increase zinc concentrations and metallothionein expression in the rat liver. Br J Nutr 1093:425–432. https://doi.org/10.1017/S0007114512001195
Calvo J, Jung H, Meloni G (2017) Copper metallothioneins. IUBMB Life 694:236–245. https://doi.org/10.1002/iub.1618
Kang YJ, Jiang Y, Saari JT (2007) Changes in copper and zinc status and response to dietary copper deficiency in metallothionein-overexpressing transgenic mouse heart. J Nutr Biochem 1811:714–718. https://doi.org/10.1016/j.jnutbio.2006.10.009
Brugger D, Windisch WM (2017) Short-term subclinical zinc deficiency in weaned piglets affects cardiac redox metabolism and zinc concentration. J Nutr 1474:521–527. https://doi.org/10.3945/jn.116.240804
Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M (2011) Zinc, metallothioneins and immunosenescence: effect of zinc supply as nutrigenomic approach. Biogerontology 125:455–465. https://doi.org/10.1007/s10522-011-9337-4
Wang X, Wang ZY, Gao HL, Danscher G, Huang L (2006) Localization of ZnT7 and zinc ions in mouse retina–immunohistochemistry and selenium autometallography. Brain Res Bull 711–3:91–96. https://doi.org/10.1016/j.brainresbull.2006.08.002
Zhang LH, Wang X, Stoltenberg M, Danscher G, Huang L, Wang ZY (2008) Abundant expression of zinc transporters in the amyloid plaques of Alzheimer’s disease brain. Brain Res Bull 771:55–60. https://doi.org/10.1016/j.brainresbull.2008.03.014
Zhong M-L, Guo C, Chi Z-H, Shan Z-Y, Teng W-P and Wang Z-Y (2013) Distribution of zinc and zinc transporters in the mouse ovarian follicles and corpus luteum. Histology and Histopathology :1517–1527. https://doi.org/10.14670/HH-28.1517
Goldschmidt J, Wanger T, Engelhorn A, Friedrich H, Happel M, Ilango A, Engelmann M, Stuermer IW, Ohl FW, Scheich H (2010) High-resolution mapping of neuronal activity using the lipophilic thallium chelate complex TlDDC: protocol and validation of the method. Neuroimage 491:303–315. https://doi.org/10.1016/j.neuroimage.2009.08.012
Yokokawa H, Fukuda H, Saita M, Miyagami T, Takahashi Y, Hisaoka T, Naito T (2020) Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. J Gen Fam Med 216:248–255. https://doi.org/10.1002/jgf2.377
Iwaya H, Kashiwaya M, Shinoki A, Lee JS, Hayashi K, Hara H, Ishizuka S (2011) Marginal zinc deficiency exacerbates experimental colitis induced by dextran sulfate sodium in rats. J Nutr 1416:1077–1082. https://doi.org/10.3945/jn.111.138180
Adamo AM, Liu X, Mathieu P, Nuttall JR, Supasai S, Oteiza PI (2019) Early developmental marginal zinc deficiency affects neurogenesis decreasing neuronal number and altering neuronal specification in the adult rat brain. Front Cell Neurosci 13:62. https://doi.org/10.3389/fncel.2019.00062
Croxford TP, McCormick NH, Kelleher SL (2011) Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr 1413:359–365. https://doi.org/10.3945/jn.110.131318
Gupta S, Brazier AKM, Lowe NM (2020) Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet 335:624–643. https://doi.org/10.1111/jhn.12791
Ozeki I, Arakawa T, Suii H, Tatsumi R, Yamaguchi M, Nakajima T, Kuwata Y, Toyota J (2020) Zinc deficiency in patients with chronic liver disease in Japan. Hepatol Res 503:396–401. https://doi.org/10.1111/hepr.13465
Maruyama Y, Nakashima A, Fukui A, Yokoo T (2021) Zinc deficiency: its prevalence and relationship to renal function in Japan. Clin Exp Nephrol 257:771–778. https://doi.org/10.1007/s10157-021-02046-3
Jing MY, Sun JY, Wang JF (2008) The effect of peripheral administration of zinc on food intake in rats fed Zn-adequate or Zn-deficient diets. Biol Trace Elem Res 1242:144–156. https://doi.org/10.1007/s12011-008-8132-9
Kambe T, Hashimoto A, Fujimoto S (2014) Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci 7117:3281–3295. https://doi.org/10.1007/s00018-014-1617-0
Kumari D, Nair N, Bedwal RS (2011) Effect of dietary zinc deficiency on testes of Wistar rats: morphometric and cell quantification studies. J Trace Elem Med Biol 251:47–53. https://doi.org/10.1016/j.jtemb.2010.11.002
Madej D, Pietruszka B, Kaluza J (2021) The effect of iron and/or zinc diet supplementation and termination of this practice on the antioxidant status of the reproductive tissues and sperm viability in rats. J Trace Elem Med Biol 64:126689. https://doi.org/10.1016/j.jtemb.2020.126689
Tubek S (2007) Zinc supplementation or regulation of its homeostasis: advantages and threats. Biol Trace Elem Res 1191:1–9. https://doi.org/10.1007/s12011-007-0043-7
Maret W (2015) Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 72:202–211. https://doi.org/10.1039/c4mt00230j
Danscher G, Stoltenberg M (2006) Silver enhancement of quantum dots resulting from (1) metabolism of toxic metals in animals and humans, (2) in vivo, in vitro and immersion created zinc-sulphur/zinc-selenium nanocrystals, (3) metal ions liberated from metal implants and particles. Prog Histochem Cytochem 412:57–139. https://doi.org/10.1016/j.proghi.2006.06.001
Davis S R and Cousin R J (2000) Metallothionein expression in animals: a physiological perspective on function. American society for nutritional sciences : 1085-1088. https://doi.org/10.1093/jn/130.5.1085.
K E J, J.Q C, F G J and P R D (1996) Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. Am Inst Nutr 1782-1790. https://doi.org/10.1093/jn/126.7.1782
Liu C, He X, Hong X, Kang F, Chen S, Wang Q, Chen X, Hu D, Sun Q (2014) Suppression of placental metallothionein 1 and zinc transporter 1 mRNA expressions contributes to fetal heart malformations caused by maternal zinc deficiency. Cardiovasc Toxicol 144:329–338. https://doi.org/10.1007/s12012-014-9256-0
Szczurek E I, Bjornsson C S and Taylor C G (2001) Dietary zinc deficiency and repletion modulate metallothionein immunolocalization and concentration in small intestine and liver of rats1,2. Nutr Metabol 2132-2138. https://doi.org/10.1093/jn/131.8.2132
Beckman JS, Estévez AG, Crow JP, Barbeito L (2001) Superoxide dismutase and the death of motoneurons in ALS. A Trends Guide to Neurodegenerative Disease and Repair 24:S15–S20. https://doi.org/10.1016/s0166-2236(00)01981-0
Ermilova IP, Ermilov VB, Levy M, Ho E, Pereira C, Beckman JS (2005) Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci Lett 3791:42–46. https://doi.org/10.1016/j.neulet.2004.12.045
Yin LL, Zhang Y, Guo DM, An K, Yin MS, Cui X (2013) Effects of zinc on interleukins and antioxidant enzyme values in psoriasis-induced mice. Biol Trace Elem Res 1553:411–415. https://doi.org/10.1007/s12011-013-9799-0
Deng B, Zhou X, Wu J, Long C, Yao Y, Peng H, Wan D, Wu X (2017) Effects of dietary supplementation with tribasic zinc sulfate or zinc sulfate on growth performance, zinc content and expression of zinc transporters in young pigs. Anim Sci J 8810:1556–1560. https://doi.org/10.1111/asj.12788
Cao Y, Li YS, Li ZJ, Wang F, Li CM (2015) Dietary zinc may attenuate heat-induced testicular oxidative stress in mice via up-regulation of Cu-Zn SOD. Genet Mol Res 144:16616–16626. https://doi.org/10.4238/2015.December.11.9
Gowanlock DW, Mahan DC, Jolliff JS, GM H, (2015) Evaluating the influence of National Research Council levels of copper, iron, manganese, and zinc using organic (Bioplex) minerals on resulting tissue mineral concentrations, metallothionein, and liver antioxidant enzymes in grower-finisher swine diets. J Anim Sci 93(3):1149–1156. https://doi.org/10.2527/jas2014-8173
Mattei D, Pietrobelli A (2019) Micronutrients and brain development. Curr Nutr Rep 82:99–107. https://doi.org/10.1007/s13668-019-0268-z
Elgazar V, Razanov V, Stoltenberg M, Hershfinkel M, Huleihel M, Nitzan YB, Lunenfeld E, Sekler I, Silverman WF (2005) Zinc-regulating proteins, ZnT-1, and metallothionein I/II are present in different cell populations in the mouse testis. J Histochem Cytochem 537:905–912. https://doi.org/10.1369/jhc.4A6482.2005
Hennigar SR, Olson CI, Kelley AM, McClung JP (2021) Slc39a4 in the small intestine predicts zinc absorption and utilization: a comprehensive analysis of zinc transporter expression in response to diets of varied zinc content in young mice. J Nutr Biochem 101:108927. https://doi.org/10.1016/j.jnutbio.2021.108927
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 579:399–411. https://doi.org/10.1016/s0753-3322(03)00081-7
Acknowledgements
Thanks to all members of Professor Wang CH’s lab at the School of Public Health, Wuhan University for their generous help. We would like to thank the animal facilities of Wuhan University Animal Experiment Center for the maintenance of the mouse population.
Funding
This study was supported by grants from Angel Nutritech Nutrition Fund (Grant NO. AF2019004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Peng, C., Zhang, Y. et al. Characteristics of Zn Content and Localization, Cu–Zn SOD, and MT Levels in the Tissues of Marginally Zn-Deficient Mice. Biol Trace Elem Res 201, 262–271 (2023). https://doi.org/10.1007/s12011-022-03119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03119-4