Abstract
Purpose of Review
This review summarizes the most recent evidence regarding the effects of micronutrients on brain development.
Recent Findings
Emerging evidence indicates that nutrition in the early life can profoundly influence neurodevelopment, affecting later life health outcomes, neurocognitive performances, and disease risks. Inadequate early life nutrition has been associated with some neuropsychiatric disorders. Epigenetic mechanisms could play a crucial role, imprinting the genomes in early life making the individual more susceptible to develop diseases later in life.
Summary
Children adequately nourished are more likely to reach their developmental potential in cognitive, motor, and socioemotional abilities, with positive societal repercussions. Data from further clinical trials are needed before more definitive conclusions can be drawn regarding the efficacy of dietary interventions for improving neurocognitive and social outcomes and preventing some neuropsychiatric illnesses. Nevertheless, it is reasonable to make recommendations to our patients to adopt certain dietary habits to optimize early life nutritional status in order to avoid long-term adverse consequences. Strategies of prevention should focus on ensuring more quality food to preconceptional, pregnant, lactating women and to children in their early life, not only in those areas where malnutrition is common but also in developed countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The period included between the conception and the first 2 years of life, known as the “first 1000 days”, is fundamental to influence long-term child health outcomes. Particularly, this time period constitutes a golden opportunity to program normal brain development [1]. Neurodevelopment is a highly complex process. Although it is mostly genetic, preprogrammed, and experience-independent, there are environmental factors that can profoundly influence early brain development. Some of these factors are beyond our control, but nutrition is not [2, 3]. In the last decades many studies showed that nutrition plays a critical role: firstly, being responsible of providing those substances required for the creation of the early brain structure and, secondly, being responsible of supporting and preserving its healthy functioning [4]. In addition, accumulating evidence suggests that diet influences gene expression through epigenetic mechanisms. These epigenetic changes, especially if experienced during the early development, could be responsible for later life diseases [5,6,7]. Several preclinical and clinical studies have helped to elucidate the role and mechanism of individual macro- and micronutrients on brain development [4]. While almost all nutrients are required, a subset of nutrients plays a particularly significant role in a variety of critical neurodevelopmental processes across brain regions, supporting the high rate of brain metabolism during early life. These include macronutrients (e.g., protein, long-chain polyunsaturated fatty acids, and glucose) and micronutrients (e.g., iron, iodine, zinc, and vitamins) [8••]. Over time, evidence has demonstrated that, regardless of the cause, impairments in the early life neurodevelopment have long-term consequences on health, with respect to education, job potential, and adult mental health [9, 10]. Considering the worldwide diffusion of early life malnutrition in all its forms—undernutrition, micronutrient deficiencies, obesity, and diet-related noncommunicable diseases—and the related unacceptably high long-term economic and societal repercussions, policy makers should focus their attention on planning and making appropriate assessments and interventions, in order to optimize nutrient supplies during the most vulnerable period of brain development. This review will focus on the importance of early life nutrition to ensure optimal brain development, first by describing the important milestones of neurodevelopment, overviewing the basic principles that regulate the nutrient-brain interactions, and then presenting recent findings about the most common micronutrient deficiencies (iron, zinc, and iodine) and the associated risk of neuropsychiatric adverse consequences.
Early Brain Development
Human brain development is a protracted process that begins in the third gestational week with the differentiation of the neural progenitor cells and extends at least through late adolescence, arguably throughout the lifespan [11]. By the end of the embryonic period (eighth week post conception), the rudimentary structures of the brain are established and the major compartments of the central and peripheral nervous systems are defined [11]. After the external form is established, a complex series of processes begins. Between the second and fourth months of gestation, major proliferative events occur: progenitors of neurons and glia are produced and proliferate. From approximately 5 months of gestation to early postnatal life, there is glial multiplication [12]. As progenitors cells proliferate and differentiate, they migrate from their sites of origin to different brain areas where they need to make connections with other neurons. The peak time for this occurrence is from the third to fifth months of gestation [12]. Once the cells have reached their target region of the brain, in order to become integrated into neural networks, they have to develop neural processes (dendritic and axonal ramifications) that allow them to communicate through synaptic contacts. These organizational events, which occur from approximately the fifth month of gestation to several years after birth, are crucial to establish the awesome circuitry that identifies the human brain. The efficiency of information transmission in the pathways is greatly enhanced by myelin which ensheathes the axons. The period of myelination in humans is long, being rapid and dramatic from the second trimester of pregnancy to the first 2 years of postnatal life and continuing into adult life [11].
Principles of Nutrient Effects on Brain Development
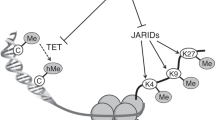
In the first 1000 days of life, the brain faces an extraordinary growth, increasing its dimension, differentiating gradually in a highly specialized organ, and slowly losing plasticity. The rate of growth in this period is the highest throughout the lifespan. In general, the higher the rate of an organ’s growth, the greater is its risk to be damaged by insufficient supply of nutrients. This makes the developing brain greatly susceptible to damages [13]. The brain is a heterogeneous organ. It is comprised of distinct anatomical regions (hippocampus, cortex, and striatum) and processes (e.g., myelination, neurotransmitters), each with unique developmental trajectories and a set of nutrient requirements [13]. Many of these regions or processes have developmental trajectories that begin and accelerate in fetal life or shortly after birth. Furthermore, every region and every process have two crucial moments: the critical period and the sensitive period. The borders between the two periods are blurred. However, they can conceptually be defined as follows: the former is an early life epoch where irreversible long-term consequences follow insults; the latter represents broader epochs when the brain is more susceptible to environmental factors, such as nutrient deficiencies, but the effect is not inevitably permanent [14, 15]. As such, there are a series of nutrient- and tissue-specific critical periods throughout the development, and the effects that nutrient deficiencies have on the vulnerability of the brain are determined by two factors: the timing of the nutrient deficiency and the specific region’s requirement for that nutrient at that time [16••]. Failure to construct a brain region during its critical period can lead to permanent consequences, such as residual structural defects [17], persistent neurochemical and electrophysiological abnormalities, and altered gene expression [18,19,20, 21•]. These mechanisms potentially explain the biological bases of long-term effects of early life nutritional perturbations (Fig. 1). Thus, ensuring adequate nutrient intake is necessary to allow a time-coordinated brain development and to create an integrated healthy working brain structure.
Interactions between genes and environment shape human development. Early life nutrition (prenatal and neonatal) represents a fundamental environmental factor that can profoundly affect brain development. Early life malnutrition, during critical periods of neurodevelopment, can create an altered brain structure and induce altered patterns of epigenetic markers, leading to brief- and long-term adverse health consequences with respect to cognitive, social, emotional, neurological, and psychiatric performances
Epigenetics
The first epidemiological evidence of a link between inadequate early nutrition and diseases emerged from cohort studies examining health status of offspring whose mothers were pregnant during periods of severe famine, such as the Dutch Hunger Winter. These studies demonstrated that individuals prenatally exposed to severe famine were more likely to have coronary heart disease, atherogenic lipid profile, disturbed blood coagulation, increased stress responsiveness, obesity, and glucose intolerance during adulthood [7]. Among all the diseases, there was also an increased risk of neuropsychiatric diseases, such as schizophrenia and affective disorders [22,23,24]. In the last few years, growing attention has been put on epigenetics. The emerging hypothesis is that part of these adverse health consequences could be mediated by epigenetic changes, induced by early life environmental risk factors such as inadequate or inappropriate nutrition. There is convincing evidence that early malnutrition has an impact on genomes with long-term consequences on health outcomes. By DNA methylation, histone modifications, and noncoding microRNA, epigenetics modulates the intensity and the timing of gene expression throughout the entire life course, potentially leading to later life behavioral consequences and diseases [6]. Several animal studies have demonstrated that prenatal exposure to stressing events (including malnutrition) induces lasting epigenetic changes in the brain, which have been linked to changes in brain gene expression, stress reactivity, and behavior [25]. On the contrary, in humans, it is very challenging to establish this epigenetic link, for different reasons: (i) they are exposed to a mixture of environmental factors that can have confounding effects; (ii) brain tissue is inaccessible in living humans. Although some studies showed epigenetics changes in neurodevelopmental-related genes, it still remains unclear if these alterations are the actual cause of the neurodevelopmental disorders [26, 27]. Many human diseases are the result of an interaction between genetic and environmental factors. Understanding the mechanisms through which dietary affects epigenomes and how these epigenetic changes are involved in determining neuropsychological phenotypes could potentially offer an opportunity for preventing or treating some mental illnesses [28]. Thus, more and better-designed studies need to be done, to deepen our knowledge about the relationship between nutrition, epigenetics, and neurodevelopment.
Further Research
All these considerations offer the opportunity not only to focus on ensuring the child with adequate nutrition to promote normal brain development but also to consider nutrition as a powerful instrument to optimize cognitive, social, emotional behavioral development and health outcomes. Yet, the literature related to this vast topic is frequently contradictory due to several reasons: critical and sensitive periods are challenging to define and often used interchangeably; timing dose duration of supplementation principle is not always clearly definable; assessment of connections with neurobehavioral tests and outcome measures is not always made with the finest tests at the correct assessment age. Studies often are extremely different from each other (e.g., in terms of age and population examined, duration and doses of supplementation, assessment tests, general health status, and sociocultural habits), explaining why meta-analyses are not the best tool to assess nutrient-brain relationships. In the attempt to increase statistical power by enlarging the sample size, all the nutrient nuances are lost and the type II error increases, inevitably leading to inconclusive results [29•]. As a result, finding or excluding nutrient-brain decisive connection remains still problematic [30••]. Further studies should take into account these factors, aiming to reduce confounding variables in order to obtain definite indications.
Nutrients That Influence Neurodevelopment
During pregnancy, macro- and micronutrient requirements increase. Adequate nutritional diet is a key to avoid adverse health consequences for the developing fetus. The 2014 Italian RDA, specifically, indicate an additional requirement of 69 kcal/d for the first trimester, 266 kcal/day for the second trimester, and 496 kcal/day in the third trimester of pregnancy (for a grand total of an additional 76,530 kcal). Very similar amounts have been established by the EFSA (70 kcal/day in the first trimester to 260 and 500 kcal/day in the second and third trimesters, respectively), with an increase of about 500 kcal/day during the first 6 months of exclusive breastfeeding [31]. A comprehensive review of macronutrients requirements is beyond the scope of this article. The following sections will focus on the most common micronutrient deficiencies. In Table 1, the estimated average requirements for the most common micronutrients are listed [32, 33].
Iron
Iron is an essential micronutrient involved in many biological processes, including neurodevelopment. It is necessary for normal anatomic brain development [34,35,36], myelination, and neurotransmission [37]. Iron deficiency is the most common micronutrient deficiency worldwide. Approximately 2 billion people around the world are estimated to suffer from this condition, roughly half of the preschool-aged children and pregnant women [38]. While during adolescence this condition is frequently reversible without consequences, early life iron deficiency can lead to long-lasting and potentially permanent effects, resulting in later life neurocognitive and behavioral disorders [39]. Many animal and human studies demonstrated a crucial role of iron in brain development [37]. Overall, it is well accepted that prevention of iron deficiency is preferable to treatment [40]. A set of randomized clinical trials conducted in Nepal demonstrated the known positive effect of prenatal iron to support hippocampal and striatum development. School-aged children whose mothers were supplemented with iron/folic acid during gestation and early neonatal life had better neurocognitive performances (working memory, inhibitory control, and fine motor functioning) than those born from not supplemented mothers [41]. Furthermore, the supplementation between the 12th and 36th months of life did not confer additional benefits on general neurointellectual functioning [42] nor cause a neurocognitive catch-up growth in those children who were not supplemented during gestation [43]. A Vietnamese study demonstrated that preconception supplementation with iron and folic acid, compared with exclusively folic acid supplementation, improved linear growth and fine motor development at 2 years of age [44]. A Chinese study proved that infants who received iron supplementation in infancy (between 6 weeks and 9 months) exhibited better gross motor scores at 9 months than children who did not receive iron [45]. In addition, infants born iron-deficient exhibited slower recognition of their mothers’ voice at 2 months of age, as measured by event-related potentials, than children born iron-sufficient. This is in line with the effect of iron deficiency on hippocampal development [46]. Formerly, iron-deficient infants were demonstrated to have significant slower reaction times and poorer inhibitory control 8 to 9 years after supplementation [47], as well as different patterns of functional brain connectivity [48]. A 10-year follow-up Chilean study verified that mistimed or excessive iron might lead to worse neurodevelopmental outcomes at 10 years [49]. In another trial conducted in Nepal, it was found that delayed cord clamping (≥ 180 s after delivery) reduces anemia at 8 and 12 months of age in a high-risk population, which may have major positive effects on infants’ health and development [50]. Longitudinal cohort studies showed that infants who experienced iron deficiency were more likely to experience cognitive and socioemotional impairments throughout infancy, childhood, and adolescence, with slower perceptual speed, poorer understanding of quantitative concepts, poorer spatial memory [51], impaired language abilities [52], and worse recognition memory [53]. Furthermore, at 25 years, a higher proportion of the group with chronic iron deficiency did not complete secondary school, were single, and reported poorer mental health and more negative emotions [54]. Moreover, in retrospective population-based cohorts, an association was found between maternal iron deficiency and risk of schizophrenia spectrum disorders among offspring [55], as well as an association between low iron intake and increased risk of autism spectrum disorders [56]. Collectively, these studies confirm the timing-dose-duration principles previously explained [30••]. In addition, they are consistent with the long-term consequences of early iron deficiency on neurodevelopmental processes and on neuropsychiatric susceptibility to diseases. Lastly, new insights have been provided into the mechanisms through which iron deficiency could affect neurocognitive performance. Among these, epigenetics seems to play a role. In a study performed on pigs, even though the sample size was relatively low, it was demonstrated that neonatal iron deficiency led to altered hippocampal DNA methylation and gene regulation, potentially leading to effects on neurodevelopment mediated by increased hypoxia-induced angiogenesis and increased blood-brain barrier permeability [57]. Another study, conducted on mice, showed that chronic iron deficiency during development alters the adult hippocampal transcriptomes and that restoring iron status during a known critical period of hippocampal neurodevelopment incompletely normalized these changes [21•]. Further detailed studies need to be designed to better understand the implication of such biological process.
Iodine
Gestation involves significant changes in maternal thyroid function. Even though fetal thyroid starts to produce hormones around the 18th–20th weeks of gestation, the major supply of thyroxine remains the mother. Since the first epidemiological studies, the association between severe iodine deficiency in pregnant women and fetal neurological damage has been demonstrated [58, 59]. Thyroidal hormones are involved directly and indirectly in essentially all key neurodevelopmental processes [60, 61]. Both neurons and glial cells (astrocytes and oligodendrocytes) are greatly provided with thyroid hormone receptors. A recent review from Velasco et al. masterfully enumerates trials, meta-analyses, and reviews that demonstrate which brain areas are affected by iodine deficiency and the cognitive and neurodevelopmental consequences [62••]. In the last two decades, increasing evidence has proved that adverse neurological outcomes are linked not only to maternal hypothyroidism but also to a condition known as hypothyroxinemia [63]. This is defined as the presence of a free thyroxine value below the 2.5th percentile with a thyrotropin level within the reference range. Hypothyroxinemia is not related to rural-insufficient iodine intake areas; evidence has showed that it exists even in iodine-sufficient regions [64,65,66]. The phenotypes related to iodine deficiency have evolved from goiter and severe mental disability to a new clinical spectrum of neuropsychological disorders associated with maternal hypothyroxinemia. In biopsies performed in experimental rodents, gestational thyroid hormone deficiency was found to cause dendritic and axonal growth limitation, neural abnormal location, synaptic function alteration, hystogenesis, and cerebral cortex cytoarchitecture alteration. As such hypothyroxinemia causes disrupted neocortical layering [67, 68]. Cognitive deficits and poor psychomotor development in the progeny of mothers who were hypothyroxinemic during the first half of gestation have been shown [67]. In two observational studies conducted in the UK and Australia, it was found that children of mothers with UIC < 150 μg/g creatinine were more likely to score within the lowest quartile on verbal IQ, reading accuracy, and reading comprehension at age 8–9 years [69] and had lower educational outcomes at age 9 years [65]. These findings were confirmed in a population-based prospective cohort of earlier children, where a UIC below ~ 100 μg/L was associated with lower infant language skills up to 18 months [70]. A prospective study from the Netherlands reported that low maternal UIC during pregnancy (< 10th percentile) was associated with impaired executive function in children at age 4 years [71]. A large Norwegian population-based prospective observational study concluded that a suboptimal maternal iodine intake (below the estimated average requirement of 160 μg/day) during pregnancy was associated with symptoms of child language delay, behavior problems, and reduced fine motor skills at 3 years of age. Surprisingly, no beneficial effect was associated with iodine supplementation during pregnancy [66]. A recent RCT in India and Thailand found no benefit of iodine supplementation on 5–6-years child cognition born to mothers with mild-to-moderate deficiency; however, it should be observed that the Indian women recruited were actually iodine-sufficient and that both countries adhere to iodized salt programs [72]. In the last years, some evidence emerged, though not consistent, that offspring of mothers with abnormal serum thyroid hormone concentrations during early pregnancy might present an increased risk of attentional problems, such as attention-deficit/hyperactivity disorder (ADHD). A Norwegian study found that a low iodine intake (< 200 g/L) was associated with increased child ADHD symptom scores, but not with ADHD diagnosis. Iodine supplementation did not reduce the risk [73]. Additionally, Román et al. [74] found a consistent association between severe, early gestation, maternal hypothyroxinemia and autistic symptoms in offspring. The possibility of preventive interventions must be further investigated.
Zinc
Zinc is an essential trace mineral for all forms of life because of its universal role in keeping cells operating. Inadequate zinc intake is common, especially in individuals and population whose regular diets do not include readily bioavailable zinc sources or are overreliant on cereals (rich with zinc inhibitors) [75]. Gestation and older infancy constitute periods of increased risk of zinc deficiency. Many preclinical studies demonstrated its key role in neurodevelopmental processes (such as neurogenesis, neuronal migration, synaptic genesis, and myelination) and modulation of intra- and intercellular signaling (GABAergic neurons) [75, 76]. While it has been well established that severe deficiencies cause serious brain structural malformations [77], less is known about mild-to-moderate deficiency affecting sensorimotor and cognitive development. An Indian DB-RCT showed that zinc supplementation till 3-month corrected age in preterm breastfed infants significantly improves alertness and attention patterns; it decreases signs of hyperexcitability and abnormal patellar and bicipital reflexes [78]. Among 8–18-month infant adoptees coming from three global regions (post-Soviet states, Ethiopia, and China), zinc deficiency was the second most common micronutrient deficiency and was associated with compromised memory functioning [79]. In 2002, a study investigated the effects of iron-folic acid and/or zinc supplementation on the results of the Fagan Test of Infant Intelligence and the A-not-B task of executive functioning among 367 Nepali infants living in Sarlahi District. Neither the combined nor the individual micronutrient supplements improved the performance five indicators of information processing [80]. A recent double-blind trial conducted in Tanzania randomized infants at 6 weeks of age to zinc, multivitamins, zinc and multivitamins, or placebo. At approximately 15 months, a subsample of children underwent developmental assessment using the cognitive, language (receptive and expressive), and motor (fine and gross) scales of the Bayley Scales of Infant and Toddler Development Third Edition. Neither daily zinc nor multivitamin (vitamins B-complex, C, and E) supplementation led to improvements in any of the developmental domains assessed [81]. A DB-RCT was conducted on Peruvian infants aged 6–18 months in order to determine the effects of prevention of zinc deficiency on cognitive and sensorimotor development during infancy. A set of assessments measured the cognitive development (attention, memory, and learning) and the developmental status, finding zinc to be supportive in sustaining normative neurodevelopment in the first 2 years of life [82]. Intriguingly, among individuals with autism spectrum disorders, the incidence rate of zinc deficiency in very young age has been reported to be significantly increased compared with age-matched healthy control subjects [83, 84]. In addition, two small case-control studies showed a correlation between low levels of zinc and attention-deficit/hyperactivity disorder [85]. Further studies are needed to clarify good interventions in terms of neurocognitive outcomes.
Conclusions
The studies reviewed here suggest that early life environment, particularly the fetal and early postnatal environment, influences later life health outcomes and disease risks across multiple organ systems. Among all the environmental factors, nutrition plays a fundamental role. From conception to approximately 3 years of age, the basis for lifespan brain functions is established. Children adequately nourished are more likely to reach their developmental potential in cognitive, motor, and socioemotional abilities, with positive societal repercussions. The restricted development of these skills during early life increases the risk for later neuropsychological problems, psychiatric illnesses, poor school achievement, early school dropout, low-skilled employment, and poor care of future children, thus contributing to the intergenerational transmission of poverty.
Epigenetics seems to play a leading role, explaining, at least in part, how early life stimuli can have these long-term consequences. Despite recent advances in technologies, our knowledge regarding nutritional epigenetics is still limited. Further studies are needed to better understand the use of nutrition for maintaining health and preventing diseases through modifiable epigenetic mechanisms.
In order to draw more evidence-based, relevant, and ready-to-use conclusions on nutrition, data collection and research must be conducted more regularly and more rigorously, with a higher focus on national and regional idiosyncrasies. Disaggregation of data based on wealth, age, gender, disabilities, and geography could lead to the establishment of more specific standards for diagnosis, treatment, and prevention of all forms of malnutrition. Being more aware of how nutritional status might vary within households even in the same region could support drawing policies to tackle long-term consequences of early life malnutrition.
Strategies of prevention should focus on ensuring more quality food to preconceptional, pregnant, lactating women and to children in their early life, not only in those areas where malnutrition is common but also in developed countries.
Lastly, although it is premature to propose diet as therapy for mental illnesses and more studies are needed to deepen our knowledge, optimizing nutrition could potentially help in preventing or attenuating some mental disorders.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pietrobelli A, Agosti M, MeNu Group. Nutrition in the first 1000 days: ten practices to minimize obesity emerging from published science. Int J Environ Res Public Health. 2017;14:1491. https://doi.org/10.3390/ijerph14121491.
Barks A, Hall AM, Tran PV, Georgieff MK. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr Res. 2018;85:176–82. https://doi.org/10.1038/s41390-018-0204-8.
Pereira-da-Silva L, Rêgo C, Pietrobelli A. The diet of preschool children in the Mediterranean countries of the European Union: a systematic review. Int J Environ Res Public Health. 2016;13:572. https://doi.org/10.3390/ijerph13060572.
Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–84. https://doi.org/10.1111/nure.12102.
Levi RS, Sanderson IR. Dietary regulation of gene expression. Curr Opin Gastroenterol. 2004;20:139–42.
Canani RB, Costanzo MD, Leone L, Bedogni G, Brambilla P, Cianfarani S, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24:198–205. https://doi.org/10.1017/S0954422411000102.
Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–91. https://doi.org/10.1016/j.earlhumdev.2006.07.001.
•• Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27:411–23. https://doi.org/10.1017/S0954579415000061 This is an excellent paper about neural plasticity and nutrient effects on brain development.
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9. https://doi.org/10.1016/j.biopsych.2010.05.028.
Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–57. https://doi.org/10.1016/S0140-6736(07)60076-2.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. https://doi.org/10.1007/s11065-010-9148-4.
Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016;89:248–68. https://doi.org/10.1016/j.neuron.2015.12.008.
Fox SE, Levitt P, Nelson CA. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81:28–40. https://doi.org/10.1111/j.1467-8624.2009.01380.x.
Bornstein MH. Sensitive periods in development: structural characteristics and causal interpretations. Psychol Bull. 1989;105:179–97.
Colombo J. The critical period concept: research, methodology, and theoretical issues. Psychol Bull. 1982;91:260–75.
•• Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J Pediatr. 2016;175:16–21. https://doi.org/10.1016/j.jpeds.2016.05.013 This is an excellent review about recent evidence on nutrition effects on brain development.
Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. https://doi.org/10.1159/000075667.
Tyagi E, Zhuang Y, Agrawal R, Ying Z, Gomez-Pinilla F. Interactive actions of Bdnf methylation and cell metabolism for building neural resilience under the influence of diet. Neurobiol Dis. 2015;73:307–18. https://doi.org/10.1016/j.nbd.2014.09.014.
Zeisel S. Choline, other methyl-donors and epigenetics. Nutrients. 2017;9:445. https://doi.org/10.3390/nu9050445.
Ly A, Ishiguro L, Kim D, Im D, Kim S-E, Sohn K-J, et al. Maternal folic acid supplementation modulates DNA methylation and gene expression in the rat offspring in a gestation period-dependent and organ-specific manner. J Nutr Biochem. 2016;33:103–10. https://doi.org/10.1016/j.jnutbio.2016.03.018.
• Barks A, SJB F, Georgieff MK, Tran PV. Early-life neuronal-specific iron deficiency alters the adult mouse hippocampal transcriptome. J Nutr. 2018;148:1521–8. https://doi.org/10.1093/jn/nxy125 This is an excellent paper about epigenetic changes induced by early life malnutrition.
Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry. 1992;49:983–8.
Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31.
St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–62. https://doi.org/10.1001/jama.294.5.557.
Kundakovic M, Jaric I. The epigenetic link between prenatal ad-verse environments and neurodevelopmental disorders. Genes (Basel). 2017;8:104. https://doi.org/10.3390/genes8030104.
Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1350–4. https://doi.org/10.1002/ajmg.b.31109.
Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–61. https://doi.org/10.1073/pnas.1214056110.
Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–4. https://doi.org/10.1016/S2215-0366(14)00051-0.
• Barnard ND, Willett WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA. 2017;318:1435–6. https://doi.org/10.1001/jama.2017.12083 This is an excellent paper about meta-analysis and the potential consequences on diet and health policies.
•• Georgieff MK, Ramel SE, Cusick SE. Nutritional influences on brain development. Acta Paediatr. 2018;107:1310–21. https://doi.org/10.1111/apa.14287 This is an excellent review about effects of nutrient on neurodevelopment.
Marangoni F, Cetin I, Verduci E, Canzone G, Giovannini M, Scollo P, et al. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients. 2016;8:629. https://doi.org/10.3390/nu8100629.
Società di Nutrizione Umana (SINU). LARN—Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana; IV Revisione. Milano: SICS; 2014. p. 1–655.
Ross AC, Taylor CL, Yaktine AL, et al. Dietary reference intakes for calciumand vitaminD. Institute ofMedicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. National Academies Press (US); 2011. Summary tables.
Greminger AR, Lee DL, Shrager P, Mayer-Pröschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J Nutr. 2014;144:1058–66. https://doi.org/10.3945/jn.113.187732.
Carlson ES, Tkac I, Magid R, O’Connor MB, Andrews NC, Schallert T, et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–9. https://doi.org/10.3945/jn.108.096354.
Tran PV, Kennedy BC, Lien Y-C, Simmons RA, Georgieff MK. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Regul Integr Comp Physiol. 2015;308:R276–82. https://doi.org/10.1152/ajpregu.00429.2014.
Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–91. https://doi.org/10.1301/nr.2006.may.S34-S43.
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444–54. https://doi.org/10.1017/S1368980008002401.
Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69:S43–8. https://doi.org/10.1111/j.1753-4887.2011.00432.x.
Cusick SE, Georgieff MK. Nutrient supplementation and neurodevelopment: timing is the key. Arch Pediatr Adolesc Med. 2012;166:481–2. https://doi.org/10.1001/archpediatrics.2012.199.
Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304:2716–23. https://doi.org/10.1001/jama.2010.1861.
Christian P, Morgan ME, Murray-Kolb L, LeClerq SC, Khatry SK, Schaefer B, et al. Preschool iron-folic acid and zinc supplementation in children exposed to iron-folic acid in utero confers no added cognitive benefit in early school-age. J Nutr. 2011;141:2042–8. https://doi.org/10.3945/jn.111.146480.
Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, et al. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med. 2012;166:404–10. https://doi.org/10.1001/archpediatrics.2012.37.
Nguyen PH, Gonzalez-Casanova I, Young MF, Truong TV, Hoang H, Nguyen H, et al. Preconception micronutrient supplementation with iron and folic acid compared with folic acid alone affects linear growth and fine motor development at 2 years of age: a randomized controlled trial in Vietnam. J Nutr. 2017;147:1593–601. https://doi.org/10.3945/jn.117.250597.
Angulo-Barroso RM, Li M, Santos DCC, Bian Y, Sturza J, Jiang Y, et al. Iron supplementation in pregnancy or infancy and motor development: a randomized controlled trial. Pediatrics. 2016;137:e20153547. https://doi.org/10.1542/peds.2015-3547.
Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr. 2015;167:1226–32. https://doi.org/10.1016/j.jpeds.2015.08.035.
Algarín C, Nelson CA, Peirano P, Westerlund A, Reyes S, Lozoff B. Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Dev Med Child Neurol. 2013;55:453–8. https://doi.org/10.1111/dmcn.12118.
Algarin C, Karunakaran KD, Reyes S, Morales C, Lozoff B, Peirano P, et al. Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Front Aging Neurosci. 2017;9:54. https://doi.org/10.3389/fnagi.2017.00054.
Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med. 2012;166:208–15. https://doi.org/10.1001/archpediatrics.2011.197.
Kc A, Rana N, Målqvist M, Jarawka Ranneberg L, Subedi K, Andersson O. Effects of delayed umbilical cord clamping vs early clamping on anemia in infants at 8 and 12 months: a randomized clinical trial. JAMA Pediatr. 2017;171:264–70. https://doi.org/10.1001/jamapediatrics.2016.3971.
Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. https://doi.org/10.1056/NEJM199109053251004.
Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70. https://doi.org/10.1067/mpd.2002.120688.
Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. https://doi.org/10.1179/147683010X12611460763689.
Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr. 2013;163:1260–6. https://doi.org/10.1016/j.jpeds.2013.05.015.
Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry. 2008;65:1136–44. https://doi.org/10.1001/archpsyc.65.10.1136.
Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol. 2014;180:890–900. https://doi.org/10.1093/aje/kwu208.
Schachtschneider KM, Liu Y, Rund LA, Madsen O, Johnson RW, Groenen MA, et al. Impact of neonatal iron deficiency on hippocampal DNA methylation and gene transcription in a porcine biomedical model of cognitive development. BMC Genomics. 2016;17:856. https://doi.org/10.1186/s12864-016-3216-y.
Pharoah P, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Int J Epidemiol. 2012;41:589–92. https://doi.org/10.1093/ije/dys070.
Thilly CH, Delange F, Lagasse R, Bourdoux P, Ramioul L, Berquist H, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab. 1978;47:354–60. https://doi.org/10.1210/jcem-47-2-354.
Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. https://doi.org/10.1016/j.neuroscience.2015.09.070.
Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A, et al. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol. 2012;237:477–88. https://doi.org/10.1016/j.expneurol.2012.07.019.
•• Velasco I, Bath SC, Rayman MP. Iodine as essential nutrient during the first 1000 days of life. Nutrients. 2018;10:290. https://doi.org/10.3390/nu10030290. This is an excellent review about iodine effects on brain development.
Furnica RM, Lazarus JH, Gruson D, Daumerie C. Update on a new controversy in endocrinology: isolated maternal hypothyroxinemia. J Endocrinol Investig. 2015;38:117–23. https://doi.org/10.1007/s40618-014-0203-5.
Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol. 2013;79:152–62. https://doi.org/10.1111/cen.12227.
Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab. 2013;98:1954–62. https://doi.org/10.1210/jc.2012-4249.
Abel MH, Caspersen IH, Meltzer HM, Haugen M, Brandlistuen RE, Aase H, et al. Suboptimal maternal iodine intake is associated with impaired child neurodevelopment at 3 years of age in the Norwegian mother and child cohort study. J Nutr. 2017;147:1314–24. https://doi.org/10.3945/jn.117.250456.
Min H, Dong J, Wang Y, Wang Y, Teng W, Xi Q, et al. Maternal hypothyroxinemia-induced neurodevelopmental impairments in the progeny. Mol Neurobiol. 2016;53:1613–24. https://doi.org/10.1007/s12035-015-9101-x.
Lavado-Autric R, Ausó E, García-Velasco JV, Arufe M del C, Escobar del Rey F, Berbel P, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111:1073–82. https://doi.org/10.1172/JCI16262.
Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;382:331–7. https://doi.org/10.1016/S0140-6736(13)60436-5.
Markhus MW, Dahl L, Moe V, Abel MH, Brantsæter AL, Øyen J, et al. Maternal iodine status is associated with offspring language skills in infancy and toddlerhood. Nutrients. 2018;10:1270. https://doi.org/10.3390/nu10091270.
van Mil NH, Tiemeier H, Bongers-Schokking JJ, Ghassabian A, Hofman A, Hooijkaas H, et al. Low urinary iodine excretion during early pregnancy is associated with alterations in executive functioning in children. J Nutr. 2012;142:2167–74. https://doi.org/10.3945/jn.112.161950.
Gowachirapant S, Jaiswal N, Melse-Boonstra A, Galetti V, Stinca S, Mackenzie I, et al. Effect of iodine supplementation in pregnant women on child neurodevelopment: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:853–63. https://doi.org/10.1016/S2213-8587(17)30332-7.
AbelMH, Ystrom E, Caspersen IH, Meltzer HM, Aase H, Torheim LE, et al. Maternal iodine intake and offspring attention-deficit/hyperactivity disorder: results froma large prospective cohort study. Nutrients. 2017;9:1239. https://doi.org/10.3390/nu9111239.
Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VWV, Hofman A, de Rijke YB, et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013;74:733–42. https://doi.org/10.1002/ana.23976.
Sandstead HH. Zinc deficiency. A public health problem? Am J Dis Child. 1991;145:853–9.
Sandstead HHWO. Atwater memorial lecture. Zinc: essentiality for brain development and function. Nutr Rev. 1985;43:129–37.
Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998;68:464S–9S.
Mathur NB, Agarwal DK. Zinc supplementation in preterm neonates and neurological development, a randomized controlled trial. Indian Pediatr. 2015;52:951–5.
Fuglestad AJ, Kroupina MG, Johnson DE, Georgieff MK. Micronutrient status and neurodevelopment in internationally adopted children. Acta Paediatr. 2016;105:e67–76. https://doi.org/10.1111/apa.13234.
Siegel EH, Kordas K, Stoltzfus RJ, Katz J, Khatry SK, LeClerq SC, et al. Inconsistent effects of iron-folic acid and/or zinc supplementation on the cognitive development of infants. J Health Popul Nutr. 2011;29:593–604.
Locks LM, Manji KP, McDonald CM, Kupka R, Kisenge R, Aboud S, et al. The effect of daily zinc and/or multivitamin supplements on early childhood development in Tanzania: results from a randomized controlled trial. Matern Child Nutr. 2017;13:e12306. https://doi.org/10.1111/mcn.12306.
Colombo J, Zavaleta N, Kannass KN, Lazarte F, Albornoz C, Kapa LL, et al. Zinc supplementation sustained normative neurodevelopment in a randomized, controlled trial of Peruvian infants aged 6-18 months. J Nutr. 2014;144:1298–305. https://doi.org/10.3945/jn.113.189365.
Pfaender S, Grabrucker AM. Characterization of biometal profiles in neurological disorders. Metallomics. 2014;6:960–77. https://doi.org/10.1039/c4mt00008k.
Yasuda H, Tsutsui T. Assessment of infantile mineral imbalances in autism spectrum disorders (ASDs). Int J Environ Res Public Health. 2013;10:6027–43. https://doi.org/10.3390/ijerph10116027.
Elbaz F, Zahra S, Hanafy H. Magnesium, zinc and copper estimation in children with attention deficit hyperactivity disorder (ADHD). Egypt J Med Human Genet. 2017;18(2):153–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Davide Mattei and Angelo Pietrobelli declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition and the Brain
Rights and permissions
About this article
Cite this article
Mattei, D., Pietrobelli, A. Micronutrients and Brain Development. Curr Nutr Rep 8, 99–107 (2019). https://doi.org/10.1007/s13668-019-0268-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-019-0268-z