Abstract
Mild to moderate dopaminergic (DA) neuronal death in substantia nigra pars compacta (SNc) as the main pathological hallmark of Parkinson’s disease (PD) is usually silent and does not produce marked clinical symptoms. In this study, we investigated the association between SNc DA neuronal loss and serum levels of total bilirubin (TB), selenium (Se), and zinc (Zn) in 6-hydroxydopamine (6-OHDA) animal model of PD. The neurotoxin of 6-OHDA was injected into the medial forebrain bundle of right hemisphere by stereotaxic surgery. Two conventional behavioral tests were carried out in several steps after the toxin to confirm the model reproduction and quantify severity and progress of 6-OHDA-induced PD. Blood samples were collected within 1 week before the toxin and in the second, fifth, and eighth weeks thereafter. Immunohistochemistry (IHC) assessments were performed on the rat’s brain to determine the severity of DA neuronal loss in SNc. The severity of behavioral symptoms and TB levels were progressively increased in 6-OHDA-treated rats. On the other hand, Se and Zn levels in them were lower than control. These changes were observed in rats with severe or mild behavioral symptoms. Also, IHC revealed that changes in TB, Se, and Zn associate with SNc DA neuronal loss but do not correlate with its severity. Significant changes in serum levels of TB, Se, and Zn in the mild SNc DA neuronal loss suggest them as valuable parameters for establishment of a serum profile for early detection of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive loss of dopaminergic (DA) neurons in substantia nigra pars compacta (SNc) is the main pathophysiological mechanism underlying Parkinson’s disease (PD). The pathogenic mechanism responsible for this neuronal death is unclear but a large body of evidence suggests that oxidative stress or reduced capability of endogenous anti-oxidant mechanisms in the brain tissue plays an important role [1,2,3]. Mild to moderate death of DA neurons in human has no clinical symptom and extrapyramidal signs such as tremor, bradykinesia, and rigidity appear when about 70% of DA neurons in SNc already loss [4, 5]. Therefore, detection of biomarkers signalizing DA neuronal death in SNc can assist in the early diagnosis and thus better management of PD. As oxidative stress is the main cause of DA neuronal death, biochemical parameters reflecting this stress are of primary importance.
Studies have shown that bilirubin acts as a natural bodily anti-oxidant in human [6,7,8,9]. It is a tetrapyrrolic compound which be able to scavenge reactive oxygen species (ROS). Indeed, bilirubin is the only endogenous lipophilic antioxidant in human body which effectively protects lipids from oxidation. Wu et al. showed that bilirubin is almost three times more potent than vitamin E analog Trolox in the preventing of low-density lipoprotein (LDL) oxidation [7]. Plasma total bilirubin (TB) is mainly related to heme-oxygenase (HO) enzyme system [10, 11]. This pathway is activated early in DA cells exposed to oxidative stress [11]. Heme is catabolized to free iron, carbon monoxide (CO), and biliverdin in the presence of HO, with biliverdin reductase converting biliverdin into bilirubin [12].
The two essential trace elements of selenium and Zinc are necessary for proper functioning of the brain mainly due to their antioxidant activity. Se incorporates into selenoproteins which have a selenocysteine residue in their active site. They play a variety of important functions such as selenium transport and control of the cellular redox state [13]. Through their antioxidant and redox activities, selenoproteins play neuroprotective role in CNS [14]. Additionally, studies on the different animal models have shown that disbalance in Se level probably modifies dopamine turnover [15]. Zn is also an essential functional component of many enzymes counteracting oxidative stress such as superoxide dismutase, which catalyzes the redox conversion of superoxide anions to hydrogen peroxide and oxygen. This enzyme is commonly found in high concentrations in melanized dopaminergic neurons of SNc where it functions as a scavenger of free radicals, thereby protecting these neurons from oxidative stress [16].
In spite of the well-known role of TB, Se, and Zn in protection of DA neurons against oxidative stress, studies on the association between serum TB, Se, and Zn with PD are limited and the few existing reports in this issue have conflicting results [17,18,19,20,21]. More important, almost all of them are clinical or epidemiological studies and are unable to clarify whether the association between these parameters and PD is merely related to their pre-existing high levels or that progressive DA neuronal death produces dynamic changes in their plasma levels. Furthermore, pharmacological therapy and comorbidity remarkably disturb clinical data and prevent the precise evaluation of these associations. Therefore, in this study we investigated the association between death of SNc DA neurons and serum levels of TB, Se, and Zn in 6-OHDA animal model of PD. In this model, although the behavioral symptoms are different from the clinical signs of PD in human, but the model reproduces the main cellular processes such as oxidative stress, neurodegeneration, neuroinflammation, and neuronal death by apoptosis which involve in DA neuronal death in PD [22]. Previously, we demonstrated that change in serum urate level, another endogenous natural anti-oxidant, can predict severity of DA neuronal death in 6-OHDA-treated rats [23].
Experimental Procedures
Animals and Experimental Groups
Adult male Wistar rats (Razi Institute, Karaj, Iran), weighing 250–300 g in beginning of the study, were housed in large cages (38 × 59 × 20 cm) at a temperature-controlled colony room under 12 h light/12 h dark cycle with full access to tap water and standard food in the form of pellet (Pars Animal Feed Co, Karaj, Iran). All procedures of the present study carried out according to the guidelines of animal experiments of the Research Council at Qazvin University of Medical Sciences. Three groups of rats were included: control (n = 10), sham (n = 9), and 6-OHDA (n = 19). n in each group is number of rats that were alive in the end of study and yield enough serum samples. Control group comprised healthy rats that did not experience stereotaxic surgery. Rats in sham group received 6-OHDA solvent by the same procedure that 6-OHDA group received the toxin itself.
Experimental Schedule
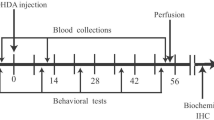
Figure 1 displays the time schedule of the experiments. Apomorphine-induced rotational test was carried out first and animals with less than 10 net left or right rotations within 30 min were selected. After a few days, asymmetry forelimb use (cylinder) test was performed on the selected rats and then 6-OHDA or its solvent was intracerebrally administrated by stereotaxic surgery. The post-toxin behavioral tests were carried out within the second, fourth, sixth, and eighth weeks. Rotational and cylinder tests were carried out on separate days with at least 1-day interval to avoid the effect of apomorphine on the forelimbs use. Blood samples were collected before the toxin and in the second, fifth, and eighth weeks thereafter.
Experiment design. Only rats showed less than 10 rotations in 30 min before the toxin were selected for study. Behavioral tests were carried out within 1 week before the toxin and in the second, fourth, sixth, and eighth weeks after that. Blood collection and serum extractions were carried out within 1 week before the toxin and at the second, fifth, and eighth weeks after that. After blood collection in the eighth week, selected rats in each group were perfused and their brains were removed for IHC studies. Numbers show days after the toxin
Stereotaxic Surgery
6-OHDA (Sigma) was injected into medial forebrain bundle (MFB) of the right hemisphere using stereotaxic surgery and through a 10-μl Hamilton syringe. Rats were first anesthetized with a combination of ketamine and xylazine (K/X, 70 and 6 mg/kg, respectively, i.p.) and then, 4 μl of 6-OHDA (4 μg/μl) dissolved in the saline (NaCl) containing ascorbic acid (0.2%) was injected into two sites in the right hemisphere with the following coordinates: anterior–posterior (AP) − 4, lateral (L) − 1.8, dorso-ventral (DV) 9 and AP − 4.4, L − 2, DV 8.8 according to the atlas of Paxinos and Watson [24]. Bregma was the reference for calculation of AP and L and surface of skull was the reference for calculation of the DV.
Behavioral Tests
Apomorphine-Induced Circling Behavior
Apomorphine (APO)-induced rotational test was carried out according to the method as described previously [23, 25]. Briefly, rats were first allowed to acclimate for 5 min with a cylindrical container (diameter, 28 cm; height, 38 cm), then apomorphine hydrochloride (Sigma, 0.5 mg/ kg) was injected intraperitoneally. After a minute, the number of full rotations during 30 min was recorded by expert persons. Contralateral (toward left side) and ipsilateral (toward right side) rotations were recorded as positive and negative scores, respectively. The net number of rotations was calculated by subtraction of the negative scores from positive scores. High number of net contralateral rotations indicates that the model is reproduced well.
Cylinder Test
Drug-free spontaneous forelimb use was examined by cylinder test based on previous report [26]. The rats were placed in a cylindrical container (diameter, 28 cm; height, 38 cm) and were allowed to move freely and explore the environment for 10 min. During this period, right and left or both forelimbs use for weight-bearing wall contacts was counted directly by a person who was trained for this experiment. Afterwards, an asymmetry score was calculated using the following equation:
I and C indicate number of ipsilateral (unimpaired) and contralateral (impaired) forelimb contacts, respectively, and B indicates number of contacts by both forelimbs. Scores on the forelimb asymmetry ratio range from − 1 to 1; the positive and negative scores indicate that unimpaired (right) forelimb and impaired (left) forelimb were dominant for wall contact. Thus, in a severe hemiparkinsonian rat, a significant positive ratio is expected.
Histological Assessments
IHC was carried out to assess the severity of DA neuronal loss in SNc. Three rats of each control and sham groups and eight rats of 6-OHDA group were used for this experiment. After anesthesia with K/X, rat’s brain was transcardially perfused with 50 ml phosphate-buffered saline (PBS), followed by about 100 ml paraformaldehyde 4%. After that, the brain was removed and postfixed overnight in paraformaldehyde at 4 °C. The midbrain segment was isolated bilaterally and embedded in paraffin wax and then the coronal Sects. (8-μm thickness) were prepared using a microtome (Thermo Shandon Microtome, UK) and one out of every serial three was selected. After overnight drying, sections were deparaffinized, rehydrated, and permeabilized with 0.2% Triton X-100 and non-specific binding was blocked using 10% normal goat serum. Following that, the sections were incubated overnight at 4 °C with an anti-tyrosine hydroxylase (TH) antibody (1:200, Santa Cruz) and then they were incubated with secondary antibody (60 min, rabbit IgG, Santa Cruz). Subsequently, horseradish peroxidase conjugate was applied to the sections for 60 min, then diaminobenzidine was applied until staining was optimal for light microscopy observation. Afterwards, the sections were dehydrated, cleared by xylene, and coverslipped. They were visualized on an Olympus microscope at 10 × magnification, and sections including the SN (AP, − 4.8 to − 5.2 relative to the bregma) were selected. Then six sections from each animal were selected from totally 50 sections which had been divided into five series rostrocaudally. There was at least five-section interval between two selected sections. TH-positive cells were visualized and counted manually using a light microscope at 400 × magnification. The number of TH neurons was expressed as average of the counts obtained from the six representative sections.
Blood Collection and Measurement of Serum Levels of TB, Se, and Zn
Blood samples were collected in four steps: within 1 week before the toxin and at the second, fifth, and eighth weeks after that (Fig. 1). The first, second, and third samples were collected from caudal vein and the fourth samples were collected from the heart of animals under deep anesthesia. Blood was allowed to coagulate and then serum was separated and kept in − 80 °C until the measurement time. The Alpha Classic Auto analyzer was used to determine the TB levels. Zn and Se levels were determined using calorimetric and atomic absorption techniques, respectively.
Statistical analysis
Data were expressed as the mean ± standard error (SE), in spite of the probable non-normality of the distribution of data. Data were initially analyzed by the Kolmogorov–Smirnov test to define their normality. Then, repeated measure analysis of variance (ANOVA) followed by the Newman-Keuls test was used for analysis of behavioral and biochemical data to obtain probable significant differences within and between the groups. Also, Pearson’s correlation coefficient was used to assess the possible correlation between behavioral and IHC data and serum levels of TB, Se, and Zn. Statistically significant differences were considered at a p ≤ 0.05.
Results
Behavioral Tests
Two behavioral tests were conducted to confirm the model reproduction and quantify the severity and progress of 6-OHDA-induced PD.
6-OHDA-Treated Rats Showed Marked Circling Behavior in Response to Apomorphine
In response to apomorphine, 6-OHDA-treated rats showed more than 200 rotations contralaterally directed to lesioned (right) hemisphere (upper plot in Fig. 2). The severity of circling behavior increased progressively such that in eighth week after the toxin, number of rotations was more than twice of that in the second week. Sham and control groups did not show significant circling behavior.
Behavioral symptoms in 6-OHDA-treated rats. Upper diagram displays findings of apomorphine-induced rotational test and lower diagram displays findings of cylinder test. 1st, 2nd, 3rd, and 4th refer to tests carried out in the second, forth, sixth, and eighth weeks after the toxin, respectively. Note that the intensity of behavioral symptoms increased progressively
6-OHDA-Treated Rats Showed Marked Asymmetry Score
In the absence of toxin, rats showed no significant preference to use left or right hand for contact the wall of container and their asymmetry scores were close to zero. On the other hand, 6-OHDA-treated rats showed significant scores. Their scores were 0.18 ± 0.04 and 0.66 ± 0.15 in the second and eighth weeks, respectively (Fig. 2, lower plot).
Serum Bilirubin, Selenium, and Zinc Levels
Serum TB Levels Increased in 6-OHDA-Treated Rats
Upper plot in Fig. 3 displays TB levels before the toxin and in second (1st), fifth (2nd), and eighth (3rd) weeks thereafter. TB levels before the toxin were 0.22 ± 0.03, 0.18 ± 0.09, and 0.25 ± 0.07 mg/dl in control, sham, and 6-OHDA groups, respectively. The differences were not statistically significant. In control and sham groups, TB levels did not change significantly in the second and fifth weeks but in the eighth week, the levels increased significantly and reach to 0.76 ± 0.09 and 0.68 ± 0.14, respectively.
In 6-OHDA-treated rats, TB levels increased by 170, 308, and 392% in the second, fifth, and eighth weeks, respectively. The differences between 6-OHDA-treated rats and control or sham group after the toxin were statistically significant even in the cardiac samples. However, in control group the level increased by 400% from fifth week to eighth week but in 6-OHDA group it increased only 13%.
6-OHDA-Treated Rats Had Lower Levels of Se and Zn
These elements were measured only in the cardiac samples. In contrast to TB, the levels of both Se and Zn in the 6-OHDA-treated rats were significantly lower than those in control and sham groups (Fig. 3, lower plots). Se levels in control, sham, and 6-OHDA groups were 194 ± 23, 184 ± 15, and 123 ± 10 µg/l, respectively. Levels for Zn were 214 ± 22, 200 ± 18, and 134 ± 4 µg/dl, respectively.
Association Between Severity of the Behavioral Symptoms and TB, Se, and Zn Levels
The statistical analysis indicates that there was marked correlation between TB levels and mean number of rotations in the first, third, and fourth post-toxin tests (r = 0.985, p < 0.05, n = 3, Pearson’s correlation coefficient, two-tailed). However, no significant correlation was observed between TB levels and intensity of circling behavior in a single rotational test (r = − 0.127, p = 0.302, n = 19, between number of rotations and TB levels in the second week; r = 0.19, p = 0.46, n = 19, between number of rotations in the sixth week and TB levels in fifth week; and r = − 0.171, p = 0.36, n = 19, between number of rotations and TB levels in eighth week). Similarly, no significant correlation was observed between TB levels before the toxin and number of rotations after the toxin. Serum levels of Se and Zn were not correlated with number of rotations in the eighth week too.
6-OHDA-treated rats did not respond equally to apomorphine and number of rotations varied remarkably. Most of rats showed severe circling behavior but some of them showed the mild severity of rotations. Based on this, these rats were divided into two subgroups: symptomatic (n = 10) including rats showed more than 200 contralateral rotations during a test and asymptomatic (n = 9) including rats showed no rotations or lower than 30 rotations (Fig. 4 upper plot). TB levels in asymptomatic subgroup were higher than that in symptomatic subgroup but the difference was not statistically significant. However, in both subgroups TB level increased progressively after the toxin (Fig. 4 middle plot). Our analysis showed that in symptomatic subgroup there was a marked but insignificant correlation between TB levels after the toxin and mean number of rotations in the first, third, and fourth post-toxin tests (r = 0.911, p = 0.136, n = 3). However, no significant correlation was observed between TB levels and intensity of circling behavior in a single test (r = − 0.246, p = 0.25, n = 10 between number of rotations and TB levels in second week). Also, no significant difference was observed in serum levels of Se and Zn between symptomatic and asymptomatic subgroups (Fig. 4 middle and lower plots).
Number of rotations and serum TB, Se, and Zn levels in symptomatic and asymptomatic subgroups of 6-OHDA-treated rats. Difference in number of rotations between these subgroups was strongly significant but difference in serum TB, Se, and Zn levels was not statistically significant. However, difference between both subgroups and sham group was significant. *: p < 0.05, **: p < 0.01, ***: p < 0.001 relative to control or sham group
Association Between Number of TH-Positive Neurons in SNc and TB, Se, and Zn Levels
For further assessment, we analyzed the association between number of TH-positive neurons in SNc of the lesion hemisphere with serum levels of TB, Se, and Zn. Figure 5 displays the rate of survival of these neurons in 6-OHDA-treated rats. In symptomatic subgroup (n = 6, Fig. 5 middle micrograph), number of TH-positive neurons was 83 ± 16% less than that in control group. A marked loss of DA neurons was also observed in the asymptomatic rats (n = 3) such that TH-positive neurons in them were 45 ± 10% less than that in control (Fig. 5 lower micrograph). However, statistical analysis revealed no correlation between number of TH-positive neurons and serum TB, Se, and Zn levels in 6-OHDA-treated rats.
Micrographs show the rate of survival of TH-positive neurons in SNc of the right hemisphere in sham (upper micrograph) group and also symptomatic (middle micrograph) and asymptomatic (lower micrograph) subgroups of 6-OHDA-treated rats. Diagram quantifies number of TH-positive neurons in the right SNc. ***: p < 0.001 relative to sham group. ###: p < 0.001 relative to symptomatic group. Note that the number of TH-positive neurons in asymptomatic subgroup was significantly lower than that in sham group but significantly higher than that in symptomatic subgroup. Scale bar: 100 µm
Discussion
In the present study, we investigated the association between 6-OHDA-induced PD in rat and the serum levels of TB, Se, and Zn. We found that TB level increases in 6-OHDA-treated rats and Se and Zn levels them were lower than that in control or sham group.
Several clinical and case–control studies have shown that serum TB level in patients with PD is higher than that in control subjects [19,20,21, 27]. However, some other clinical studies indicate that either TB level decreases [28, 29] or does not change in these patients [18]. This confliction might be due to confounding factors such as presence of HO polymorphisms which modifies TB level in patients with different genetic backgrounds [30]. Several comorbidities might also affect TB level especially because patients with PD have usually higher proportion of comorbidities [27]. Here we used an animal model to explore the association between SNc DA neuronal death, as the basic pathophysiological mechanism underlying PD, and TB level. Animal model studies provide opportunity to extrude all, or almost all of confounding factors disturbing clinical findings. Similar to PD in humans, the main pathophysiological mechanism underlying 6-OHDA-induced Parkinsonism is the loss of DA neurons in SNc. Moreover, this model reproduces the main cellular processes involved in DA neuronal loss in PD, such as oxidative stress, neurodegeneration, neuroinflammation, and neuronal death by apoptosis [22]. Furthermore, remarkable evidences have been shown that behavioral symptoms especially asymmetrical circling in 6-OHDA-treated rats are the behavioral outcome of unilateral DA degeneration in the SNc [2, 31, 32]. In line with this, our IHC data revealed that 6-OHDA-treated rats with severe behavioral symptoms had severe DA neuronal loss in SNc. Thus, we provide a basic evidence indicating that PD associates with increase in serum TB level. This evidence is contrast to proposals associating higher plasma TB level to L-dopa-induced increase in nigral oxidative stress [19]. Our data also challenge the hypothesis considering intestinal dysbiosis as the cause of TB increase in PD [33]. We also provided evidences that support the hypothesis explaining HO pathway in DA neurons of SNc is upregulated as an adaptive response to oxidative stress which in turn increased systemic TB level [10, 11, 20].
We followed up 6-OHDA-treated rats for 8 weeks after the toxin. Findings showed that TB as well as intensity of behavioral symptoms increased progressively. This is in contrast to clinical data indicating a negative or no correlation between TB level and disease duration [20, 21, 27]. It is important to note that the follow-up in these clinical studies was for several years and all of them have reported increase in TB in the first year of PD. Therefore, the mechanisms increase TB level in the first year of PD possibly also involve in progressive increase of TB level in 6-OHDA-treated rats. On the other hand, it has been shown that progressive increase in intensity of circling behavior in 6-OHDA-induced PD is not in direct correlation with DA cell loss and apparently other mechanisms involve too [32, 34, 35]. In line with this, it has been reported that rotational test differentiates just partial lesion from a near complete (> 90%) lesion and cannot discriminate lesion sizes of 50–80% [31]. This describes why symptomatic and asymptomatic subgroups in our study showed severe and mild circling behavior, respectively. Thus, progressive increase in TB level after the toxin has not been necessarily developed by progressive increase in DA neuronal loss in SNc. In addition, our data show that the intensity of asymmetrical circling in a single test and also DA neuronal loss in SNc did not correlate with TB level. Furthermore, TB level in asymptomatic subgroup (with 45 ± 10% DA cell loss) was insignificantly higher than that in symptomatic subgroup (with 83 ± 16% DA cell loss). These findings are consistent with clinical findings suggesting that TB level in mild and moderate PD disease severity is higher than that in severe cases of PD [20, 27]. Taken together, it seems that there is a marked limitation in capacity of bilirubin production so that it is saturated in early stages of DA neuronal death and thus, fails to prevent its progress. However, since TB level increased in mild to moderate neuronal death, our data suggest that serum TB level can be candidate as a potential biomarker for early diagnosis of PD in human.
The data analysis demonstrated that serum Se level in 6-OHDA-treated rats was significantly lower than that in control or sham group. One particular attribute of Se biology is that the brain has the highest priority to receive and retain this element even in conditions with Se deficiency. Evidences from animal models indicate that selenoproteins and glutathione peroxidase prevent PD development through Se transportation into the brain and reduced oxidative stress [36]. Thus, 6-OHDA-induced oxidative stress in SNc was possibly the cause of decrease in Se levels. We measured Se level 8 weeks after the toxin indicating that 6-OHDA-induced decrease in Se level is persistent. However, there was no correlation between Se level and intensity of both behavioral symptoms and DA neuronal loss in the 6-OHDA-treated rats. Accordingly, it might be as upregulation of HO pathway and bilirubin production, the system of Se transportation into the brain becomes saturated early in 6-OHDA-induced neurotoxicity and cannot further support DA neurons in the severe neurotoxicity. Furthermore, there was no significant difference in Se level between symptomatic and asymptomatic subgroups indicating that serum Se level cannot discriminate severe SNc DA neuronal loss from mild or moderate ones. However, our data show that serum Se level decreases in early stage of DA neuronal loss.
Similar to Se, zinc level in 6-OHDA-treated rats was significantly lower than that in control group. This finding is in agreement with several human studies indicating that serum Zn level in patients with PD was significantly lower than that in aged-match control subjects [16, 17]. Zn is the main cofactor of the antioxidant enzymes of SOD, catalase, and peroxidase; each of these enzymes plays an important role in the protection of neurons against oxidative stress [37, 38]. Also, it has been hypothesized that zinc competes with metal ions like Cu and Fe for binding to specific sites within proteins and other macromolecules which in turn leads to inhibition of the production of oxygen radicals or even their elimination [39]. Additionally, it is presumed that zinc binds to free sulfhydryl groups of proteins and protects them from oxidation [16]. Therefore, it is possible that zinc is transported to the brain and spent in SNc to protect DA neurons against 6-OHDA neurotoxicity. However, no correlation was found between serum Zn level and severity of DA cell death in 6-OHDA-treated rats. Moreover, there was no difference in Zn level between symptomatic and asymptomatic subgroups indicating that DA neuronal loss in early stages is along with a decrease in serum Zn level.
An alternative finding of this study was remarkable difference in TB levels between cardiac and tail samples in control and sham groups. Significant influence of bleeding site on the blood levels of glucose and lipid profile has been reported previously [40, 41]. Some authors also described influence of bleeding site on the serum bilirubin level. For example, Seibel et al. [42] reported that in Sprague–Dawley rats, TB level in sublingual vein is significantly higher than that in vena cava. Or, Uemura et al. [43] reported that TB level in cardiac samples was significantly higher than that collected from femoral vein in postmortem cases. Since liver has critical role in bilirubin metabolism, hepatic circulation might involve in this effect. Anesthesia might also involve. Chauhan and Pandey [44] reported a significant increase in TB after fentanyl-ketamine anesthesia in dogs. Nonetheless, we believe that this technical issue could not disturb our findings because no comparison of data from cardiac samples of one experimental group with tail samples of other groups was performed.
Our study had some limitations. First of all, the limited volume of blood can be collected from the tail of conscious rats. So, we could not devote tail samples for measuring of Se and Zn. Also, the values of direct and indirect bilirubin in rat’s sera were too small and consequently, our measurements were unable to provide valid data. Thus, we report just total bilirubin. Furthermore, we did not assess the activity of HO enzyme system which caused it was not possible to evaluate mechanistically changes in serum TB level. Finally, because of ethical issues, number of rats in each group was too low in comparison to number of cases in clinical studies. Low number of rat in each group might underlie the insignificance of the values in our statistical comparisons.
In conclusion, we provided evidences indicating that mild and severe death of DA neurons in SNc associate with increase in serum TB level. 6-OHDA-treated rats also showed lower levels of Se and Zn compared to control. Increase in severity of DA neuronal loss did not change serum levels of these parameters indicating that their bodily production systems cannot adapt, to an appropriate extent to counteract neurodegenerative mechanisms. On the other hand, significant changes in serum levels of these parameters in mild to moderate DA neuronal loss give us an insight that it is possible to establish a serum profile for early detection of SNc DA neuronal death and PD.
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Change history
29 May 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s12011-024-04247-9
References
Tsang AHK, Chung KKK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochem Biophys Acta 1792:643–650. https://doi.org/10.1016/j.bbadis.2008.12.006
Raza C, Anjum R, Shakeel NUA (2019) Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci 226:77–90. https://doi.org/10.1016/j.lfs.2019.03.057
Dorszewska J, Kowalska M, Prendecki M et al (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391. https://doi.org/10.4103/1673-5374.300980
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Sedlak TW, Saleh M, Higginson DS et al (2009) Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A 106(13):5171–5176. https://doi.org/10.1073/pnas.0813132106
Wu TW, Fung KP, Yang CC (1994) Unconjugated bilirubin inhibits the oxidation of human low density lipoprotein better than Trolox. Life Sci 54(25):477–481. https://doi.org/10.1016/0024-3205(94)90140-6
Soto Conti CP (2021) Bilirubin: the toxic mechanisms of an antioxidant molecule. J Atheroscler Thromb 26(8):688–696. https://doi.org/10.5551/jat.RV17035
Fujiwara R, Haag M, Schaeffeler E et al (2018) Systemic regulation of bilirubin homeostasis: potential benefits of hyperbilirubinemia. Hepatology 67(4):1609–1619. https://doi.org/10.1002/hep.29599
Schipper HM, Song W, Zukor H et al (2009) Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110(2):469–485. https://doi.org/10.1111/j.1471-4159.2009.06160.x
McCarty MF (2013) Serum bilirubin may serve as a marker for increased heme oxygenase activity and inducibility in tissues—a rationale for the versatile health protection associated with elevated plasma bilirubin. Med Hypotheses 81(4):607–610. https://doi.org/10.1016/j.mehy.2013.07.013
Jin J-N, Liu X, Li M-J et al (2020) Association between serum bilirubin concentration and Parkinson’s disease: a meta-analysis. Chin Med J 134(6):655–661. https://doi.org/10.1097/CM9.0000000000001300
Ellwanger JH, Franke SIR, Bordin DL et al (2016) Biological functions of selenium and its potential influence on Parkinson’s disease. An Acad Bras Cienc 88:1655–1674
Maassa F, Michalke B, Willkommen D et al (2010) Selenium speciation analysis in the cerebrospinal fluid of patients with Parkinson’s disease. Trace Elem Med Biol 57:110–115. https://doi.org/10.1016/j.jtemb.2019.126412
Castaño A, Ayala A, Rodrı́guez-Gómez JA, et al (1997) Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem Int 30(6):549–555. https://doi.org/10.1016/s0197-0186(96)00123-4
Sun H, Liu X, Ge H et al (2017) Association between serum zinc levels and the risk of Parkinson’s disease: a meta-analysis. Biol Trace Elem Res 179:45–51. https://doi.org/10.1007/s12011-017-0941-2
Barmaki H, Morovati A, Eydivandi Z et al (2021) The association between serum oxidative stress indexes and pathogenesis of Parkinson’s disease in the northwest of Iran. Iran J Public Health 50(3):606–615. https://doi.org/10.18502/ijph.v50i3.5621
Qin XL, Zhang QS, Sun L et al (2015) Lower serum bilirubin and uric acid concentrations in patients with Parkinson’s disease in China. Cell Biochem Biophys 72:49–56. https://doi.org/10.1007/s12013-014-0402-x
Scigliano G, Girotti F, Soliveri P et al (1997) Increased plasma bilirubin in Parkinson patients on L-dopa: evidence against the free radical hypothesis? Ital J Neurol Sci 18(2):69–72. https://doi.org/10.1007/BF01999565
Moccia M, Picillo M, Erro R et al (2015) Increased bilirubin levels in de novo Parkinson’s disease. Eur J Neurol 22(6):954–959. https://doi.org/10.1111/ene.12688
Macias-Garcia D, Mendez-Del Barrio C, Jesus S, Labrador MA et al (2019) Increased bilirubin levels in Parkinson’s disease. Parkinson Relat Disord 63:213–216. https://doi.org/10.1016/j.parkreldis.2019.01.012
Hernandez-Baltazar DH, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurologia 32(8):533–539. https://doi.org/10.1016/j.nrl.2015.06.011
Sarukhani MR, Haghdoost-Yazdi H, Khandan-Chelarci G (2018) Changes in the serum urate level can predict the development of Parkinsonism in the 6-hydroxydopamine animal model. Neurochem Res 43(5):1086–1095. https://doi.org/10.1007/s11064-018-2522-y
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Sarukhani M, Haghdoost-Yazdi H, Sarbazi Golezari A et al (2018) Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res 1–9. https://doi.org/10.1080/01616412.2017.1390903
Schallert T, Kozlowski DA, Humm JL et al (1997) Use-dependent structural events in recovery of function. Adv Neurol 73:229–38 (Review)
Songsomboon C, Tanprawate S, Soontornpun A et al (2020) Serum uric acid, serum uric acid to serum creatinine ratio and serum bilirubin in patients with Parkinson’s disease: a case-control study. J Clin Med Res 12:172–179. https://doi.org/10.14740/jocmr4079
Hatano T, Saiki S, Okuzumi A et al (2016) Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J Neurol Neurosurg Psychiatry 87:295–301. https://doi.org/10.1136/jnnp-2014-309676
Li J, Zhao L, Wang Z et al (2019) Association of serum indirect bilirubin concentrations with motor subtypes of Parkinson’s disease. Neurodegener Dis 19:155–161. https://doi.org/10.1159/000505852
Ayuso P, Martínez C, Pastor P et al (2014) An association study between heme oxygenase-1 genetic variants and Parkinson’s disease. Front Cell Neurosci 8:298. https://doi.org/10.3389/fncel.2014.00298
Iancu R, Mohapel P, Brundin P et al (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10. https://doi.org/10.1016/j.bbr.2005.02.023
Yuan H, Sarre S, Ebinger G et al (2005) Histological, behavioral and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144(1):35–45. https://doi.org/10.1016/j.jneumeth.2004.10.004
Hasuike Y, Endo T, Koroyasu M et al (2020) Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson’s disease. Med Hypotheses 134:109436. https://doi.org/10.1016/j.mehy.2019.109436
Minaei A, Haghdoost-Yazdi H (2019) Dexmedetomidine attenuates the induction and reverses the progress of 6-hydroxydopamine-induced parkinsonism; involvement of KATP channels, alpha 2 adrenoceptors and anti-inflammatory mechanisms. Toxicol Appl Pharmacol 382:114743. https://doi.org/10.1016/j.taap.2019.114743
Minaei A, Sarookhani MR, Haghdoost-Yazdi H et al (2021) Hydrogen sulfide attenuates induction and prevents progress of the 6-hydroxydopamine-induced Parkinsonism in rat through activation of ATP-sensitive potassium channels and suppression of ER stress. Toxicol Appl Pharmacol 423:115558. https://doi.org/10.1016/j.taap.2021.115558
Zhang X, Liu RP, Cheng WH et al (2019) Prioritized brain selenium retention and selenoprotein expression: nutritional insights into Parkinson’s disease. Mech Ageing Dev 180:89–96. https://doi.org/10.1016/j.mad.2019.04.004
Ooi TC, Mohammad NH, Sharif R (2014) Zinc carnosine protects against hydrogen peroxide-induced DNA damage in WIL2-NS lymphoblastoid cell line independent of poly (ADP-ribose) polymerase expression. Biol Trace Elem Res 162(1–3):8–17. https://doi.org/10.1007/s12011-014-0153-y29
Kara E, Gunay M, Cicioglu I et al (2010) Effect of zinc supplementation on antioxidant activity in young wrestlers. Biol Trace Elem Res 134(1):55–63. https://doi.org/10.1007/s12011-009-8457-z30
Eide DJ (2011) The oxidative stress of zinc deficiency. Metallomics 3(11):1124–1129. https://doi.org/10.1039/c1mt00064k
Wang Z, Yang Y, Xiang X et al (2010) Estimation of the normal range of blood glucose in rats. 39(2):133–7, 142
Chan YK, Davis PF, Poppitt SD et al (2012) Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab Anim 46(2):142–147. https://doi.org/10.1258/la.2011.011136
Seibel J, Bodié K, Weber S et al (2010) Comparison of haematology, coagulation and clinical chemistry parameters in blood samples from the sublingual vein and vena cava in Sprague-Dawley rats. Lab Anim 44(4):344–351. https://doi.org/10.1258/la.2010.009049
Uemura K, Shintani-Ishida K, Saka K et al (2008) Biochemical blood markers and sampling sites in forensic autopsy. J Forensic Leg Med 15(5):312–317. https://doi.org/10.1016/j.jflm.2007.12.003
Chauhan A (2006) Pandey SK (2006) Haemato-biochemical effects of epidural fentanyl-ketamine combinations in dogs. J Bombay Vet Coll 14:96–99
Acknowledgements
Authors thank Miss Minaei for her assistance in immunohistochemistry studies and also Mrs. Dargahi for her assistance in blood collections.
Funding
This study was supported by a grant-in-aid for scientific research from the Research Council of Qazvin University of Medical Sciences (grant number: IR.QUMS.REC.1397.083).
Author information
Authors and Affiliations
Contributions
Study was designed by Haghdoost-Yazdi H and Sophiabadi M. Blood sampling and measurements of the bilirubin, Se, and Zn were managed by Rastgoo N. All authors involved in modeling of the animals and immunohistochemical assessments. Data was analyzed by Rastgoo N and manuscript wrote by all authors.
Corresponding author
Ethics declarations
Ethics Approval
All procedures of the present study were carried out according to the guidelines of animal experiments of the Research Council at Qazvin University of Medical Sciences. All authors confirm all data presented in this manuscript and consent for its submission in Biological Trace Element Research.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12011-024-04247-9
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sophiabadi, M., Rastgoo, N. & Haghdoost-Yazdi, H. RETRACTED ARTICLE: Dopaminergic Neuronal Death in Substantia Nigra Associates with Serum Levels of Total Bilirubin, Selenium, and Zinc: Evidences from 6-Hydroxydopamine Animal Model of Parkinson’s Disease. Biol Trace Elem Res 200, 4058–4067 (2022). https://doi.org/10.1007/s12011-021-03012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03012-6