Abstract
The objective of the study is to investigate the correlation between bilirubin and uric acid (UA) concentrations and symptoms of Parkinson’s disease (PD) in Chinese population. A total of 425 PD patients and 460 controls were included in the current study. Patients were diagnosed by a neurologist and assessed using the Hoehn & Yahr (H&Y) scale. Venous blood samples were collected, and bilirubin and UA concentrations were analyzed. Compared to controls, indirect bilirubin (IBIL) and UA concentrations were lower in PD patients (P IBIL = 0.015, P UA = 0.000). Serum IBIL in different age subgroups and H&Y stage subgroups were also lower compared to the control group (P IBIL = 0.000, P UA = 0.000) but were not significantly different among these subgroups. Females in the control group had significantly lower serum IBIL and UA concentrations than males (P IBIL = 0.000, P UA = 0.000) and the PD group (P IBIL = 0.027, P UA = 0.000). In early PD (patients with <2-year medical history and no treatment), serum IBIL and UA concentrations were also lower than the controls (P IBIL = 0.013, P UA = 0.000). Although IBIL concentration was positively correlated with UA concentration in controls (R IBIL = 0.229, P IBIL = 0.004), this positive association was not observed in the PD group (R IBIL = −0.032, P IBIL = 0.724). Decreased levels of serum IBIL and UA were observed in PD patients. It is possible that individuals with decreased serum bilirubin and UA concentrations lack the endogenous defense system to prevent peroxynitrite and other free radicals from damaging and destroying dopaminergic cells in the substantia nigra. Our results provide a basis for further investigation into the role of bilirubin in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress in the substantia nigra leads to lipid peroxidation, protein oxidation, and DNA oxidation, which has been implicated in the onset of Parkinson’s disease (PD) [1]. Notably, PD patients have decreased antioxidant defense, thus rendering them susceptible to damage from reactive oxygen (ROS) and reactive nitrogen species (RNS) formed during cell metabolism and oxidative stress [2].
Bilirubin belongs to a tetrapyrrolic compound superfamily, one of the most highly conserved groups of molecules in nature. It was long considered a potentially toxic metabolite of heme catabolism, but it may also be able to scavenge ROS. Although an early report on the antioxidant effects of bilirubin was published in 1954 by Bernard et al., several decades passed before this effect got any attention [3]. Indeed, bilirubin is the only endogenous lipophilic antioxidant in the human body. It is more effective as an antioxidant protecting lipids from oxidation than hydrophilic antioxidants [4]. Bilirubin is almost three times more potent in preventing low-density lipoprotein (LDL) oxidation compared to the vitamin E analog Trolox [5]. More importantly, bilirubin is a major contributor to the total antioxidant capacity of plasma [6, 7].
Urate is the anionic form of uric acid (UA) and acts as a hydrophilic antioxidant in humans. This property is mainly attributed to its capacity to react with ROS and chelate transition metal ions [8]. Indeed, longitudinal studies have shown that individuals with higher levels of serum urate have a decreased risk of PD [9, 10].
Here, we describe a case–control study to investigate the correlation between serum bilirubin and UA concentrations and PD.
Methods
We conducted a case–control study to assess the relationship between serum bilirubin concentrations and the prevalence of PD. Consecutive PD patients were recruited between July 2007 and November 2013 at the Department of Neurology of Xuzhou Central Hospital, and (425 total, 282 men and 143 women) met the inclusion criteria. Controls (460 total, 298 men and 162 women) were recruited from the Medical Examination Center at the same period of time. Controls with heart disease, liver and gallbladder disease, kidney disease, or a history of surgeries were excluded. All PD patients met the published criteria of idiopathic PD (UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria). For further investigation, PD patients were divided into three subgroups according to sex (men and women subgroups), age (<65 years old, 65–75 years old, and >75 years old), and pathogenetic condition [Hoehn & Yahr (H&Y) stage]. All participants were local residents with similar diets and living habits and were not taking any other than anti-PD medication. All participants provided informed written consent prior to participating in the study.
Venous blood was collected in the morning after an overnight fasting. Serum total bilirubin (TBIL), direct bilirubin (DBIL), and indirect bilirubin (IBIL) concentrations (normal range: 3.4–20, 0–8, and 0–17 μmol/L respectively) and UA concentrations (normal range: 100–420 μmol/L) were measured using a Clinical Analyzer 7600-ISE (Hitachi High-Technologies, Tokyo, Japan). Concurrently, concentrations of alanine transaminase (ALT, normal range: 1–40 U/L), aspartate aminotransferase (AST, normal range: 1–40 U/L), γ-glutamyl transpeptidase (GGT, normal range: 5–60 U/L), creatinine (Cr, normal range: 15–120 U/L) were also evaluated using the same analyzer. Individuals with abnormal ALT, AST, GGT, or Cr concentrations were excluded from the present study. Color ultrasound was also conducted to exclude participants with liver or gallbladder or kidney disease. Those with cardiovascular, or other brain diseases were also excluded.
Information related to sex, age, smoking habits, and the use of dopaminergic agents or other drugs was obtained from all participants. The duration of PD at the time of testing was estimated from the time of PD diagnosis. Clinical history, interview, and laboratory data were all collected on standardized forms and manually entered into a password-protected database.
Statistical Analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS) statistical software (version 18.0, SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). Statistical significance was set at P < 0.05. The effect of age and sex on serum bilirubin and UA concentrations in different groups was analyzed using one-way analysis of variance.
Results
The clinical characteristics and laboratory findings of patients and controls are summarized in Table 1. Overall, there were 460 controls (298 men and 162 women) and 425 PD cases (282 men and 143 women), who were ultimately included in the study. The mean age of the PD cases and controls were 67.32 ± 9.15 (range: 48–87 years) and 67.84 ± 8.18 (range: 45–86 years), respectively, with sex ratios of 282/143 and 298/162 (men/women). The PD and control groups were not significantly different with respect to age and sex. The mean disease duration for all cases was 3.50 ± 3.23 years (3.54 ± 3.44 years for the 82 men, and 3.30 ± 2.95 years for the 43 women). The disease duration was not significantly different between the sexes. Cases were further divided into age subgroups: 165 cases were <65 years, 189 cases between 65 and 75 years, and 71 cases older than 75 years. Cases were also divided into H&Y stage subgroups: 203 cases were stages I–II, 150 cases were stage III, and 72 cases were at stages IV-V. A total of 82 patients (55 men and 27 women) were not treated and had disease durations of <2 years. The rest of the patients were only treated with levodopa and benserazide.
Compared to controls, significantly lower serum UA concentrations were observed in PD subjects (P = 0.000). Females in the control group had considerably lower serum UA concentrations than males (P = 0.000), and this trend was observed in the PD group as well (P = 0.000). Female PD patients had lower serum UA concentrations compared to control females (P = 0.000), and the same was observed for control and PD males (P = 0.000). The serum UA concentrations were lower in the different age and H&Y stage subgroups compared to controls (P = 0.000). But the differences among the subgroups were not significantly different. UA concentration was also significantly decreased in early PD (P = 0.000) (Table 1; Figs. 1–4).
Higher DBIL but a lower IBIL concentrations were found in PD cases compared to controls (P DBIL = 0.000, P IBIL = 0.015). No significant difference in TBIL concentrations was observed in PD cases and controls (P TBIL = 0.650). Females in the control group were found to have lower TBIL, DBIL, and IBIL concentrations than males (P TBIL = 0.000, P DBIL = 0.000, P IBIL = 0.000), and the similar differences were observed in the PD group (P TBIL = 0.007, P DBIL = 0.008, P IBIL = 0.027). Females in the PD group had higher DBIL concentrations than those in the control group. Males in the PD group also had higher DBIL concentrations compared to the control group. The mean IBIL concentration in the female PD group was not different from that of control group. However, the IBIL concentration in males with PD was lower than control males. Serum DBIL levels in the different age and H&Y stage subgroups were higher than the control group (P = 0.000), but the differences were not significant among subgroups. Serum IBIL in different age subgroups and H&Y stage subgroups were lower than control (P = 0.000), with no notable difference among subgroups. In early PD patients, IBIL concentration was lower than the control subjects (P = 0.013), while the DBIL concentration was higher (P = 0.012) (Table 1; Figs. 1–4).
Finally, we analyzed the correlation between serum UA and bilirubin concentrations. In the control group, TBIL, DBIL, and IBIL had positive relationships with UA (R TBIL = 0.224,P TBIL = 0.005; R DBIL = 0.163, P DBIL = 0.04; R IBIL = 0.229, P IBIL = 0.004), but these positive associations were not observed in the PD group (R TBIL = −0.029, P TBIL = 0.752; R DBIL = −0.023, P DBIL = 0.796; R IBIL = −0.032, P IBIL = 0.724) (Table 2 and Figs. 5–10).
Correlation between serum UA and TBIL, DBIL, and IBIL in the control and PD groups. Serum TBIL, DBIL, and IBIL concentrations were positively correlated with UA concentration in the control group (TBIL R = 0.224, P = 0.005; DBIL R = 0.163, P = 0.04; IBIL R = 0.229, P = 0.004), but there were no significant associations in the PD group (TBIL R = −0.029, P = 0.752; DBIL R = −0.023, P = 0.796; IBIL R = −0.032, P = 0.724)
Discussion
The major finding of this study in Chinese PD patients and healthy controls was that the serum UA and IBIL concentrations were lower in PD patients than controls, while the mean serum DBIL concentration was significantly higher in PD patients. Moreover, these trends were also observed when males and females were assessed separately and when PD patients were further divided into age and H&Y subgroups. In addition, serum TBIL, DBIL, and IBIL concentrations showed a positive correlation with the serum UA concentration in the control group, while no such correlation was seen in PD group. To our knowledge, this is the first study reporting an assessment of bilirubin concentrations in patients with PD.
Bilirubin has long been considered as simply the metabolic end product of heme catabolism; however, it is increasingly recognized as a potent antioxidant under physiological conditions and has recently been reported to play an important role in protecting from oxidative stress. The first report on the antioxidant effects of bilirubin was published as early as 1954, but several decades later the antioxidant properties of the bilirubin have attracted major scientific attention. The pioneering study of Stocker et al. in 1987 introduced the concept that unconjugated bilirubin (UCB) at low “physiological” plasma concentrations has a beneficial role by acting as a potent antioxidant that scavenges peroxyl radicals as efficiently as α-tocopherol [3]. Bilirubin may, in fact, be the most abundant endogenous antioxidant in mammalian tissues [3, 11]. UCB has been demonstrated to protect neurons [12] and hippocampus [13] from the oxidative stress damage by acting as an antioxidative neuroprotective. The highly lipophilic bilirubin molecule might interact with cell membranes to protect against lipid peroxidation [14, 15]. Bilirubin also appears to explain the neuroprotection offered by heme oxygenase (HO), as the detrimental effect of HO deletion is reversed by restoring even low concentrations of bilirubin [16]. In an experimental model of acute ischemic stroke, overexpression of HO attenuates injury, and HO knockout substantially worsens neuronal damage. Bilirubin may limit stroke-induced neurologic injury, a hypothesis that is supported by the observation of increased neuroprotection of biliverdin reductase in experimental stroke [17]. Notably, as expected the cytoprotection offered by bilirubin greatly exceeds that of glutathione (GSH) based on its intracellular 10,000-fold excess of oxidants [4]. Indeed, bilirubin has been shown to be more effective for protection of lipids from oxidation than the water-soluble antioxidants, such as GSH, which primarily protects proteins from oxidation [10]. However, bilirubin has also been demonstrated to be about 30 times more potent toward the prevention of LDL oxidation compared with the lipid-soluble vitamin E analog, Trolox [5].
Multiple substances are thought to act as antioxidants in the human body, including bilirubin, UA, GSH, ascorbate, vitamins A and E, ergothioneine, and possibly melatonin. Some are endogenous, such as bilirubin, melatonin, GSH, and UA, while others are exogenous, such as vitamins A and E, ascorbate, and ergothioneine. Some of these are largely lipophilic, such as bilirubin and vitamins A and E, whereas GSH, ascorbate, UA, and ergothioneine are more hydropholic. Bilirubin is the only endogenous, lipophilic antioxidant in human body [4].
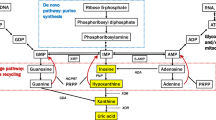
Uric acid is the end product of adenosine, guanosine, and purine metabolism and is thought to be a potent antioxidant that effectively scavenges ROS and RNS, and its potential protective effects in PD have been reported previously. Epidemiological and clinical studies reported that individuals with lower serum urate levels have a markedly higher risk of developing PD [18, 19]; conversely, those with higher serum urate levels have a lower risk of developing PD [20, 21]. In addition, UA levels in patients with PD are lower than those measured in healthy controls [22]. Experimental studies have demonstrated that the UA has protective effects on neural cells, including dopaminergic neurons [8, 23, 24]. UA levels are related with disease onset and development, which suggests that it could serve as a novel PD biomarker to aid diagnosis and prognosis [25]. In our study, the mean serum UA concentration was dramatically lower in patients with PD, which is in accordance with previously published results. However, we found that female patients had even lower UA concentrations compared to male patients, and that the lower value was not associated with age or disease stage. We also collected data from patients who were recently diagnosed with PD and with less than 2 years disease duration and were not taking any PD medications, seemed to have the same results, implying that UA might decrease prior to or coincident with disease onset.
Although the exact causes of selective dopaminergic cell death underlying PD remain unknown, oxidative stress is regarded as a potential mechanism in disease pathogenesis [26], and evidence of oxidative damage, such as DNA and protein damage and lipid peroxidation, have been observed in post mortem PD brains [27, 28]. Brain tissue is rich in polyunsaturated fats, which are one of the substrates for lipid peroxidation, and these are especially sensitive to ROS [29]. Autopsy studies have demonstrated an increased lipid peroxidation and higher levels of free radicals in substantia nigra neurons of PD patients [29]. Lipid peroxidation seems to have an important role in the pathological processes of PD; consequently preventing peroxidation might be a useful therapeutic strategy to combat PD.
Since the oxidative stress in PD is important, the concept of discrete lipophilic and hydrophilic domains of protection has clinical implications. The major antioxidative defense system is the GSH redox cycling system, which is comprised of GSH and its two redox enzymes, glutathione peroxidase and glutathione reductase. This system is primarily effective for hydrophilic ROS because of the hydrophilic nature of GSH. Vitamin E was previously thought to neutralize lipophilic ROS; however, extensive studies have yielded disappointing results. In contrast, the biliverdin and bilirubin pathway is expected to be effective against lipophilic ROS, because of the lipophilic nature of bilirubin. Therefore, this biliverdin to bilirubin pathway system is presumed to be complementary to the GSH redox cycling system for scavenging both hydrophilic and lipophilic ROS.
TBIL consists of direct (conjugated) and indirect (unconjugated) bilirubin. UCB is derived from the reduction of biliverdin, a product of heme degradation by biliverdin reductase [30]. UCB is strongly bound to serum albumin, which transports it to the liver. There, bilirubin is conjugated with one or two molecules of glucuronic acid, a reaction catalyzed by bilirubin UDP–glucuronosyltransferase in hepatocytes [31]. In comparison to unconjugated bilirubin, conjugated bilirubin is soluble in serum and only weakly binds to albumin, making it more easily available in its active form compared to IBIL [32]. Most of the previous studies have reported that UCB exerts anti-ROS properties and therefore had protective effects; whereas conjugated bilirubin was rarely mentioned.
In our study, serum IBIL concentration was found to exhibit an inverse association with PD incident. While serum DBIL concentration was positively associated with PD, the relationship is not fully elucidated and requires further investigation. The same result was found in early stage PD patients, implying that IBIL might decrease before or coincident with disease onset rather than in response to disease. Lower IBIL levels were not related with patients’ age or disease stage, nor were UA concentrations. To our knowledge, there is limited data showing a differentially protective effect of IBIL in PD; most previous studies assessing bilirubin levels in certain diseases (e.g., metabolic syndrome, diabetes, etc.) only measured TBIL without separation of various types of bilirubin. As far as the effect of lipid peroxidation in PD pathogenesis is concerned, lower serum IBIL levels influence PD development by reducing endogenous anti-lipid peroxidation resistance still requires further investigation.
Bilirubin and UA are endogenous antioxidants with neural protective effect. It has been confirmed that UA plays a protective role against PD. The fact that both IBIL and UA concentrations were decreased in PD patients indicates that IBIL could also protect against PD. Our results demonstrated a correlation between bilirubin and UA levels. Levels of IBIL and UA were positively correlated in the control group but not the PD group.
PD pathogenesis is influenced by multiple factors, and bilirubin and UA might affect different mechanisms or pathways. The relationship between bilirubin concentration and PD warrants further research with larger patient sample size.
Limitations
Our study has some limitations. Our results must be interpreted cautiously as the study population, although well defined, was relatively small. Moreover, reduced serum UA and bilirubin concentrations are not specific for PD; as they have been linked to a variety of diseases, including metabolic syndrome, diabetes, cardiovascular disease, and cancer [33–35]. An important factor to consider is that patients with PD are usually older and often have one or more comorbid conditions.
Conclusions
In conclusion, our findings indicate decreased levels of serum bilirubin and UA in PD patients. Although there is some uncertainty as to whether these changes are cause or consequence of PD. But it is possible that persons with decreased serum bilirubin and UA concentrations lack endogenous defense system to prevent peroxynitrite and other free radicals from damaging and destroying dopaminergic cells in the substantia nigra. Our results provide a basis for further investigation into the role of bilirubin in PD and other neurodegenerative conditions.
References
Jenner, P. (2003). Oxidative stress in Parkinson’s disease [J]. Annals of Neurology, 53(suppl 3), S26–S36.
Ames, B. N., Cathcart, R., Schwiers, E., et al. (1981). Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 78(11), 6858–6862.
Stocker, R., Yamamoto, Y., McDonagh, A. F., et al. (1987). Bilirubin is an antioxidant of possible physiological importance [J]. Science, 235(4792), 1043–1046.
Sedlak, T. W., Saleh, M., Higginson, D. S., et al. (2009). Bilirubin and glutathione have complementary antioxidant and cytoprotective roles [J]. Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5171–5176.
Wu, T. W., Fung, K. P., & Yang, C. C. (1994). Unconjugated bilirubin inhibits the oxidation of human low density lipoprotein better than Trolox. Life Sciences, 54(25), 477–481.
Frei, B., Stocker, R., & Ames, B. N. (1988). Antioxidant defenses and lipid peroxidation in human blood plasma. Proceedings of the National Academy of Sciences of the United States of America, 85(24), 9748–9752.
McGeary, R. P., Szyczew, A. J., & Toth, I. (2003). Biological properties and therapeutic potential of bilirubin[J]. Mini-Reviews in Medicinal Chemistry, 3(3), 253–256.
Gong, L., Zhang, Q. L., Zhang, N., et al. (2012). Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: Linking to Akt/GSK3βsignaling pathway [J]. Journal of Neurochemistry, 123(5), 876–885.
Logroscino, G., Gao, X., Chen, H., et al. (2008). Dietary Iron intake and risk of Parkinson’s disease [J]. American Journal of Epidemiology, 168(12), 1381–1388.
Weisskopf, M. G., O’Reily, E., Chen, H., et al. (2007). Plasma urate and risk of Parkinson’S disease [J]. American Journal of Epidemiology, 166(5), 561–567.
Gopinathan, V., Miller, N. J., Milner, A. D., & Rice-Evans, C. A. (1994). Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Letters, 349, 197–200.
Dore, S., Takahashi, M., Ferris, C. D., et al. (1999). Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury [J]. Proceedings of the National Academy of Sciences of the United States of America, 96(5), 2445–2450.
Dore, S., & Snyde, S. H. (1999). Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures [J]. Annals of the New York Academy of Sciences, 890, 167–172.
Tomaro, M. L., & Batlle, A. M. (2002). Bilirubin: Its role in cytoprotection against oxidative stress[J]. International Journal of Biochemistry & Cell Biology, 34(3), 216–220.
Granato, A., Gores, G., Vilei, M. T., et al. (2003). Bilirubin inhibits bile acid induced apoptosis in rat hepatocytes[J]. Gut, 52(12), 1774–1778.
Dore, S., Sampei, K., Goto, S., et al. (1999). Heme oxygenase-2 is neuroprotective in cerebral ischemia. Molecular Medicine, 5, 656–663.
Panahian, N., Huang, T., & Maines, M. D. (1999). Enhanced neuronal expression of the oxidoreductase-biliverdin reductase-after permanent focal cerebral ischemia. Brain Research, 850, 1–13.
De Vera, M., Rahman, M. M., RamKin, J., et al. (2008). Gout and the risk of Parkinson’s Disease: A cohort study[J]. Arthritis and Rheumatism, 59(11), 1549–1554.
Annanmaki, T., Muuronen, A., & Murros, K. (2007). Low plasma uric acid level in Parkinson’s disease [J]. Movement Disorders, 22(8), 1133–1137.
Gao, X., Chen, H., Choi, H. K., et al. (2008). Diet, urate, and Parkinson’s disease risk in men. American Journal of Epidemiology, 167(7), 831–838.
De Lau, L. M., Koudstaal, P. J., Hofman, A., et al. (2005). Serum uric acid levels and the risk of Parkinson’s disease. Annals of Neurology, 58(5), 797–800.
Cipriani, S., Chen, X., & Schwarzschild, M. A. (2010). Urate: A novel biomarker of Parkinson’s disease risk, diagnosis and prognosis[J]. Biomarkers in Medicine, 4(5), 701–712.
Cipriani, S., Desjardins, C. A., Burdett, T. C., et al. (2012). Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease [J]. PLoS One, 7(5), e37331.
Haberman, F., Tang, S. C., Arumugam, T. V., et al. (2007). Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice [J]. Neuromolecular Medicine, 9(4), 315–323.
Schwarzschild, M. A., Schwid, S. R., Marek, K., et al. (2008). Serum urate as a predictor of clinical and radiographic progression in Parkinson’s disease J]. Archives of Neurology, 65(6), 716–723.
Schlesinger, I., & Schlesinger, N. (2008). Uric acid in Parkinson’s disease. Movement Disorders, 23, 1653–1657.
Jenner, P. (2007). Oxidative stress and Parkinson’s disease. Handbook of Clinical Neurology, 83, 507–520.
Lotharius, J., Brundin, P. (2002). Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nature Reviews Neuroscience, 3(12):932–42.
Testa, C. M., Sherer, T. B., & Greenamyre, J. T. (2005). Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures[J]. Brain Research. Molecular Brain Research, 134(1), 109–118.
Tenhunen, R., Marver, H. S., & Schmid, R. (1968). The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proceedings of the National Academy of Sciences of the United States of America, 61, 748–755.
Schachter, D. (1957). Nature of the glucuronide in direct-reacting bilirubin. Science, 126, 507–508.
Nakagami, T., Toyomura, K., Kinoshita, T., et al. (1993). A beneficial role of bile pigments as an endougenous tissue protector: Anti-complement effects of biliverdin and conjugated bilirubin. Biophysica Acta, 1158, 189–193.
Hunt, S. C., Kronenberg, F., Eckfeldt, J. H., et al. (2001). Association of plasma bilirubin with coronary heart disease and segregation of bilirubin as a major gene trait: The NHLBI family heart study [J]. Atherosclerosis, 154(3), 747–754.
Djousse, L., Levy, D., Cupples, L. A., et al. (2001). Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study[J]. American Journal of Cardiology, 87(10), 1196–1200.
Gourley, G. R. (1997). Bilirubin metabolism and kernicterus [J]. Advances in Pediatrics, 44, 173–229.
Conflict of interest
Authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, Xl., Zhang, Qs., Sun, L. et al. Lower Serum Bilirubin and Uric Acid Concentrations in Patients with Parkinson’s Disease in China. Cell Biochem Biophys 72, 49–56 (2015). https://doi.org/10.1007/s12013-014-0402-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0402-x