Abstract
The release of heavy metals to the environment increased dramatically with industrialization and rapid economic development, and they have accumulated in aquatic organisms. The current study aimed toe valuate the physiological, immunological, and histological changes of crayfish (Procambarus clarkii) as bio-indicator for water quality. Crayfishes of the filed study group were collected from a polluted area (Rosetta branch, Egypt), where the highest concentration for heavy metals in water was for zinc (Zn). Besides the field study group, other crayfishes were exposed to different doses of ZnSO4 (0, 203, and 406 mg L−g) corresponding to Zn concentration (0, 46.03, and 92.06 mg L−1) respectively in aquariums for consecutive 4 days. Heavy metal concentrations in field water sample were arranged as follows: Zn > Fe> Pb > Cu and Mn > Ni > Co > Cd. The result revealed that Zn bioaccumulation increases significantly with the increase of water Zn concentration among the tested groups compared to the control group, where the highest bioaccumulation in all studied tissues (hepatopancreas, gills, and muscles) was observed in the field group and Zn high-dose group. Also, there was a significant increase in the levels of hemolymph uric acid, urea, creatinine, glucose, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase. Their highest concentrations were observed in the Zn high-dose group and the field group, while the levels of total protein, albumin, and cholesterol showed a significant decrease among the tested groups as compared with the control group. Their determined lowest concentrations were in the Zn high-dose group and field group. Among tested groups, total hemocytes and granulated hemocytes decreased significantly while hyaline hemocytes increased as compared with the control group. Histological damages were observed in hepatopancreas, gills, and muscles in the field and Zn groups. The present study showed that exposure to Zn caused physiological and histological changes in Procambarus clarkia. We assumed that Procambarus clarkia could be used as a sensitive bioindicator for monitoring water quality criteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The arrival of weighty metals to the environment expanded significantly with industrialization and quick financial turn, and they have gathered in marine and freshwater organisms [1]. The principal destructive impact of heavy metals includes inhibition of enzymes, cancer, and birth defects [2]. During the previous years, the food industry has tended to a great extent towards the use of marine and river sources due to its high nutritional value [3]. Therefore, it is necessary to study the concentration of heavy metals that show potential toxic effects in aquatic animals [4]. The danger of heavy metals is their inability to be biodegradable and their ability to accumulate in the soil, where they are transferred to the organisms that live in the water through either respiration or nutrition, and then, they reach the higher-level organisms, including those of humans [5]. One of the most important heavy metals is zinc (Zn), which is a stimulant of activation of mineral enzymes in the body, such as carbonic anhydrase, but this element represents a danger to the organism at high concentrations [6]. Zinc is one of the most widely known pollutants in the aquatic environment [7, 8]. Organisms can counteract zinc toxicity by distributing it in an orderly manner over specific tissues. This strategy is one of the most important detoxification methods used by crayfish [9]. Exposure of living organisms such as crayfish to zinc toxicity stimulates abnormal physiological reactions within the body [10]. Therefore, the study of physiological, immunological, and histological responses resulting from exposure to heavy metals is receiving increased attention due to the possibility of using them as vital indicators of the presence of pollution [11,12,13].
Crayfish animals are found in a prime location within the food web, as they are one of the most important methods of transporting toxic metals to higher predators, including humans, through tropical transport [6]. Many researchers have used them as good biomarkers for detecting mineral pollution in the environment [14]. The crayfishes have been widely chosen due to its ability to withstand environmental stress better than other organisms. Also, its life spans are long (2–5 years), and it can stay in physical contact with water and contaminated soil. It is huge to obtain samples from various tissue organs, making it a suitable study sample for environmental monitoring studies [15,16,17]. The crayfish, Procambarus clarkii, is a freshwater crustacean found naturally and extensively in some lakes, pools, and rivers in Egypt [2]. Therefore, the current study was aimed at assessing the effect of Zn toxicity on the aquatic environment by using crayfish, Procambarus clarkii, as a fundamental bioindicator in the freshwater system.
Materials and Methods
Experimental Animal
Sixty healthy crayfishes were collected from the Damietta branch in El-Qanater El-Khyria (31° 8′ E and latitude 30° 11′ N, Fig. 1), Egypt. The weight of animals was 15–25 g, in the intermolt stage, and physically intact, possessing a complete set of legs including chelae. The animals adapted to the laboratory conditions for 14 days before the experiment which continued for 4 days starting at temperature 19–22 °C, pH 7.3 ± 0.4, with dissolved oxygen 8.1 ± 0.7 mg L−1. The studied crayfishes were classified into groups in glass aquaria (25 L) (40 × 60 × 25 cm) with a flat gravel bottom and constantly aerated fresh dechlorinated tap water, under 12:12 light-dark cycle. Water was changed every day, and the crayfishes were fed 40 min before each water change with commercial shrimp feed: 45–55% protein, 10–11% lipid, 20–30% carbohydrate, and 1.5–2% fiber, which meets the crayfish’s nutritional needs [19]. Another 20 adult crayfishes were collected from the Rosetta branch in Cairo, as a polluted area to make field study.
Map of the studied sites [18]
Experimental protocols and procedures used in this study were approved by the Cairo University Institutional Animal Care and Use Committee (CU-IACUC) (Egypt), (approval no. CU/I/F/86/18) in accordance with the international guidelines for care and use of laboratory animals.
Water Heavy Metal Determination
Surface water samples of the Rosetta branch as a polluted area for field study and El-Qanater El-Khyria as reference area were collected using a plastic container. Water sampling was achieved according to standard methods for examination of water and wastewater [20]. Water samples (n = 4) from each site (reference and field site) were collected in various containers that were preserved for metal determining via adding concentrated nitric acid to reduce the pH below 2 to prevent any microbial reactions. Five milliliters of concentrated HCl was added to 250 ml of water sample and evaporated to 25 ml. The concentrated HCl was transferred to a 50 ml flask and diluted to mark with distilled water. Metal contents were determined by using a 304 u/c atomic absorption spectrometer.
Experimental Design
The 20 adult crayfishes were collected from the Rosetta branch in Cairo, as a polluted area for making field study; once they are transferred to the university lab, our studies were done directly, while the 60 healthy crayfishes were distributed into 3 aquariums with two replications, and chemically pure salt of zinc sulfate (ZnSO4.7H2O) dissolved in distilled water was used as toxicant. The test crayfishes were subjected to different Zn concentrations. The control group (N = 10) crayfishes were only maintained in de-chlorinated water, and Zn low-dose (N = 10) crayfishes were subjected to 1/4 of 96 h of Zn Lc50 [21]. Zn high-dose (N = 10) crayfishes were exposed to 1/2 of 96 h of Zn Lc50.
Hemolymph Sampling
Hemolymph was collected aseptically from the base of one of the second walking legs using a 3-ml syringe fitted with a 23-gauge needle under ice anesthesia to determine (i) total hemocyte count (THC) and (ii) differential hemocyte count (DHC) (Fig. 2). To prevent coagulation, the syringe was filled with an equal volume of the anticoagulant (510 mM NaCl; 0.1 M glucose; 30 mM trisodium citrate; 20 mM citric acid; 10 mM EDTA, pH 4.6). Then, fresh hemolymph was centrifuged immediately at 2000 rpm for 15 min at 4 °C and the supernatant was used in the biochemical analyses. Supernatant was collected by aspiration and stored at 4 °C until use.
Total Hemocyte Count
The total hemocyte count (THC; number of hemocytes per mm3) was determined using a Neubauer hemocytometer chamber. Hemocytes were classified according to [22] using the presence or absence of cytoplasmic granules as simple criteria.
Differential Hemocyte Count
To perform the differential hemocyte count (DHC;%), a small drop of hemolymph was smeared on a slide, fixed in absolute methanol for 6 min, stained with diluted May–Grünwald–Giemsa (3 min in 10-fold diluted May–Grünwald and then 10 min in 10-fold diluted Giemsa), dehydrated with absolute ethanol (1 min) and xylene (6 min), and then mounted in Permount mounting medium (Bioscience, San Diego, CA, USA). Cells were counted in random areas on each slide, and the relative proportions of various classes were computed [23]. A total of 200 cells were counted on each slide. DHCs were calculated using the following equation:
Biochemical Parameters
The aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assessed by the technique in [24], alkaline phosphatase (ALP) [25], total protein [26], albumin [27], cholesterol [28], glucose [29], creatinine [30], urea [31], and uric acid [32] according to the manufacturer’s instructions using bio-diagnostic kits (Giza, Egypt).
Determination of Zn Tissue Concentration
Approximately 2.0 g each of the samples (hepatopancreas, gills, and muscles) was weighed and dried in the oven at 100 °C for 12 h then ashed in the muffle at 650 °C for 12 h. The ash was dissolved in 2 ml of concentrated HCl and completed to 25 ml volume. Concentrations of zinc was determined by flam atomic absorption spectrophotometry, model PerkinElmer-2280, according to APHA [20]. The detection limits of the instrument in parts per million were <0.005 for Zn. The measuring accuracy was checked by standard reference material (Lake Superior Fish 1946 NIST, National Institute of Standards and Technology, USA), and the recovery ranges were between 90 and 110%.
Histopathological Investigations
Tissue specimens from the hepatopancreas, gills, and muscles were fixed in 10% buffered neutral formalin, dehydrated, embedded, and sectioned at 4 μm, then stained with hematoxylin-eosin (HE), and observed under the light microscope.
Statistical Analysis
SPSS (version18.0) was used for the statistical analysis. All values were expressed as means ± SE. The obtained data were subjected to one-way ANOVA test using with the Duncan post hoc test to compare between groups. The significance of the means was tested at P < 0.05.
Results
Metal Concentration (ppm) in Water
Table 1 details the metal concentrations in water samples in the Rosetta branch as a polluted area for making field study and El-Qanater El-Khyria as a reference area. Heavy metal concentrations in water samples of the Rosetta branch were arranged as follows: Zn > Fe > Pb > Cu and Mn > Ni > Co > Cd.
Accumulation of Zn in Procambarus clarkia Tissues
Table 2 shows a significant increase (P < 0.05) in Zn concentration in hepatopancreas, gills, and muscles among the tested groups compared with the control group, where the highest hepatopancreas and muscle concentrations of Zn were observed in the Zn high-dose group, then the field group, and then the Zn low-dose group, while in the gills, the highest concentration was observed in the field group, followed by the Zn high-dose group, then the Zn low-dose group.
Biochemical Parameters
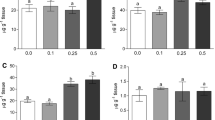
According to the data represented in Table 3, the levels of uric acid, urea, creatinine, glucose, ALT, AST, and ALP increased significantly (P < 0.05) among the studied groups as compared with the control group. Their highest concentrations were observed in the Zn high-dose group and the field group, while levels of total protein, albumin, and cholesterol showed a significant decrease (P < 0.05) among the studied groups as compared with the control group. Their determined lowest concentrations were in the Zn high-dose group and field group.
Hemocyte Count
Table 4 demonstrates that total hemocytes and granulated hemocytes decreased significantly (P < 0.05) while hyaline hemocytes increased in the tested groups as compared with the control group. These changes were clearly observed in the field and the Zn high-dose group.
Histopathological Examination of Hepatopancreas
The hepatopancreas sections of crayfish in the control group showed a normal structure with compactly arranged epithelial cells (Fig. 3 (1)). While hepatopancreas sections of the tested groups (Fig. 3 (2, 3, 4)) showed lumen dilation, eosinophilic deposition, vacuolization, degenerated tubule, and hyperplasia. The highest rate of tissue degeneration was noticed in the Zn high-dose group, then the field group, and then the Zn low-dose group.
Photomicrograph of hematoxylin and eosin–stained hepatopancreas sections from the (1) control group, (2) Zn low-dose group, (3) Zn high-dose group, and (4) field group (H&E × 400). E epithelial cell, L lumen, LD lumen dilation, ED eosinophilic deposition, V vacuolization, DT degenerated tubule, H hyperplasia
Histopathological Examination of Gills
Histological sections of the gills in the control group showed well-structured gill lamella (Fig. 4-1). While gill sections of tested groups showed hyperemia, necrotic epithelium, and vacuolization (Fig. 4 (2, 3, 4)). The histological degeneration of the gills was more pronounced in the Zn high-dose group, then the field group, and then the Zn low-dose group.
Histopathological Examination of Muscles
The control group showed a normal muscle structure with well-ordered myofibers and oval nuclei (Fig. 5 (1)), while the muscle sections of tested groups showed a fracture and lysis myofibers (Fig. 5 (2, 3, 4)). Histological degenerations were observed clearly in the Zn high-dose group, then the field group, and then the Zn low-dose group.
Discussion
The Rosetta branch suffers from several environmental problems. It receives pollutants from three main sources; the first source is El-Rahawy drain which receives domestic and agriculture wastes from Giza city and pours more than 1,900,000 m3 day-1 of its effluents into the Rosetta branch. The second source results from Kafr El-Zayat industrial area, and the third source of pollution is several small agricultural drains that discharge their wastes into the branch in addition to sewage discharged from several cities [33, 34], while the reference site selection (El-Qanater El-Khyria) was based on standardized values of several water parameters (pH, electrical conductivity, dissolved oxygen, BOD, ammonia, nitrite, and nitrate content) in addition to the concentrations of six metals (Cu, Zn, Fe, Mn, Pb, and Cd) in water, sediment, and fish tissues [18, 33].
The current study reported that there was a significant increase of Zn bioaccumulation in the studied tissues (hepatopancreas, gills, and muscles) with the increase of water Zn concentration among the tested groups compared with the control group, where the highest bioaccumulation of Zn was observed in the Zn high-dose group and the field group. In addition, the average concentrations of the toxic Zn in the three studied tissues indicate that Zn accumulates mainly in the hepatopancreas, gills, and muscle tissues with the lowest accumulations. Many researchers have reported the same accumulation trend for other heavy metals [35,36,37]. Differences in the concentration of zinc recorded between the body tissues of crustaceans are conditioned by physiological nature of each organ [4], where the hepatopancreas has an important role in the absorption and disposal of zinc. One of the most important strategies for dealing with zinc toxicity is either by limiting its absorption, storing it in an inactive form, or by actively excreting it from the body [16, 38, 39]. As for the accumulation of zinc in the gills, this is a result of direct contact with water, which makes it more vulnerable to water pollutants [40, 41].
Crustaceans contain a fluid called hemolymph, which is similar to the blood in mammals. This tissue expresses the physiological changes inside the body, so it is of great value for the detection of environmental toxin exposure [42]. Uric acid, urea, and creatinine concentration in the present study increased significantly with the increase of Zn concentration among the tested groups as compared with the control group. Their highest concentration was observed in the Zn high-dose and the field groups. The apparent increase in the concentration of uric acid is due to the effect of zinc that causes pathological changes in the green glands [43], while the high level of urea is mainly associated with a defect in the gills as a result of the accumulation of zinc, which leads to a loss of its ability to regulate the spread of urea between water and hemolymph, while kidney damage leads to an increase in creatinine level.
One of the most important sensitive indicators of environmental stress is a change in glucose level [44], where heavy metal toxicity leads to an increased level of glucose [12, 45, 46]. The crustacean hyperglycemic hormone (CHH) controls glucose regulation in crustaceans [47, 48]. Increased synthesis and secretion of this hormone have been observed in crustaceans in response to environmental pollutants, which leads to glycogen breakdown and increases glucose production [49].
In the present study, AST, ALT, and ALP increased significantly with the increase of Zn concentration among the tested groups compared with the control group, where the highest concentration was recorded in the Zn high-dose group and the field group. Their elevation represents damage to the hepatopancreas tissue, considering that these enzymes attach to the hemolymph after cellular lysis occurs [44]. Total proteins are important biochemical components that play an essential role in metabolic pathways and biochemical reactions. Therefore, an assessment of the total protein contents could be used as a diagnostic tool for determining the physiological status of an organism [50, 51].
Total protein currently showed a significant decrease with the increase of Zn concentration among the tested groups, where the lowest concentration was recorded in the field group, then the Zn high-dose group, and then the Zn low-dose group. These results were in agreement with previous reports that examined the effects of heavy metal exposure on invertebrates [52]. Abd El-Atti and Saied [53] attributed the depletion of the total protein levels in hemolymph and the tissues of hepatopancreas and muscle of test crayfish due to the enhanced proteolytic activity in these organs. Zn plays in protein metabolism where many proteases, such as carboxypeptidases A and B, contain Zn as an essential structural element [54, 55].
Cholesterol concentration in the actual study recorded a significant decrease with the increase of Zn concentration among the tested groups. The low level of cholesterol is attributed to the poor ability to absorb it from food [56] or increase the activity of the enzyme lipase responsible for breaking down cholesterol [53]. Zn involved in the production of metalloenzymes and plays an important role in the metabolism of lipids [57] where it acts as a cofactor for lipase enzymes [58].
Due to the lack of adaptive immunity, Procambarus clarkii defends itself against pathogens through an innate immune system [59]. Total hemocyte count (THC) and differential hemocyte count (DHC) are considered immunotoxicity biomarkers for environmental pollution [60, 61]. The present study demonstrated a significant decrease in THC with the increase of Zn concentration in the tested groups compared with the control group. Environmental pollution affects the hematopoietic tissues of the crustacean, as it leads to a decrease in the hemocytes [62]. The hemocytes in crustaceans are classified into 3 types, each of which has an immune role, and they are hyalinocytes (no granules in the cytoplasm), semi-granulocytes (a few granules in the cytoplasm), and granulocytes (plenty of granules in the cytoplasm) [63, 64]. In crustaceans, hyalinocytes, semi-granulocytes, and granulocytes are responsible for phagocytosis, encapsulation, and release of the prophenoloxidase system respectively [65]. In the current study, the hyaline hemocyte percentage significantly increased with the increase of Zn concentration among the tested groups while granulated hemocyte decreased. We can illustrate the current increase in the hyaline percentage that Zn toxicity could have been sufficiently strong to cause oxidative stress and apoptosis, which subsequently cause hemocyte (hyaline) proliferation [66]. It can be suspected that the strategy of the increased proliferation activity could be optimal to compensate for the lesser hemolymph THC [10, 46, 67], while the decrease in granulated hemocytes maybe because of the migration of hemocytes out of the hemolymph circulation to participate in the encapsulation or phagocytosis [68,69,70].
The histological damage in the present study of the different tissues (hepatopancreas, gills, and muscles) was related to the Zn concentration, where the most histological degeneration was observed clearly in the Zn high-dose group, then the field group, and then the Zn low-dose group, while the significant bioaccumulation occurs preferably in hepatopancreas and gill tissues at all Zn concentrations. The gills play an important vital role in aquatic organisms such as ammonia secretion, ion transport, and acid-base balance [71]. Therefore, the histological changes in the gills due to pollution lead to a loss of their functional capabilities [72,73,74]. Exposure of crayfish to different concentrations of zinc led to histological changes in the hepatopancreas and thus affected metabolic processes since hepatopancreas serves as the main energy reserve for growth and molting [75]. Hence, the histological changes of the gills, hepatopancreas, and muscles can be explained as secondary and compensatory responses because of zinc toxicity [76].
Finally, our results showed that the physiological, immunological, and histological changes in crayfish were more observed in the Zn high-dose group, then field groups, and then the Zn low-dose group. Although the Zn concentration in water of the field group was less than the Zn concentration in the water experimental Zn low-dose group, the toxicity in the field group was higher due to the synergism of various heavy metals present in the environment.
Conclusion
The present study showed that zinc accumulated in different parts of crayfishes (Procambarus clarkii) in a concentration-dependent manner causing changes in the content of hemolymph and structure of gills, hepatopancreas, and muscle. These changes could be used as a bio-indicator for water quality criteria.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bai J et al (2019) Arsenic and heavy metals pollution along a salinity gradient in drained coastal wetland soils: Depth distributions, sources and toxic risks. Ecol Indic 96:91–98
Zhang Y et al (2019) Cadmium- induced oxidative stress, histopathology, and transcriptome changes in thehepatopancreas of fresh water crayfish (Procambarus clarkii). Sci Total Environ 666:944–955
Śmietana N et al (2020) Variability of elements and nutritional value of spiny-cheek crayfish (Faxonius limosus, Rafinesque, 1817): variability of elements and nutritional value of Flimosus. J Food Compos Anal 94:103656
Nędzarek A, Czerniejewski P (2021) The edible tissues of the major European population of the invasive Chinese mitten crab (Eriocheir sinensis) in the Elbe River, Germany, as a valuable and safe complement in essential elements to the human diet. J Food Compos Anal 96:103713
Gedik K, Boran M (2013) Assessment of metal accumulation and ecological risk around Rize Harbor, Turkey (Southeast Black Sea) affected by copper ore loading operations by using different sediment indexes. Bull Environ Contam Toxicol 90(2):176–181
Anandkumar A et al (2020) Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J Food Compos Anal 88:103449
Naito W et al (2010) Exposure and risk assessment of zinc in Japanese surface waters. Sci Total Environ 408:4271–4284
Brinkman SF, Johnston WD (2012) Acute toxicity of zinc to several aquatic species native to the Rocky Mountains. Arch Environ Contam Toxicol 62:272–281
Wang WX, Rainbow PS (2008) Comparative approaches to understand metal bioaccumulation in aquatic animals. Comp Biochem Physiol C Toxicol Pharmacol 148:315–323
Stara A et al (2019) Effects of S-metolachlor and its degradation product metolachlor OA on marbled crayfish (Procambarus virginalis). Chemospere 224:616–625
Bini G, Santini G, Chelazzi G (2015) Pre-exposure to cadmium or zinc alters the heart rate response of the crayfish Procambarus clarkii towards copper. Bull Environ Contam Toxicol 95:12–17
Aliko V et al (2018) Antioxidant defence system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol 76:101–109
Freitas R et al (2019) Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to salicylic acid. Aquat Toxicol 214:105258
Gedik K et al (2017) Distribution of arsenic and other metals in crayfish tissues (Procambarus clarkii) under different production practices. Sci Total Environ 574:322–331
Alcorlo P et al (2006) The use of the red swamp crayfsh (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci Total Environ 366(1):380–390
Suárez-Serrano A et al (2010) Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol Environ Saf 73:280–286
Adebiyi FM, Ore OT, Ogunjimi IO (2020) Evaluation of human health risk assessment of potential toxic metals in commonly consumed crayfish (Palaemon hastatus) in Nigeria. Heliyon, v. 6 (1): e03092
Abdel-Khalek AA et al (2016) Assessment of metal pollution around Sabal drainage in river Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turk J Fish Aquat Sci 16:227–239
Yonar MS et al (2017) Effects of dietary propolis on the number and size of pleopadal egg, oxidative stress and antioxidant status of freshwater crayfish (Astacus leptodactylus Eschscholtz). Anim Reprod Sci 184:149–159
APHA. American Water Works Association (2005) Standard methods for the examination of water and wastewater. American Public Health Association, New York
Bini G (2006) Cardiac responses of marine and freshwater invertebrates induced by natural and anthropogenic stresses: potentials for their use as biomarkers of metal pollution in aquatic environments. PhD Thesis, University of Florence (in Italian)
Lanz H, Tsutsumi V, Aréchiga H (1993) Morphological and biochemical characterization of Procambarus clarkia blood cells. Dev Comp Immunol 17:389–397
Mahmood A, Yousaf M (1985) Effect of some insecticides on the haemocytes of Gryllus bimaculatusde Geer. Pak J Zool 17:71–84
Reitman SS, Frankel A (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Belfield A, Goldberg DM (1971) Normal ranges and diagnostic value of serum 5′ nucleotidase and alkaline phosphatase activities in infancy. Arch Dis Child 46:842–486
Tietz NW, Burtis CA, Ashwood ER (1994) Tietz textbook of clinical chemistry. Saunders, Philadelphia, pp 122–133
Tietz NW, Finley P, Pruden E, Amerson (1990) Clinical Guide to Laboratory Tests Saunders. Philadelphia, p. 232-233
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19(12):1350–1356
Freund A et al (1986) Subjective symptoms, blood glucose estimation, and blood glucose concentrations in adolescents with diabetes. Diabetes Care 9:236–243
Schirmeister J (1964) Determination of creatinine in serum. Dtsch Med Wschr 89:1940
Fawcett JK, Soctt JE (1960) A Rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Barham D, Trinder P (1972) An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 151:142–145
Mohamed MF et al (2020) Impact of heavy metals on Oreochromis niloticus fish and using electrophoresis as bio-indicator for environmental pollution of Rosetta Branch, River Nile. Egypt Eur Chem Bull 9(2):48–61
El Bouraie MM et al (2011) Water quality of Rosetta branch in Nile delta, Egypt. Suo 62(1):31–37
Annabi A, El Mouadeb R, Herrel A (2018) Distinctive accumulation patterns of heavy metals in Sardinella aurita (Clupeidae) and Mugil cephalus (Mugilidae) tissues. Environ Sci Pollut Res Int 25(3):2623–2629
Dan Z et al (2019) Cu accumulation, detoxification and tolerance in the red swamp crayfish Procambarus clarkii. Ecotoxicol.Environ Saf 175:201–207
Subotić S et al (2019) Metal accumulation in muscle and liver of the common nase (Chondrostoma nasus) and vimba bream (Vimba vimba) from the Danube River, Serbia: bioindicative aspects. Bull Environ Contam Toxicol 103(2):261–266
Rainbow PS (2002) Trace metal concentrations in aquatic invertebrates: why and so what? Environ. Pollut. 120:497–507
Zhao D et al (2019) Cu accumulation, detoxification and tolerance in the red swamp crayfish Procambarus clarkii. Environ Saf 175:201–207
Svobodová J et al (2017) Toxic and heavy metals as a cause of crayfish mass mortality from acidified headwater streams. Ecotoxicol. 26(2):261–270
Anandkumar A et al (2018) Human health risk assessment and bioaccumulation of trace metals in fish species collected from the Miri coast, Sarawak, Borneo. Mar Pollut Bull 133:655–663
Faggio C et al (2016) Cytotoxicity, haemo-lymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat Toxicol 180:258–265
Abd El-Atti MS (2005) Histological and ultrastructural alterations in the antennal gland of the crayfish Procambarus clarkii exposed to mercury. Egypt J Aquat Biol Fish 9:489–503
Stara A, Kouba A, Velisek J (2018) Biochemical and histological effects of sub-chronic exposure to atrazine in crayfish Cherax destructor. Chem Biol Interact 291:95–102
Machale RP et al (1989) Copper and cadmium induced changes in blood sugar level of the crab, Barytelphusa cunicularis. Uttar Pradesh J Zool 9:113–115
Aliko V et al (2015) Copper induced lysosomal membrane destabilisation in hemolymph cells of Mediterranean green crab (Carcinus aestuarii, Nardo, 1847) from the Narta Lagoon (Albania). Braz Arch Biol Technol 58(5):750–756
Chang ES (2005) Stressed-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr. Comp. Biol. 45(1):43–50
Lorenzon S et al (2004) Variation of crustacean hyperglycemic hormone (cHH) level in the eyestalk and haemolymph of the shrimp Palaemon elegans following stress. J Exp Biol 207(24):4205–4213
Kim BM et al (2013) Role of crustacean hyperglycemic hormone (CHH) in the environmental stressor-exposed intertidal copepod Tigriopus japonicus. Comp Biochem Physiol C Toxicol Pharmacol 158(3):131–141
Prasath PMD, Arivoli S (2008) Biochemical study of freshwater fish Catla catla with reference to mercury chloride. Iran. J Environ Health Sci Eng 3:109–116
Abd El-Attia M et al (2019) Effects of titanium dioxide nanoparticles on red swamp crayfish, Procambarus clarkii: Bioaccumulation, oxidative stress and histopathological biomarkers. Egypt J Aquat Res 45:11–18
Shahat MMA et al (2018) Evaluation of the protective roles of synthetic zeolite on some physiological and biochemical parameters after cadmium toxicity of crayfish (Procambarus clarkii). Egypt J Hosp Med 72(11):5517–5526
Abd El-Atti MS, Saied RM (2018) Physiological and ultrastructural alterations in the crayfish Procambarus clarkii treated with spinosad (bacterial derived insecticide). Biochem Physiol 7:1
Folk JE, Schirmer EW (1963) The porcine pancreatic carboxypeptidase A system. J Phys Chem 238:3884–3894
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Martínez-Córdova LR et al (2016) Physiological and immune response of Litopenaeus vannamei undergoing the acute phase of the necrotizing hepatopancreatitis disease and after being treated with oxytetra cycline and FF. Lat Am J Aquat Res 44:535–545
Knez M et al (2019) Is there a link between zinc intake and status with plasma fatty acid profile and desaturase activities in dyslipidemic subjects? Nutrients 12(1):93
Olechnowicz J et al (2018) Zinc status is associated with inflammation, oxidative stress,lipid,and glucose metabolism. J Physiol Sci 68(1):19–31
Johansson MW et al (2000) Crustacean haemocytes and haematopoiesis. Aquac. 191:45–52
Qin Q et al (2012) Immune responses and ultrastructural changes of hemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat Toxicol 106-107:140–146
Ray S et al (2015) Immunotoxicological threats of pollutants in aquatic invertebrates. In: Larramend y ML (ed) Emerging pollutants in the environment -current and further implications. In Tech, Croatia, pp 147–165
Le Moullac G, Haffner P (2000) Environmental factors affecting immune responses in Crustacea. Aquac 191:121–131
Hose JE, Martin GC, Gerard AS (1990) A decapod hemocyte classification scheme integrating morphology, cytochemistry, and function. Biol Bull 178:33–45
Celi M et al (2013) Physiological and agonistic behavioural response of Procambarus clarkii to an acoustic stimulus. J Exp Biol 216:709–718
Lee SY, Söderhäll K (2002) Early events in crustacean innate immunity. Fish Shellfish Immunol 12:421–437
Qylia M, Alikoa V, Faggio C (2020) Physiological and biochemical responses of Mediterranean green crab, Carcinus aestuarii, to different environmental stressors: Evaluation of hemocyte toxicity and its possible effects on immune response. Comp Biochem Physiol C 231:108739
Matozzo V, Gallo C, Marin MG (2010) The role of hemocytes from the crab, Carcinus aestuarii (Crustacea, Decapoda), in immune responses: a first survey. Fish Shellfish Immunol 28:534–541
Banaee M et al (2011) Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic Biochem Physiol 99:1–6
Saravanan M, Prabhu KK, Ramesh M (2011) Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic Biochem Physiol 100:206–211
Zhou Y-L et al (2018) Hemocytes of the mud crab Scylla paramamosain: cytometric, morphological characterization and involvement in immune responses. Fish Shellfish Immunol 72:459–469
Henry RP et al (2012) Multiple functions of the crustacean gill: osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front Physiol 15:431
Desouky MM, Abdel-Gawad H, Hegazi B (2013) Distribution, fate and histopathological effects of ethion insecticide on selected organs of the crayfish, Procambarus clarkii. Food Chem Toxicol 52:42–52
Benli ACK (2014) The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): acute toxicity and sublethal effects on histology, hemolymph parameters, and total hemocyte counts. Environ Toxicol 30(8):887–894
Ikerd JL, Burnett KG, Burnett LE (2014) Effects of salinity on the accumulation of hemocyte aggregates and bacteria in the gills of Callinectes sapidus, the Atlantic blue crab, injected with Vibrio campbellii. Comp Biochem Physiol A 183:97–106
Vogt G (1994) Life-cycle and functional cytology of the hepatopancreatic cells of Astacus astacus (Crustacea, Decapoda). Zoomorphol. 231:83–101
Chupani L et al (2016) Histological changes and antioxidant enzyme activity in signal crayfish (Pacifastacus leniusculus) associated with sub-acute peracetic acid exposure. Fish Shellfish Immunol 48:190–195
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mamdouh, S., Mohamed, A.S., Mohamed, H.A. et al. The Effect of Zinc Concentration on Physiological, Immunological, and Histological Changes in Crayfish (Procambarus clarkii) as Bio-indicator for Environment Quality Criteria. Biol Trace Elem Res 200, 375–384 (2022). https://doi.org/10.1007/s12011-021-02653-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02653-x