Abstract
National water-quality criteria for the protection of aquatic life are based on toxicity tests, often using organisms that are easy to culture in the laboratory. Species native to the Rocky Mountains are poorly represented in data sets used to derive national water-quality criteria. To provide additional data on the toxicity of zinc, several laboratory acute-toxicity tests were conducted with a diverse assortment of fish, benthic invertebrates, and an amphibian native to the Rocky Mountains. Tests with fish were conducted using three subspecies of cutthroat trout (Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus, greenback cutthroat trout O. clarkii stomias, and Rio Grande cutthroat trout O. clarkii virginalis), mountain whitefish (Prosopium williamsoni), mottled sculpin (Cottus bairdi), longnose dace (Rhinichthys cataractae), and flathead chub (Platygobio gracilis). Aquatic invertebrate tests were conducted with mayflies (Baetis tricaudatus, Drunella doddsi, Cinygmula sp. and Ephemerella sp.), a stonefly (Chloroperlidae), and a caddis fly (Lepidostoma sp.). The amphibian test was conducted with tadpoles of the boreal toad (Bufo boreas). Median lethal concentrations (LC50s) ranged more than three orders of magnitude from 166 μg/L for Rio Grande cutthroat trout to >67,000 μg/L for several benthic invertebrates. Of the organisms tested, vertebrates were the most sensitive, and benthic invertebrates were the most tolerant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Extending from New Mexico in the south to British Columbia in the north, the Rocky Mountain range has been the site of extensive mining. Abandoned mines contribute to acid-mine drainage or acid-rock drainage, which have been described as the greatest water-quality problem in the region (United States Department of Agriculture Forest Service 1993; Mineral Policy Center 1997). Zinc is commonly present in high concentrations in acid-mine drainage and adversely affects aquatic life in an estimated 900 miles of streams in Colorado alone (Colorado Department of Public Health and Environment 2010). State zinc standards for the protection of aquatic life are based on national water-quality criteria, which are derived from toxicity data for many aquatic species (USEPA 1987, 1996). Species native to the Colorado Rocky Mountains are poorly represented in the toxicity data sets used to derive zinc water-quality criteria. As such, it is uncertain whether national zinc criteria adequately protect or are overprotective of species native to the Rocky Mountains. National criteria are intended to be conservatively protective of all or almost all bodies of water (Stephan et al. 1985), which assumes that species sensitivity in the national database is representative of untested species.
Recently, the mottled sculpin, which is native to the Rocky Mountain range, has been found to be sensitive to zinc and may not be protected by national zinc criteria (Woodling et al. 2002; Brinkman and Woodling 2005; Besser et al. 2007). This prompted concern about the protectiveness of zinc criteria for other aquatic species native to the Rocky Mountains. The objectives of these studies were to measure acute zinc toxicity to several aquatic species native to the Rocky Mountain region. Zinc-toxicity tests were conducted using a diverse assortment of organisms native to Rocky Mountain streams and transition zones, including fishes, benthic invertebrates, and an amphibian. Fishes tested were three subspecies of cutthroat trout native to Colorado (Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus, greenback cutthroat trout O. clarkii stomias, and Rio Grande cutthroat trout O. clarkii virginalis), mountain whitefish (Prosopium williamsoni), mottled sculpin (Cottus bairdi), longnose dace (Rhinichthys cataractae), and flathead chub (Platygobio gracilis). Aquatic invertebrates tested were mayflies (Baetis tricaudatus, Drunella doddsi, Cinygmula sp. and Ephemerella sp.), a stonefly (Chloroperlidae), and a caddis fly (Lepidostoma sp.). Tadpoles of the boreal toad (Bufo boreas) were also tested. Results of the toxicity tests were compared with national ambient water-quality criteria for zinc to determine protectiveness for Rocky Mountain native aquatic species.
Methods
Organisms
Colorado River and greenback cutthroat trout were obtained as freshly fertilized eggs from Colorado Division of Wildlife hatchery brood stock recently founded from wild populations (Lake Nanita, Grand County, CO, at the Glenwood Hatchery and Carr Creek, Garfield County, CO, at the Poudre Hatchery, respectively). Eggs from a minimum of four females were mixed with the milt from two males each. Fertilization rates were enhanced by maintaining anhydrous conditions during the process, with conception occurring in the ovarian fluid bath. Eggs were then hardened for an additional 30 min in water from the brood source before making the 2–4 h journey to the toxicology laboratory in individual 4 L coolers where they were then treated with 1600 mg/L formalin to control fungus (Piper et al. 1982). Rio Grande cutthroat trout were obtained as eyed eggs from the Colorado Division of Wildlife Pitkin Hatchery (source: Haypress Lake, Mineral County, CO). The three subspecies of cutthroat trout eggs were incubated in Ft. Collins dechlorinated municipal tap water (hardness near 50 mg/L as CaCO3, temperature 12°C, pH 7.5). After swimup, fry were fed starter salmon chow (Rangen, Buhl, ID) at approximately 5% body weight/day supplemented with brine shrimp nauplii. Toxicity tests were conducted with each subspecies at water hardnesses of 50 mg/L as CaCO3 and 150 mg/L as CaCO3. Two weeks after swimup, fry were divided randomly into two groups. To minimize size bias between the two groups, small numbers of fry were netted and transferred to alternating tanks, one to five fry at a time, until all fish from the original tanks had been transferred. Half of the fry were maintained in the 50 mg/L as CaCO3 hardness dechlorinated tap water, whereas the other half of the fry was acclimated to a mixture of on-site well water and dechlorinated tap water. Conductivity controllers maintained the tap/well water mixture near a water hardness of 150 mg/L as CaCO3 during acclimation and toxicity tests. Fry were maintained in their respective waters for 2 weeks before toxicity tests. Cutthroat fry were fasted for 48 h before initiation of toxicity tests.
Mountain whitefish eggs were collected from wild adults during a spawning run up Mad Creek near Steamboat, CO in 2006, 2007, and 2009. Eggs from a minimum of six females were stripped and fertilized in the field as before, then transported 4 h by truck in individual one-gallon coolers to the laboratory where eggs were again treated with 1600 mg/L formalin for 15 min (Piper et al. 1982). Eggs were incubated in Ft. Collins dechlorinated municipal tap water (hardness near 50 mg/L as CaCO3, temperature 7°C, pH 6.8–7.4). After hatching, mountain whitefish fry were fed ad libitum with live brine shrimp nauplii and a 1:1 mixture of freeze-dried brine shrimp and bloodworms (Hikari, Hayward, CA) sieved through 500-μm screen. Mountain whitefish fry were fasted for 48 h before initiation of toxicity tests.
Boreal toad egg masses were collected from a pond with a healthy breeding population of adults near Henderson, CO. Boreal toad larvae were acclimated to test temperature and water-quality conditions for 24 h before the start of the test. Boreal toad embryos were stage 16–17 on the Gosner amphibian developmental stage (Gosner 1960). Tadpoles were fed a mixture of Mazuri amphibian food, starter trout chow (Rangen), and frozen romaine lettuce ad libitum during acclimation to test conditions and during the toxicity test.
Toxicity tests with other fishes used organisms collected from the field Longnose dace, mottled sculpin, and flathead chub were collected using backpack electrofishing units with pulsed DC current. Longnose dace (N = 261) were collected from the Cache la Poudre River near Ft. Collins (Larimer County, CO). Mottled sculpin (N = 250) were collected from the Dolores River near Lizard Head Pass (Dolores County, CO). Longnose dace and mottled sculpin were deemed young-of-the-year age class based on length-frequency distribution. Fry were sorted in the field and transported in an aerated cooler. Juvenile flathead chub (N = 114) were collected from lower Fountain Creek (El Paso County, CO). At the laboratory, field-collected fry were placed in aquaria with water that matched the temperature and water hardness of their source locations. Water temperature and hardness were then slowly adjusted to test conditions (2°C/d and 10 mg/L as CaCO3/d). Longnose dace and mottled sculpin fry were fed starter salmon chow (Rangen) supplemented with brine shrimp nauplii. Flathead chubs were fed frozen bloodworms. Fry were maintained at test water-quality conditions for a minimum of 14 days before the start of toxicity tests. Fry were fasted for 48 h before initiation of toxicity tests.
Invertebrates were collected by picking up cobble substrate from the stream and transferring adhered nymphs with small, fine paint brushes to 2-L plastic collection containers (12.5 × 12.5 × 13.0 cm) that contained site water. Individuals were sorted and identified to genus in the field using a taxonomic key from Ward and Kondratieff (1992). Collection containers with the nymphs were transported in a cooler maintained near site water temperature with ice or a Peltier chiller (Coolworks Ice Probe; Aquatic Ecosystems, Apopka, FL). Air and water flow were provided during transport by a battery-operated pump, flexible tubing, and air stones. Transportation time to the laboratory was <1 h. At the laboratory, nymphs were carefully individually coaxed to adhere to a fine camel-hair paintbrush, transferred to aerated, glass holding tanks containing site water, and placed in a temperature-controlled incubator. Temperature was adjusted to 12°C and site water replaced with dechlorinated municipal tap water during a 48-h acclimatization period before start of the test. Invertebrates were not fed during acclimatization or during the toxicity-test exposures.
When possible, test organisms were obtained as embryos from wild adults and reared in the laboratory (cutthroat trout, mountain whitefish, and boreal toad tadpoles). Other test organisms were collected from streams with no history of zinc contamination that had robust wild populations. Although little is known about culture requirements for many of the species tested, laboratory conditions were conducive for survival and growth during the relatively short duration of holding and testing. Mortality of field-collected organisms was low (<10%) after collection, and fry increased in weight during acclimatization to test conditions. Mean weight of mottled sculpin fry increased 40% during 40 days of acclimatization, and longnose dace increased by 25% during 14 days. Flathead chubs readily consumed frozen bloodworms.
Toxicity-Test Methods
Toxicity testing was conducted at the Colorado Division of Wildlife Aquatic Toxicology Laboratory in Ft. Collins, CO. Toxicity tests methods followed established guidelines (American Society for Testing and Materials 1997) except that temperature was measured three to five times during the test and not hourly as specified in section 11.9.2.3. Tests were conducted using Ft. Collins dechlorinated municipal tap water as the dilution water (hardness near 50 mg/L as CaCO3). Each subspecies of cutthroat trout was also tested at a water hardness of 150 mg/L as CaCO3. The mottled sculpin test was conducted at a water hardness of 100 mg/L as CaCO3. Water hardness for the cutthroat trout tests at 150 mg/L as CaCO3, and the mottled sculpin test was modified by mixing Ft. Collins dechlorinated municipal tap water with on-site well water. All toxicity tests were flow-through and were conducted for 96 h except for Lepidostoma sp., which was a 96-h static test with 50% renewal at 48 h. Flow-through tests were conducted using continuous-flow serial diluters (Benoit et al. 1982) constructed of glass, Teflon, polyethylene, and polypropylene components. The diluters delivered five concentrations with a 50% dilution ratio and a control (seven concentrations for boreal toad tadpoles). A peristaltic pump delivered a stock solution of zinc sulfate heptahydrate (ZnSO4·7H2O; Mallinckrodt analytical reagent grade or Fisher American Chemical Society grade) dissolved in deionized water. Food-grade vinyl tubing delivered test solutions to exposure chambers. Standpipes allowed test solutions to overflow from exposure chambers into a temperature-controlled water bath. Exposure chambers varied among tests according to size of test organisms. Cutthroat trout, mountain whitefish, and mottled sculpin were tested in 14 × 29 × 13-cm polypropylene containers holding 2.8 L of solution. Longnose dace and flathead chub were tested in 20 × 40 × 25-cm glass aquaria holding 18 L of solution. Boreal toad tadpoles were tested in 9 × 18.5 × 15-cm glass aquaria holding 2.2 L of solution. Invertebrates were tested in 1.25-L cylindrical polypropylene containers that used an airlift to provide aeration and a continuous circular water flow as described by Brinkman and Johnston (2008). Two unglazed ceramic tiles (5 × 5 cm) and 1000 μm nylon mesh (approximately 7.5 × 7.5 cm) (Aquatic EcoSystems, Apopka, FL) were provided as substrate in each invertebrate test. The flow-through tests delivered 40 mL/min to each exposure chamber, except for tests with longnose dace and flathead chub, for which 60 mL/min was delivered. Biomass loading in test chambers was <12% of maximum recommended rates (ASTM 1997) in the cutthroat trout, mountain whitefish, sculpin, and longnose dace tests and 31% of maximum rate in the flathead chub test. Masses of boreal toad tadpoles and invertebrate nymphs were not measured but were considered negligible relative to flow rates and exposure-chamber volumes.
The 96 h static-renewal tests with Lepidostoma sp. were conducted in 1-L polypropylene tri-pour beakers containing 500 mL test solution. Five zinc concentrations and a control were prepared with four replicate chambers per concentration. Nymphs were gently transferred from the cooler using a turkey baster. Nymphs were fasted during the experiments. Exposure solutions were renewed (50%) at 48 h. After replacement, exposure solutions were pooled by treatment and retained for water quality and zinc analysis.
At the start of each test, individuals were distributed, one at a time, to each exposure chamber, and the process was repeated until the desired number of organisms was distributed to each chamber. Replication and number of individuals distributed to each exposure chamber varied among tests depending on size and availability of test organisms (Table 1). Ambient laboratory fluorescent lighting provided a 16-/8-h light-to-dark photoperiod. Lighting for boreal toad tadpoles used Reptasun full-spectrum fluorescent bulbs suspended over the aquaria. Diluters and toxicant flow rates were monitored daily to ensure proper operation. Mortality was operationally defined as the failure to respond to repeated prodding with a net (fish) or turkey baster (invertebrates). Dead organisms were removed from the test chambers. Dead fish were blotted dry with a paper towel, weighed, and recorded. At the end of the tests, surviving fish were killed with 0.5 g/L MS-222 (3-amino benzoic acid ethyl ester), blotted dry with a paper towel, weighed, and recorded.
Several of the toxicity tests were repeated: Three toxicity tests were conducted with mountain whitefish in three separate years (mountain whitefish I, II, and III), and two toxicity tests were conducted 1 year apart with Rio Grande cutthroat trout at 50 mg/L hardness as CaCO3 (Rio Grande cutthroat I and II).
Water-quality parameters were measured three times (after start of test, at 48 h, and just before test termination) or five times (daily) during flow-through tests and at 48 and 96 h for static-renewal tests. Hardness and alkalinity were determined according to standard methods (American Public Health Association 1998). Electronic meters were used to measure dissolved oxygen (Orion model 1230; Orion, Beverly, MA), pH (Thermo Orion model 635), conductivity (YSI model 35, YSI, Yellow Springs, OH) and temperature (Thermo Orion model 635). Dissolved oxygen and pH meters were calibrated before each use.
Water samples for dissolved zinc analyses were filtered through a 0.45-μm filter and preserved with high-purity nitric acid to pH <2. Metal concentrations were determined using atomic absorption (instrumentation laboratory video 22; Allied Analytical Systems, Franklin, MA) and calibrated before each use; calibration was verified using a reference standard. Chloride and sulfate concentrations were measured with a flow-injection analyzer (QuikChem 8000; Lachat, Loveland, CO) using USEPA methods 325.1 and 375.4, respectively. Sample splits were collected and spikes prepared to verify reproducibility and quantify analytical recovery. Ninety-six-hour median lethal concentrations (LC50) were estimated from measured zinc concentrations using the Trimmed Spearman–Karber technique with automatic trim (Hamilton et al. 1977, 1978).
Results
The relative SD of sample splits was 0.7% (range 0% to 3.5%). Mean recovery of sample spikes was 102% (range 96.8% to 110%). Recovery of external quality-assurance standards was 100% (range 95.8% to 105%). Measured zinc concentrations from the tests with fishes were within 15% of nominal concentrations (Table 1). Deviations of measured concentrations relative to nominal were much greater in the invertebrate tests due to the much greater concentrations used in the tests. Low SDs of measured zinc concentrations indicate that concentrations were stable during the test and consistent among replicates. Survival in control exposures was 100% except for boreal toad tadpoles (99%), Baetis tricaudatus (93%), and Lepidostoma sp. (95%). In tests where metal-related mortalities were observed, organism mortality increased monotonically with zinc concentrations or with a minor deviation due to sporadic mortality of a single organism. One exception was the B. tricaudatus test, in which intermediate zinc concentrations resulted in abnormally high mortality. Water-quality parameters were consistent within each of the toxicity tests (Table 2). Measured dissolved oxygen was maintained near saturation levels (mean 95% [range 80% to 104%]). Dissolved oxygen was not measured in the invertebrate flow-through tests. These tests used an airlift that provided aeration and continuous flow, which, in our previous experience, maintained dissolved oxygen at saturation levels (Brinkman and Johnston 2008). Water hardness ranged between 40 and 62 mg/L as CaCO3 except for the cutthroat tests and mottled sculpin test, for which water hardnesses were deliberately modified to study the effect of water hardness on zinc toxicity. Temperatures for most tests were near 12°C, except for the boreal toad tadpole test, which was conducted at 19°C.
Dissolved organic carbon (DOC) was not measured in these studies. Previous studies at our laboratory using the same water sources had measured DOC concentrations that were low (average 1.7 mg/L [range 0.8–4.0, N = 27]). There are conflicting reports whether DOC concentrations at this low range appreciably affect zinc toxicity. Paulauskis and Winner (1988) found that as little as 0.75 mg/L humic acid decreased zinc toxicity to Daphnia magna. In contrast, the protective effect of DOC against zinc toxicity to Atlantic salmon and fathead minnow was minimal at DOC concentrations <10 mg/L (Zitko et al. 1973; Bringolf et al. 2006).
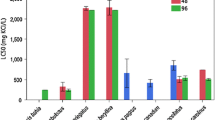
The range of median lethal concentrations spanned two orders of magnitude (Table 3). To facilitate direct comparisons, median lethal concentrations were normalized to 50 mg/L as CaCO3 water hardness using the pooled-species mean-hardness slope (USEPA 1987). Invertebrates were the most tolerant organisms tested, with normalized LC50s ranging from 10,020 μg/L for Baetis tricaudatus to >60,000 μg/L for Chloroperlidae, D. doddsi, Ephemerella sp. and Cinygmula sp. The caddisfly Lepidostoma sp. was also tolerant, with no mortality at 48,500 μg/L, which was the highest zinc concentration used in the test. LC50s for vertebrates were much lower than those of invertebrates but also spanned a wide range. Normalized LC50 values ranged from 166 μg/L for Rio Grande cutthroat trout to 2492 μg/L for flathead chub. Boreal toad tadpoles were intermediate in sensitivity, with a normalized LC50s of 755 μg/L.
Increasing water hardness decreased toxicity of zinc. In the cutthroat tests, increasing water hardness by a factor of three increased the LC50 by factors of 4.8, 5.6, and 7.7 for Rio Grande, greenback, and Colorado River cutthroat trout, respectively. Greenback cutthroat trout were approximately 1.4 and 1.7 times more tolerant than Rio Grande cutthroat trout at 50 and 150 mg/L hardness as CaCO3, respectively. Colorado River cutthroat trout were intermediate in sensitivity. The LC50s of replicated toxicity tests were generally close. The three mountain whitefish tests (mountain whitefish I, II, III), each conducted in separate years, had normalized LC50s that ranged from 404 to 505 μg/L. The relative SD of the LC50 of the three replicate mountain whitefish tests was 11.7%. The two Rio Grande cutthroat trout tests conducted 1 year apart (Rio Grande cutthroat I and II) had normalized LC50s of 166–193 μg/L (10.6% relative SD).
Discussion and Conclusion
The native species tested in our study were selected based in part on recreational importance (e.g., mountain whitefish, cutthroat trout) or because the species were state listed as rare, threatened, or endangered (e.g., cutthroat trout, boreal toad tadpoles). Field-collected species were selected based in part on our ability to capture sufficient numbers of young-of-the-year or small juvenile fish for testing (mottled sculpin, longnose dace, flathead chub). These small sizes can be expected to be more sensitive to zinc than adults, although they may be more tolerant than larval or swimup fry (see, e.g., Besser et al. 2007). Invertebrates from Ephemeroptera, Plecoptera, and Tricoptera were selected because of their reported sensitivity to metals (Clements et al. 2000) and the lack of zinc-toxicity data. This diverse assortment of organisms exhibited a wide range of zinc sensitivity with toxicity values that spanned more than two orders of magnitude. Salmonids and mottled sculpin were most sensitive, followed by other vertebrates (boreal toad tadpoles, longnose dace, and flathead chub). Benthic invertebrates were most tolerant.
The three native subspecies of Colorado native cutthroat trout tested in this study have experienced significant decreases in population and distribution due to both competition and hybridization with nonnative trout (Behnke 2002). Greenback cutthroat trout are listed as threatened, and Rio Grande cutthroat trout was found warranted for listing under the Endangered Species Act (United States Fish and Wildlife Service [USFWS] 1978, 2008). Given the importance of cutthroat trout in Rocky Mountain streams, surprisingly few zinc-toxicity tests have been published. An unspecified strain of cutthroat trout strain had a 670 μg/L 14-day LC50 obtained by diluting zinc-contaminated river water with well water (Nehring and Goettl 1974). Another study with an unspecified strain of cutthroat trout reported a 96-h LC50 of 90 μg/L (Rabe and Sappington 1970). These toxicity values were not used in derivation of zinc criteria because water-quality characteristics varied too much during the test or were not reported. Normalized LC50 values from the present study were between the values reported previously and ranged from 166 to 611 μg/L (geometric mean 315) and are in agreement with unpublished Colorado Division of Wildlife data (Davies et al. 2000; Brinkman and Hansen 2004; Brinkman and Vieira 2008) (Fig. 1).
Mountain whitefish have a wide distribution in the Rocky Mountains ranging from the Colorado River to the MacKenzie River basin in northern Canada. The toxicity of zinc to many salmonids has been well studied and documented; however, we were unable to find any published data for mountain whitefish. Normalized LC50s for the three mountain whitefish tests ranged between 404 and 505 μg/L (geometric mean 464). Mountain whitefish were slightly more zinc-tolerant than were cutthroat trout.
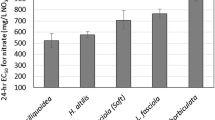
Mottled sculpin are reported to be sensitive to zinc (Woodling et al. 2002; Brinkman and Woodling 2005; Besser et al. 2007 [see Fig. 2]). Besser et al. (2007) reported that mottled sculpin from both Mississippi River and Missouri River drainages varied significantly in their metal sensitivity and suggested that tests with local populations may be necessary to protect sculpin in drainages affected by metal pollution. Zinc-toxicity tests at high and low water hardness were previously conducted using Colorado mottled sculpin collected from the White River (Woodling et al. 2002; Brinkman and Woodling 2005). Mottled sculpin for this study were collected from a different drainage (Dolores River) and tested at an intermediate hardness (100 mg/L as CaCO3) to better characterize the water hardness–toxicity relation. Based on limited data, the sensitivity of sculpin from the two drainages appears to be similar (Fig. 2).
Boreal toads were once common in montane areas of the Southern Rocky Mountains but have experienced significant decreases during the last two decades. Boreal toads are listed as endangered by Colorado and New Mexico and are protected in Wyoming (Loeffler 2001). The normalized LC50 of early stage tadpoles was 755 μg/L. This result is consistent with limited results from a previous study that reported complete survival at 100 μg/L but rapid and complete mortality at 39,000 μg/L (Porter and Hakenson 1976).
Longnose dace and flathead chub were the most tolerant of the vertebrates tested. The normalized LC50s were 1908 and 2492 μg/L, respectively, for longnose dace and flathead chub. Longnose dace and flathead chub were more sensitive to zinc than all but one of the cyprinids reported in the USEPA criteria document (1987).
Invertebrates used in the tests were tolerant to acute zinc exposures. LC50s ranged between 10,020 μg/L for Baetis tricaudatus to >67,543 μg/L for Chloroperlidae and Ephemerella sp. In general, the relatively high LC50 values are consistent with the sparsely published data on toxicity of zinc to mayflies, stoneflies, and caddisflies, although direct comparisons are complicated by different durations of exposure. Ephemerella sp. from our study had a 96-h LC50 >67,543 μg/L compared with LC50s of 16,000 μg/L after 10 days (Warnick and Bell 1969) and >6900 μg/L after 14 days (Nehring 1976). The mayfly Rhithrogena hageni had a 96-h LC50 of 50,500 μg/L (Brinkman and Johnston 2008). The stonefly Pteronarcys californica had a 14-day LC50 >13,900 μg/L (Nehring 1976), and the caddisflies Acroneuria and Hydropsyche had an LC50 of 32,000 μg/L after 14 and 11 days, respectively (Warnick and Bell 1969).
The current USEPA freshwater acute criterion for zinc is 67 μg/L (at 50 mg/L as CaCO3 water hardness) based on data from 36 genera (USEPA 1996). This study reports toxicity data for 11 additional genera and 1 family. National acute criteria appear generally protective of Rocky Mountain native species tested in the present study. Although our zinc-toxicity data will have minimal effect on national criteria, they could be important for site-specific zinc standards. National criteria can be adjusted on a site-specific basis using the recalculation procedure, which is designed to protect local aquatic communities (USEPA 1994). The recalculation procedure calculates site-specific standards using the same methodology as national criteria but uses a modified subset of the toxicity database based on species that occur or are expected to occur at the site. Toxicity data from related species are used as surrogates when toxicity data are unavailable for species present at the site. When the recalculation procedure was used to derive site-specific zinc standards in several streams in Colorado, zinc-sensitive Daphnia sp. and Ceriodaphnia sp. were not considered resident species due to their inability to persist in rapidly moving water. Their toxicity data were not used in the recalculation, resulting in a significant numerical increase in the zinc standard for those sites. Thus, it was more important to us to measure toxicity of zinc to Rocky Mountain native species not only to ensure protectiveness of national criteria but also to ensure protective recalculated standards. The species we tested were often more sensitive than their potential surrogates. The species mean acute values (SMAV) for cutthroat trout and mountain whitefish were 315 and 464 μg/L, respectively. The SMAV for other salmonids were 446 (Chinook salmon), 689 (rainbow trout), 1502 (sockeye salmon), 1628 (coho salmon), 2176 (Atlantic salmon), and 2100 μg/L (brook trout) (USEPA 1987). Similarly, longnose dace and flathead chub were more sensitive than other cyprinids with the exception of longfin dace. Invertebrate toxicity values were generally too high to affect recalculated standards.
In conclusion, zinc-toxicity values from a diverse array of Rocky Mountain native species—including fishes, an amphibian, and three orders of insects—spanned a wide range. Cutthroat trout, mottled sculpin, and mountain whitefish were found to be somewhat sensitive to zinc. Invertebrates were found to be tolerant. Boreal toad tadpoles, longnose dace, and flathead chub were intermediate in sensitivity. The results of the present studies found that national acute criteria are protective of Rocky Mountain native species. Toxicity data from resident species become important when sensitive nonresident species are deleted from the data sets used to recalculate site-specific standards
References
American Public Health Association (1998) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, American Water Works Association, and Water Pollution Control Federation, Washington, DC
American Society for Testing, Materials (1997) Standard practice for conducting acute test toxicity tests with fish, macroinvertebrates and amphibians. Standard E729 in volume 11.05 of the annual book of ASTM standards. ASTM, West Conshohocken
Behnke RJ (2002) Trout and salmon of North America. Free Press, New York
Benoit DA, Mattson VR, Olsen DC (1982) A continuous flow mini-diluter system for toxicity testing. Water Res 16:457–464
Besser JM, Mebane CA, Mount DR, Ivey CD, Kunz JL, Greer IE et al (2007) Sensitivity of mottled sculpin (Cottus bairdi) and rainbow trout (Onchorhynchus mykiss) to acute and chronic toxicity of cadmium, copper, and zinc. Environ Toxicol Chem 26:1657–1665
Bringolf RB, Morris BA, Boese CJ, Santore RC, Allen HC, Meyer JS (2006) Influence of dissolved organic matter on acute toxicity of zinc to larval fathead minnows (Pimephales promelas). Arch Environ Contam Toxicol 51:438–444
Brinkman SF, Hansen DL (2004) Federal aid in fish and wildlife restoration. Job Progress Report F-243R-11. Colorado Division of Wildlife, Fort Collins
Brinkman SF, Johnston WD (2008) Acute toxicity of copper, cadmium and zinc to the mayfly Rhithrogena hageni. Arch Environ Contam Toxicol 54:466–472
Brinkman SF, Vieira N (2008) Federal aid in fish and wildlife restoration. Job Progress Report F-243R-15. Colorado Division of Wildlife, Fort Collins
Brinkman SF, Woodling JD (2005) Zinc toxicity to the mottled sculpin (Cottus bairdi) in high-hardness water. Environ Chem Toxicol 24:1515–1517
Clements WH, Carlisle DM, Lazorchak JM, Johnson PC (2000) Heavy metals structure benthic communities in Colorado mountain streams. Ecol Appl 10:626–638
Colorado Department of Public Health and Environment (2010) Status of water quality in Colorado―2010. The update to the 2008 305(b) report. CDPHE, CO
Davies PH, Brinkman SF (2002) Federal aid in fish and wildlife restoration. Job Progress Report F-243R-9. Colorado Division of Wildlife, Fort Collins
Davies PH, Brinkman SF, Hansen DL (2000) Federal aid in fish and wildlife restoration. Job Progress Report F-243R-7. Colorado Division of Wildlife, Fort Collins
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologia 16:183–190
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Hamilton MA, Russo RC, Thurston RV (1978) Correction. Environ Sci Technol 12:417
Loeffler C (2001) Boreal toad conservation plan and agreement. Colorado Division of Wildlife, Denver
Mineral Policy Center (1997) Golden dreams poisoned streams. MPC, Washington, DC
Nehring RB (1976) Aquatic insects as biological monitors of heavy metal pollution. Bull Environ Contam Toxicol 15:147–154
Nehring RB, Goettl JP (1974) Acute toxicity of a zinc contaminated stream to four species of salmonids. Bull Environ Contam Toxicol 12:464–469
Paulauskis JD, Winner RW (1988) Effects of water hardness and humic acid on zinc toxicity to Daphnia magna Straus. Aquat Toxicol 12:273–290
Piper RG, McElwain IB, Orme JP, McCraren JP, Fowler LG, Leonard JR (1982) Fish hatchery management. United States Fish and Wildlife Service, Department of the Interior, Washington, DC
Porter KR, Hakenson DE (1976) Toxicity of mine drainage to embryonic and larval boreal toads (Bufonidae: Bufo boreas). Copeia 1976:327–331
Rabe RW, Sappington CW (1970) Biological productivity of the Coeur d’Alene River as related to water quality. Project A-024-Ida. Water Resources Research Institute, Moscow
Stephan CE, Mount DI, Hansen DJ, Gentile JH, Chapman GA, Brungs WA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. Accession No. PB-85-227049. National Technical Information Service, Springfield
United States Department of Agriculture Forest Service (1993) Acid mine drainage from mines on the National Forests: a management challenge. Program Aid 1505
United States Environmental Protection Agency (1987) Ambient water quality for zinc―1987. USEPA EPA-440/5-87-003
United States Environmental Protection Agency (1994) EPA interim guidance on determination and use of water-effect ratios for metals. EPA-823-B-94-001. Office of Water, Washington, DC
United States Environmental Protection Agency (1996) 1995 Updates: water quality criteria documents for the protection of aquatic life in ambient water. EPA-820-B-96-001.Office of Water, Washington, DC
United States Fish Wildlife Service (1978) Final determination of threatened status for the greenback cutthroat trout. Fed Reg 43:16343–16345
United States Fish Wildlife Service (2008) Endangered and threatened wildlife and plants: status review for Rio Grande cutthroat trout. Fed Reg 73:27900–27923
Ward JV, Kondratieff BC (1992) An illustrated guide to the mountain stream insects of Colorado. University Press of Colorado, Niwot
Warnick SL, Bell HL (1969) The acute toxicity of some heavy metals to different species of aquatic insects. J Water Pollut Control Fed 41:280–284
Woodling JD, Brinkman SF, Albeke SA (2002) Acute and chronic toxicity of zinc to the mottled sculpin (Cottus bairdi). Environ Toxicol Chem 21:1922–1926
Zitko P, Carson WV, Carson WG (1973) Prediction of incipient lethal levels of copper to juvenile Atlantic salmon in the presence of humic acid by cupric electrode. Bull Environ Contamin Toxicol 10:265–273
Acknowledgments
We thank Audrey Crockett, Eric Fanning, Daria Hansen, Katherine Mitchell, Matthew McIntyre, Barry Nehring, Kevin Rogers, and others too numerous to name who assisted with toxicity tests and collection and care of the test organisms. Comments by Nicole Vieira and three anonymous reviewers significantly improved an earlier draft of this manuscript. This study was supported by USFWS Federal Aid Grant F-243 and a grant from Environmental Protection Agency Region 8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brinkman, S.F., Johnston, W.D. Acute Toxicity of Zinc to Several Aquatic Species Native to the Rocky Mountains. Arch Environ Contam Toxicol 62, 272–281 (2012). https://doi.org/10.1007/s00244-011-9698-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9698-3