Abstract
The purpose of the study was to determine metal pollution and ecological risk in the sediments around Rize Harbor. Metal concentrations (Cu, Zn, Pb, Cd, As, Hg, Ni and Al), were determined in the sediments collected from 7 sites in November, 2010. Metal levels in the sediments were varied from 33.9 to 279.1 mg/kg for Cu, 82 to 383 mg/kg for Zn, 15.9 to 33 mg/kg for Pb, 4.9 to 12.3 mg/kg for As, 0.1 to 1.4 mg/kg for Cd, 0.01 to 0.07 mg/kg for Hg, 13.1 to 17.6 mg/kg for Ni, 1.5 % to 2.98 % for Al. Concentration of metals also was evaluated by enrichment factor, Sediment Quality Guideline (SQGs) of US EPA, and two sets of SQGs indices. A factor analysis was used to determine source of contamination. Two probable source types were identified as anthropogenic and crustal for metals, explaining 86.4 % of the total variance. Six sites were heavily polluted by Cu. Copper concentration in R1 station was found significant enrichment. Current concentration of copper would be expected to frequently be associated with the toxic effect on biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Metals are brought into the sea by terrestrial runoff, atmospheric deposition, sewage discharges and others. After a series of process, metals deposited along with colloid and were accumulated from the water into the sediments, so the sediments became the main repository of metals and other chemicals (Förstner and Wittmann 1983). Many researchers believe that the sediments are an indicator for water pollution, and the metals’ concentration in the sediments can reflect the water pollution level (Selvaraj et al. 2004).
Metals, unlike other pollutants, are not biodegradable and can accumulate in sediments over time. Moreover, metal concentration in sediment can uptake to toxic levels and affect marine organisms and food chain. Accumulation is enhanced in enclosed and semi-enclosed areas (Findik and Turan 2012; Karahalil et al. 2012; Tang et al. 2008). Harbors are enclosed and limited exchange water bodies. Additionally, they are tended to take serious metal inputs from marinas, shipping activities, loading and unloading operations, industrial and urban discharges (Huerta et al. 2008). It is very important that determine the metal accumulation in harbor sediments. Because, contaminated sediment can move as point sources of contamination during dredging operations or any other activity by which contaminated sediments can be transported out of the harbors and into neighboring bays or open sea areas (Huerta et al. 2008).
Rize city is located around a small bay on north-east Turkey, Black Sea coast. Rize harbor far away 18 km from mine area is important in the area because of ore transportations. The company produces 1.2 million tons of ore, annually. Average reserve grades are 3.5 % for Cu and 4.6 % for Zn (IMC 2009). Ore processing, smelting, and refining operations can cause deposition of large quantities of trace metals, such as Pb, Zn, Cu, and As into drainage basins or direct discharge into aquatic environments (Csuros and Csuros 2002). During ore loading operations, there were many environmental events, including high metal concentrations in treated runoff at Rize harbor facility, in recent years. Because of Rize has heavy rainfall, during storms it is difficult to avoid overflows of runoff and to keep Rize harbor residuals within permit limits (IMC 2009).
The overall objectives of present study were: (1) to assess the level of contamination, (2) to assess the potential ecotoxicological sense, (3) to asses of the sources of the metals.
Materials and Methods
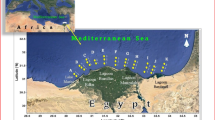
Sediment samples were taken from 7 stations located on Rize harbor and bay (Fig. 1) in November 2010. The stations were chosen so as to cover the metal pollution affected area. Three sub-sampling of sediments (the top 5 cm layer of the bottom) were sampled using 225 cm² Ekman-Birge type bottom sampler from each station. The samples were placed in polyethylene bags using a plastic spatula to prevent contamination, and homogenized to achieve a composite sample each station. After collection, samples were placed in refrigerator, and transported to the laboratory. All sediment samples were preserved at −18°C until analyzed (Csuros and Csuros 2002).The samples were dried at 45°C before analysis. For grain size distribution, sediment samples were sieved using distilled water in AS 200 vibratory sieve shaker (Retsch, Düsseldorf, Germany). Grain sizes were classified as fine (<0.063 mm), sand (0.063–2 mm) and gravel (>2 mm) according to Folk (1954). pH was determined, 1:2 w/v ratio for sediment to water and an equilibration time of 30 min, using a Hach Lange HQ40D multi meter described by Radojevic and Bashkin (2006). Total organic carbon (TOC) was carried out using a modified Walkley–Black titration method (detection limit 0.05 %) (Gaudette et al. 1974). For metal analysis, sediment samples were sieved to pass <63 μm because the metals are generally related with small grains (Morillo et al. 2004). Metal concentrations (Cu, Pb, Zn, Cd, As, Ni, Hg and Al) were analyzed using an inductively coupled plasma–mass spectrometer (ICP–MS) analysis in ACME lab., (Vancouver, BC, Canada). The accuracy of the analysis was ranged from 95.81 % to 103.50 %. The detection limits for metals were 0.1 for Cu, 1 for Zn, 0.1 for Pb, 0.1 for Cd, 0.5 As, 0.01 Hg, 0.1 Ni mg/kg and 0.01 % for Al. Factor analysis was performed to confirm the major source(s) of metals in the sampling area. The rotation method was Varimax with Kaiser Normalization in the analysis. Pearson correlation was performed to determine the relationship among metals in sediments. All data were given mg/kg dry weight (dw). All statistical analyses were performed using SPSS 15 Software package (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Percentages of sediment fractions are given in Table 1. The sand fractions were generally dominant (>50 %) in the surface sediments. Concentrations of TOC in surface sediments around Rize harbor were ranged from 0.57 % to 1.02 %. pH values were ranged from 7.12 to 7.55 in surface sediment around Rize harbor (Table 1).
Metal levels in the sediments from 7 stations varied from 33.9 to 279.1 mg/kg for Cu, 82 to 383 mg/kg for Zn, 15.9 to 33 mg/kg for Pb, 4.9 to 12.3 mg/kg for As, 0.1 to 1.4 mg/kg for Cd, 0.01 to 0.07 mg/kg for Hg, 13.1 to 17.6 mg/kg for Ni, 1.5 % to 2.98 % for Al (Table 2). The highest metals’ concentrations were at R1 station. Harbors are enclosed and low-energy water bodies (Huerta et al. 2008). It was reported in the literature that accumulation is enhanced in enclosed and semi-enclosed areas (Pekey et al. 2004; Tang et al. 2008). R1 station was in the inner harbor, water circulation in this area was not good. It can be said that concentrations of metals were higher than other stations. Mean concentrations of Cu, Zn, Pb, and Cd in sediments were higher than the shale average values whereas, the average concentration of Ni, As, Al, and Hg were less than average shale (Turekian and Wedepohl 1961) (Table 2).
According to the Sediment Quality Guideline (SQGs) proposed by US EPA, sediments were classified into three classes, non-polluted, moderately polluted and heavily polluted (Pekey et al. 2004). The average metal values of stations were compared with the SQGs (Table 2). Six sites distributed in Rize harbor and the bay heavily polluted by Cu and the remaining site located at the back of the harbor (Fig. 1) was moderately polluted. Zn concentrations in five stations were between 112 and 168 mg/kg which was classified as moderately polluted, while greater 200 mg/kg concentration, classified as heavily polluted, was determined at a station (R1). Zn concentration in R7 station was non-polluted. Concentration of As in three stations (R2, R3, R4) which is close the shore line, were classified as moderately polluted (3–8 mg/kg), while four stations (R1, R5, R6, R7) were classified as heavily polluted (>8 mg/kg). Concentrations of Pb, Cd, and Ni in all stations were classified as non-polluted (<40, <6, <20 mg/kg) respectively.
Metal concentrations in sediments of Rize harbor were compared with harbor sediments reported from different areas of the world. Concentrations of Cu were higher than Tolo, Guam, Mao, and Montevideo harbors but lower than the Naples and Rijeka harbors. The range of Zn concentrations in Rize harbor were higher than in Tolo harbor but lower than Naples, Guam, Rijeka, Mao, and Montevideo harbors. Concentrations of Pb, Hg, and As levels in this study were detected lower than reported harbors. Cadmium concentrations in the present study were higher than Naples and Mao harbors but lower than other harbor reported studies (Adamo et al. 2005; Owen and Sandhu 2000; Denton et al. 2005; Cukrov et al. 2011; Garcia et al. 2011; Muniz et al. 2004).
Enrichment factor (EF) is an index to determine anthropogenic influences of metals in sediment. It is described as the monitoring metal to aluminum (Al) ratio in the sample of interest divided by the background metal/Al ratio because Al is one of the most abundant elements on the earth and generally has no contamination concern. Aluminum has been used by many researchers (Pekey et al. 2004; Cukrov et al. 2011). The enrichment factor (EF) is expressed as:
where (Me/Al)sample is the metal/Al ratio in the sample of interest and (Me/Al) shale is the natural background value of the metal/Al ratio. In the present study, average shale (Turekian and Wedepohl 1961) was used as background value for metals because no such data was available for the study area. Five contamination categories were determined (Sutherland 2000): EF <2 depletion to minimal enrichment, 2 ≤ EF < 5 moderate enrichment, 5 ≤ EF < 20 significant enrichment, 20 ≤ EF < 40 very high enrichment, and EF ≥ 40 extremely high enrichment.
The EF values are given (Table 3). The EF of metals in the sediments around Rize harbor was: Cu > Zn > Cd > Pb > As > Ni > Hg. Significant enrichment of sediment with Cu was found 57.1 % of stations, while 42.9 % of stations were classified as moderate enrichment. The EF of Pb was classified as moderate enrichment at all stations. Significant enrichment of sediments with Zn was found at 42.9 % of sites, while four sites (57.1 % of the total sites), were classified as moderate enrichment. For Cd EF, 14.3 % of total sites were classified as being significant enrichment; three stations were classified as being moderate enrichment and three stations were classified as being depletion to minimal enrichment. Moderate enrichment of sediment with As was found at 57.1 % of stations, whereas, three stations were classified as depletion to minimal enrichment. Rize harbor and coast of Rize is contaminated by Cu, Pb, Zn, As, and Cd as showed by enrichment factor greater than 2 (Table 3). Enrichment factor of Ni, and Hg, were classified as depletion to minimal enrichment at 100 % stations, suggesting that Ni and Hg contamination are not a problem these days. Enrichment factor values tended to be greatest in R1 station. Copper and zinc mines, belonging Inmet Mining Corporation Çayeli/Rize, are transported to other countries from this harbor. Therefore, waste waters of the harbor can be a high amount of metals. Hence, It can be said that high amount of contamination caused by waste water around Rize harbor.
Factor analysis used varimax with Kaiser Normalization rotation method was performed on Rize harbor and coast of Rize surface sediment using analyzed elements to identify the major sources of metals in study area. In this analysis, two components were extracted which explained about 86.4 % of the total variance, with the first component accounting for 59.0 %, second component for 27.4 %. The first component includes Cu, Zn, Pb, As, Cd, and Hg. All the elements in first component may represent anthropogenic sources, because of waste waters of mining have a high amount of metals (Berkun 2005). First factor’s elements (Cd, Pb, and Zn) have been associated with motor vehicle emissions (Huang et al. 1994). Moreover, Cu, Pb, Zn are probably associated discharge of sewage, industrial inputs, and surface runoff in the urban environment (Tang et al. 2008). The second component, including Ni and Al may represent natural sources of the sediments because, these elements (Ni, and Al) are most often found in crustal components (Salomons and Förstner 1984).
In order to determine the possible associations between metals, Pearson correlation was performed among metals concentrations in sediments of study area. The elements having high degrees of correlations (0.80 ≤ r ≤ 0.99) were Cu, Pb, Cd, Zn, and Hg which indicates that they have similar anthropogenic sources. Nickel and Al may be the same potential contamination source and the correlation coefficient is 0.764.
In this study, metal values in sediments were evaluated by comparing to numerical SQGs which is known the effect range low (ERL) and effect range median (ERM), the threshold effect level (TEL) and probable effect level (PEL) values (Long et al. 1998; Long et al. 1995). Low-range values (i.e., ERLs or TELs) are concentrations less than which adverse effects upon sediment dwelling fauna would not be expected. In contrast, the ERMs and PELs represent chemical concentrations above which adverse effects are likely to occur (Long et al. 1998). The incidence of toxicity was determined among samples in which none of the substances equaled or exceeded the ERL concentrations, in which one or increasing numbers of substances exceeded ERL concentrations, but none exceeded any ERM; and in which one or increasing numbers of substances exceeded ERM concentrations (Pekey et al. 2004; Zheng et al. 2008). The same approach was used to evaluate the predictive ability of the TELs/PELs (Long et al. 1998). The U.S. National Oceanic and Atmospheric Administration (NOAA) guidelines provide two values for each chemical, classifying the sediment either rarely (<ERL), occasionally (≥ERL and <ERM) or frequently (≥ERM) associated with adverse biological effects (Christophoridis et al. 2009).
Mercury concentrations in all stations were below TEL values, while Cu in sediments was above TEL at 100 % stations (Table 4). Copper was above PEL value at R1 stations. Concentrations of Zn was below TEL value at 28.6 % of stations, while Zn was above TEL value at 5 stations and also Zn was above PEL value at R1 station (Table 4). Cadmium and Pb concentrations were above TEL value at R1 station, while Cd and Pb were below TEL value at the other stations. Concentrations of As and Ni were below TEL value at 42.9 % stations, the elements concentrations were above TEL value at 4 stations (Table 4).
The SQGs values for metals and a classification of sediments based on these guidelines are shown (Table 4). According to SQG, the results of sediments were classified that Hg, Pb, and Ni in sediments would rarely be expected to cause adverse effects on biota. Seventy-one percent, 57.1 %, 42.9 %, 14.3 % of sediments would be expected to occasionally be associated with the toxic adverse effects on aquatic organism because of Cu, Zn, As, and Cd. Only a small percentage (14.3 %) of sediments would be expected to frequently be associated with the toxic effect on biota due to Cu concentration.
In addition, potential acute toxicity of contaminants in sediment samples can be estimated as the sum of the toxic units (∑TUs), defined as the ratio of the determined concentration to PEL value (Pedersen et al. 1998). The sum of toxic units levels for metals (Cu, Zn, Pb, Cd, As, Hg, and Ni) in surface sediments of the sampling stations are given in Fig. 2. Metal toxic units in sampling areas decrease in the order R1 > R3 > R2 > R5 > R4 > R6 > R7. Copper and Zn are the major contributor to toxicity (46.2 %–74 %).
References
Adamo P, Arienzo M, Imperato M, Naimo D, Nardi G, Stanzione D (2005) Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere 61:800–809
Berkun M (2005) Submarine tailings placement by a copper mine in the deep anoxic zone of the Black Sea. Water Res 39:5005–5016
Christophoridis C, Dedepsidis D, Fytianos K (2009) Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. J Hazard Mater 168:1082–1091
Csuros M, Csuros C (2002) Environmental sampling and analysis for metals. Lewis, Boca Raton
Cukrov N, Frančišković BS, Hlača B, Barišić D (2011) A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Mar Pollut Bull 62(1):154–167
Denton GRW, Concepcion LP, Wood HR, Morrison RJ (2005) Trace metals in sediments of four harbours in Guam. Mar Pollut Bull 50:1133–1141
Fındık Ö, Turan M (2012) Metal concentrations in surface sediments of Beyler reservoir (Turkey). Bull Environ Contam Toxicol 88:193–197
Folk RL (1954) The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J Geol 62:344–359
Förstner U, Wittmann GTW (1983) Metal pollution in the aquatic environment, 2nd edn. Springer, Berlin
Garcia OJ, Cañas L, Masqué P, Obrador B, Olid C, Pretus J (2011) Chronological reconstruction of metal contamination in the Port of Maó (Minorca, Spain). Mar Pollut Bull 62:1632–1640
Gaudette HE, Flight WR, Toner L, Folger DW (1974) Inexpensive titration method for determination of organic carbon in recent sediments. J Sediment Petrol 44:249–253
Huang XD, Olmez I, Aras NK, Gordon GE (1994) Emissions of trace-elements from motor-vehicles: potential marker elements and source composition profile. Atmos Environ 28:1385–1391
Huerta DMA, Delgadillo HF, Hernandez AM, Segovia ZJA, Garcia EZ, Lopez ZH et al (2008) Diagnosis of trace metal contamination in sediments: the example of Ensenada and El Sauzal, two harbors in Baja California, Mexico. Mar Environ Res 66:345–358
IMC (2009) http://www.inmetmining.com/Theme/Inmet/files/pdf/2009%20Sustainability%20Report_Turkish_Cayeli.pdf. Accessed 20 Jun 2011
Karahalil B, Ulukaya M, Alp O (2012) Pilot study of environmental monitoring of konya region near abandoned mercury mine in Turkey. Bull Environ Contam Toxicol 88:150–153
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97
Long ER, Field LJ, MacDonald DD (1998) Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ Toxicol Chem 17:714–727
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55:431–442
Muniz P, Danulat E, Yannicelli B, Garcıa AJ, Medina BG, Bicego MC (2004) Assessment of contamination by heavy metals and petroleum hydrocarbons in sediments of Montevideo Harbour (Uruguay). Environ Int 29:1019–1028
Owen RB, Sandhu N (2000) Heavy metal accumulation and anthropogenic impacts on Tolo Harbour, Hong Kong. Mar Pollut Bull 40:174–180
Pedersen F, Bjørnestad E, Andersen HV, Kjølholt J, Poll C (1998) Characterization of sediments from Copenhagen Harbour by use of biotests. Water Sci Technol 37:233–240
Pekey H, Karakas D, Ayberk S, Tolun L, Bakoglu M (2004) Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Mar Pollut Bull 48:946–953
Radojevic M, Bashkin VN (2006) Practical environmental analysis, 2nd edn. Royal Society of Chemistry, Cambridge
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, Berlin
Selvaraj K, Mohan VR, Szefer P (2004) Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Mar Pollut Bull 49:174–185
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geo 39:611–627
Tang CWY, Ip CCM, Zhang G, Shin PKS, Qian PY, Li XD (2008) The spatial and temporal distribution of heavy metals in sediments of Victoria Harbour, Hong Kong. Mar Pollut Bull 57:816–825
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earths crust. Geol Soc Am Bull 72:175–191
Zheng N, Wang QC, Liang ZZ, Zheng DM (2008) Characterization of heavy metal concentrations in the sediments of three freshwater rivers in Huludao City, Northeast China. Environ Pollut 154:135–142
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gedik, K., Boran, M. Assessment of Metal Accumulation and Ecological Risk Around Rize Harbor, Turkey (Southeast Black Sea) Affected by Copper Ore Loading Operations by Using Different Sediment Indexes. Bull Environ Contam Toxicol 90, 176–181 (2013). https://doi.org/10.1007/s00128-012-0894-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0894-2