Abstract
T-2 toxin is a member of a class of mycotoxins produced by a variety of Fusarium species under appropriate temperature and humidity conditions and is a common contaminant in food and feedstuffs of cereal origin. Selenium is an indispensable element in animals, regulates a variety of biological functions of the body, and can antagonize metal and mycotoxin poisoning to a certain extent. However, the effect of selenium on kidney injury induced by T-2 toxin has not been reported. In this study, 50 New Zealand rabbits were divided into 5 groups (the control group, T-2 toxin group, low-dose Se + T-2 toxin group, medium-dose Se + T-2 toxin group, and high-dose Se + T-2 toxin group). Rabbits were examined after oral administration of different doses of selenomethionine (SeMet) for 21 days and after perfusion with 0.4 mg/kg T-2 toxin (or the same dose of olive oil in the control group) for 5 days. We found that T-2 toxin induced kidney function damage and increased the levels of ROS and the contents of inflammatory factors. Renal structure was pathologically damaged. However, we found that after pretreatment with 0.2 mg/kg SeMet, oxidative stress, the inflammatory response, and pathological damage induced by T-2 toxin were attenuated. The results indicate that a low dose (0.2 mg/kg) of SeMet effectively reversed T-2 toxin–induced kidney injury in rabbits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are highly diverse secondary metabolites produced in nature by a wide variety of fungi, and they cause food contamination, resulting in mycotoxicosis in animals and humans. Crops are susceptible to mycotoxins during growth, storage, processing, and transportation [1]. Thus, these toxins enter the food chain by contaminating crops, cause serious harm to human and animal health, and are listed by the World Health Organization (WHO) as important sources of food-borne diseases [2]. Due to their widespread presence in the environment, mycotoxins cause both acute and chronic poisoning. One of the dangerous consequences of long-term or intense exposure to these secondary fungal metabolites is kidney and liver damage resulting in the failure of these organs [3]. The genera of mycotoxin-producing fungi are Aspergillus, Fusarium, Penicillium, Phomopsis, Emericella, and so on [4]. Fusarium toxin and Aspergillus toxin are among the most important agricultural mycotoxins that present a potential hazard to health worldwide. In particular, trichothecene mycotoxins produced by the genus Fusarium is agriculturally important worldwide due to their potential health hazards.
T-2 toxin, a trichothecene, is a sesquiterpene compound that is produced by Fusarium and lives on crops in nature under certain conditions. The toxin is commonly found in crops such as corn, wheat, rice, and oats. In China, the detection rate of T-2 toxin in cereals is as high as 80%, and the content can be as high as 735 ng/g [5]. Chemically, T-2 toxin is insoluble in water but soluble in acetone, ethylacetate, chloroform, ethanol, methanol, and propylene glycol. Chemical properties of T-2 toxin are very stable, mainly due to the fact that epoxy groups are not susceptible to attack by nucleophiles. Hence, if the epoxy group is cracked, the T-2 toxicity will also be lost. Moreover, the double bond in the left six-membered ring can be reduced, and the toxicity of the T-2 toxin will be 75~80% [6]. T-2 toxin produces HT-2 toxin or corresponding alcohols after treatment with alkaline reagents, but can not destroy their epoxy groups. The toxicity decreases only when the active parts of the structure are destroyed (482 °C for 10 min or 260 °C for 30 min). T-2 toxin can cause organ and tissue damage through various routes, including via the mouth, the skin, inhalation, and injection. T-2 toxin is known to mainly harm the immune system, digestive system, nervous system, and reproductive system, and it inhibits animal growth and development, and damages multiple target organs, including the liver, spleen, thymus, testis, kidney, stomach, and skin. Because of its thiol group, the main mechanism of T-2 toxin is inhibition of DNA and protein synthesis. In addition, numerous studies have reported that oxidative stress is also an important underlying mechanism of the toxicity of T-2 toxin and that the toxin stimulates the oxidative stress response in organs and helps with free radical generation. Trichothecenes significantly increase the levels of reactive oxygen species (ROS) and deplete intracellular reduced glutathione (GSH) levels. Moreover, they increase lipid peroxidation, leading to single-stranded DNA breaks. A study reported that T-2 toxin significantly increases ROS levels and malondialdehyde (MDA) levels in a dose-dependent manner in primary cultures of chicken tibial growth plate chondrocytes [7]. In addition, T-2 toxin regulates the levels of inflammatory factors in some immune cells and in vivo, or indirectly activates inflammatory pathways to regulate the inflammatory response. It causes an increase in the inflammatory factors IL1-α, IL-1β, IL-6, and TNFα. For example, T-2 toxin can alter blood-brain barrier (BBB) permeability, activate oxidative stress, promote MMP-9 gene expression, and increase pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, and TNF-α) in the brain and spleen [8]. The mechanisms underlying the toxicity of T-2 toxin are diverse and are not yet fully understood; therefore, it is necessary to explore its mechanisms and identify an effective therapeutic agent.
Selenium is an important trace element in humans and animals. It plays an important role in the metabolic process in vivo and plays a role in mammalian development and immune function enhancement. It is an important antioxidant with excellent antioxidant function. It is the main component of glutathione peroxidase (GSH-Px). It catalyzes GSH into GSSG, reducing toxic peroxides to nontoxic hydroxy compounds to protect cell membrane structure and function from peroxide-induced damage [9]. Selenium also reduces the toxicity of mycotoxins. Dvorska et al. [10] found that organic selenium can protect the chicken liver, antagonize oxidative damage and lipid peroxidation, and reduce organ damage caused by T-2 toxin. Recently, we found that selenium can protect against T-2 toxin–induced injury in poultry and pigs, but its effects in rabbits have not been reported. In this study, rabbits were pretreated with organic selenium and then perfused with T-2 toxin to induce acute poisoning. Biochemical and antioxidation indicators, pathological changes, inflammatory factor levels, and other indicators were used to analyze rabbit kidney poisoning and recovery, and to explore the protective effect of selenium against T-2 toxin–induced oxidative stress and inflammation in the rabbit kidney.
Materials and Methods
Animals
Fifty male New Zealand white rabbits (30 days of age) were obtained from the Experimental Center of Animal Science and Technology College of Henan University of Science and Technology (Henan, China). The animals were housed at 20–26 °C and 50–60% humidity, under a 12-h light/dark cycle, and given free access to water and food. The experiments were designed to minimize animal suffering and reduce the number of experimental animals used. All animal care and experimental protocols were conducted in accordance with university policies on the use and care of animals and were approved by the Institutional Animal Experiment Committee of Henan University of Science and Technology.
Experimental Design
T-2 toxin (MSS1023; Pribolab, Singapore) was dissolved in olive oil to prepare solutions of different concentrations and selenomethionine (SeMet; A601194-0100; Sangon Biotech Co., Ltd. Shanghai, China) was mixed into basic food at different concentrations. Based on a previous study, we used a T-2 toxin dose of 0.4 mg/kg, and 3 different doses of SeMet were used [11, 12]. The animals were randomized into 5 groups (n = 10/group): the control group; the T-2 toxin–treated group (0.4 mg/kg.bw); and the low (0.2 mg/kg)-, medium (0.4 mg/kg)-, and high (0.6 mg/kg)-dose SeMet + T-2 toxin (0.4 mg/kg bw) groups. Rabbits were fed diets containing different concentrations of SeMet for 21 days. On the 17th day, the rabbits in the treatment group were perfused with 0.4 mg/kg bw T-2 toxin for 5 consecutive days, and those in the control group were perfused with or the same volume of olive oil for the same period. On the 5th day of gavage, blood samples were collected by cardiac puncture and all rabbits were euthanized by administration of chloral hydrate. Kidney tissue samples were quickly removed and collected after overnight fasting for 15 h. Urine was collected after euthanasia by applying gentle pressure over the bladder and was stored at 4 °C. The plasma was separated by centrifugation and stored at − 80 °C. Kidney tissues were divided into three parts: one part was fixed in 4% paraformaldehyde solution, one part was preserved at − 80 °C, and one part was immediately used to prepare frozen sections.
Biochemical Analysis
Assay kits for measuring plasma urea nitrogen (UREA) and creatinine (Crea) were purchased from Nanjing Jiancheng Biotech (C011-2-1 and C013-2-1; Nanjing, China). The plasma samples were tested and analyzed according to the instructions of the assay kits. Urine was used for proteinuria analysis using proteinuria analysis kit (C035-2-1; Nanjing Jiancheng Biotech, Nanjing, China).
Histopathological Analysis
Kidney samples fixed in 4% paraformaldehyde solution were dehydrated in a graded series of ethanol solutions for paraffin embedding. Five-micrometer sections of the cortical and medullary zones were prepared and stained with hematoxylin & eosin (H&E) and periodic acid-Schiff (PAS). H&E- and PAS-stained sections were examined under a light microscope (Olympus, Tokyo, Japan) and were analyzed by CaseViewer 2.1 (3DHISTECH, Budapest, Hungary).
Kidney Antioxidant Capacity Test
Three hundred milligrams of kidney tissue was mixed with 2.7 ml of normal saline (tissue: normal saline = 1: 9) for 10 min, the homogenate was centrifuged at 3000 rpm/min for 15 min, and the supernatant was taken for measurement. According to the instructions of the BCA Protein Quantification Kit (CW0014; CoWin Biosciences Co., Ltd. Beijing, China), the protein concentration of the renal supernatant was determined. The renal supernatants were analyzed by using MDA, SOD, GSH-PX, and T-AOC analysis kits (A003-1-2, A001-3-2, A005-1-2 and A015-2-1; Nanjing Jiancheng Biotech, Nanjing, Jiangsu, China).
Kidney tissue samples were embedded in OTC embedding agent (4583; SAKURA, Japan) and cut into 10-μm sections with a freezing microtome. The 10-μm sections were washed three times with 0.1 M phosphate buffer. The kidney sections were incubated with 5 μM DHE (309,800; Sigma-Aldrich, Germany) in the dark for 20 min. Cell nuclei were stained with 5 μM DAPI (10,236,276,001; Sigma-Aldrich, Germany) in the dark for 20 min. Stained sections were examined and imaged with a confocal microscope (Leica, Germany). Fluorescence was detected with 510- to 560-nm excitation and 590-nm emission filters. ImageJ was used for quantification of the red emission signal.
Enzyme-Linked Immunosorbent Assay
The kidney tissue levels of the cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) were determined by ELISA. Rabbit serum samples (50 μL) were added to wells and analyzed using rabbit ELISA kits (RB70016, RB70006, RB70001; Myhalic Biotechnological Co., Ltd. Wuhan, China) according to the manufacturer’s instructions. The results were measured at 450 nm using a microplate reader (Thermo Fisher Scientific Inc., USA).
Total RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNA was extracted from the collected kidney tissues using TRIzol reagent (CW0580; CoWin Biosciences Co., Ltd. Beijing, China) and reverse transcribed into cDNA using a reverse transcription kit (RR036A; Takara, Japan). The primer sequences used are shown in Table 1. The 10-μl total reaction mixture contained 1 μl each of the forward and reverse primers (Sangon Biotech Co., Ltd. Shanghai, China), 1 μl of cDNA, 4 μl of SYBR Green quantitative polymerase chain reaction (PCR) mix (RR820A; Takara, Japan), and 2 μl of RNAse-free water. PCR was performed at 95 °C for 30 s, 60 °C for 30 s, and 95 °C for 5 min for 45 cycles on a StepOne Plus™ Real-Time PCR System (BIO-RAD, USA). The target gene expression levels were evaluated using the comparative 2−ΔΔCt method.
Statistical Analysis
The data were analyzed with SPSS 18.0 software (SPSS, Inc. USA). Each experiment was performed at least three times. All quantitative data are presented as the mean ± standard deviation (SD). Differences between the groups were compared by one-way analysis of variance (ANOVA) and Duncan’s multiple comparison tests. P < 0.05 was considered statistically significant.
Results
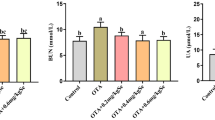
Selenomethionine Reduces T-2 Toxin–Induced Increases in Urea and Creatinine Levels in the Plasma and Protein Levels in the Urine
To evaluate the effect of SeMet on T-2 toxin–induced kidney function impairment, rabbit serum and urine were collected. Serum creatinine (Scr), urea (BUN), and protein levels in the urine were measured by using analysis kits. The results showed that the contents of Scr, BUN, and protein in the urine were significantly increased in the T-2 toxin–treated group compared with the control group (P < 0.01). The Scr, BUN, and urine protein concentrations were significantly decreased in the low-dose SeMet group compared with the T-2 toxin–treated group (P < 0.01) but were not significantly changed in the medium- and high-dose SeMet + T-2 toxin–treated groups (Fig. 1).
SeMet Reduces T-2 Toxin–Induced Kidney Histopathological Injury
The effect of SeMet on T-2 toxin–induced renal morphological changes was observed by H&E and PAS staining. Structural injuries were observed in the T-2 toxin–treated group by H&E staining, including luminal congestion, cytoplasmic vacuolization, intratubular cast formation, and the interstitial edema in the renal tubules. The accumulation of glycogen, as detected by PAS staining, was increased in the T-2 toxin–treated group compared with the control group. However, these renal structural changes and the extent of renal glycogen accumulation were significantly alleviated in the low-dose SeMet + T-2 toxin–treated group compared with the medium- and high-dose SeMet + T-2 toxin–treated groups (Fig. 2).
SeMet Reduces T-2 Toxin–Induced Oxidative Stress in the Kidney
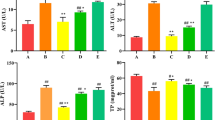
To investigate the oxidative stress level, improvements in enzymatic activity were used to indicate the antioxidant capacity of the kidney, and MDA was used as an indicator of lipid peroxidation in tissues. As shown in Fig. 3, compared with those in the control group, the levels of SOD, GSH-PX, and T-AOC in the T-2 toxin–treated group were markedly decreased, and the levels of MDA were increased (P < 0.01). The levels of SOD, GSH-PX, and T-AOC were significantly increased, and the level of MDA was decreased in the low-dose SeMet + T-2 toxin–treated group compared with the T-2 toxin–treated group, but there was no significant difference between the medium- and high-dose SeMet + T-2 toxin–treated groups and the T-2 toxin–treated group. No significant difference was found between the control group and the low-dose SeMet + T-2 toxin–treated group (Fig. 3).
The ROS levels in the kidney were assessed using the ROS fluorescent probe DHE. As shown in Fig. 4, the kidney ROS levels in the T-2 toxin–treated group were significantly higher than those in the control group (P < 0.01). The levels in the low-dose SeMet + T-2 toxin–treated group were significantly decreased compared with those in the T-2 toxin–treated group (P < 0.01). No significant difference was found between the medium- and high-dose SeMet + T-2 toxin–treated groups. Additionally, there was no significant difference between the control group and the low-dose SeMet + T-2 toxin–treated group (Fig. 4).
ROS levels in the rabbit kidney. (A) The control group. (B) The T-2-treated toxin group. (C) The low-dose SeMet + T-2 toxin–treated group. (D) The medium-dose SeMet + T-2 toxin–treated group. (E) The high-dose SeMet + T-2 toxin–treated group. (F) The ROS fluorescence intensity level. *P < 0.05, **P < 0.01 vs the control group; #P < 0.05, ##P < 0.01 vs the T-2 toxin–treated group. The scale bar represents 100 μm
SeMet Reduces T-2 Toxin–Induced Kidney Inflammation
To evaluate the effect of SeMet on T-2 toxin–induced kidney inflammation, the levels of the proinflammatory factors IL-1β, IL-6, and TNFα in the kidney were detected by qRT-PCR and ELISA. As shown in Fig. 5, the mRNA expression levels of IL-1β, IL-6, and TNF-α in the kidney in the T-2 toxin–treated group were significantly higher than those in the control group (P < 0.01). Compared with the T-2 toxin–treated group, the levels in the low-dose SeMet + T-2 toxin–treated group decreased significantly (P < 0.01). No significant difference was found between the medium- and high-dose SeMet + T-2 toxin–treated group. Additionally, no significant difference was found between the control group and the low-dose SeMet + T-2 toxin–treated group (Fig. 5).
Discussion
A variety of animals, including rabbits, have been proven to be susceptible to T-2 toxin, one of the most toxic trichothecenes. Studies have shown that after T-2 toxin is intramuscularly injected into rats and rabbits, its LD50 values are 0.85 and 1.10 mg/kg bw, respectively. Poisoning after a single injection of 0.5, 0.6, or 0.9 mg/kg of T-2 toxin into rats and rabbits is characterized by a decrease in serum alkaline phosphatase (ALP) activity [13]. Acute catarrhal gastrointestinal disease and intestinal mucosal lymphocyte necrosis are observed in rabbits after ingestion of T-2 toxin at a dose of 1–15 mg/kg bw [14]. However, in vivo studies on the toxic effects of T-2 toxin on the rabbit kidney have not been performed. The kidney is an important metabolic organ in humans and animals. It is involved in regulating acid-base balance, electrolyte concentration, extracellular fluid, blood pressure, and toxin clearance, and is an important organ for homeostatic maintenance. Clinically, creatinine and urea nitrogen are important indicators of renal function in serology. In addition, the urine protein test can also infer whether kidney function is normal. Previous studies have shown that T-2 toxin induces apoptosis of human kidney cells in vitro. It causes symptoms of hematuria in rats and induces damaging pathological changes in the kidneys. Studies have shown that poisoning by some mycotoxins increases the organ index of poultry kidneys, and researchers speculate that this may be due to swelling and necrosis of renal tubular epithelial cells. Therefore, we explored the protective effect of SeMet against the renal damage caused by T-2 toxin through serum biochemical analysis and detection of urine protein concentrations in this study. Compared with those in the control group, creatinine, urea nitrogen, and urinary protein concentrations in the T-2 toxin–treated group were significantly increased, which indicates that T-2 toxin causes impairment of kidney function in rabbits. Compared with those in the T-2 toxin–treated group, Scr, BUN, and urinary protein concentrations in the low-dose SeMet + T-2 toxin–treated group were significantly decreased and returned to the normal level. Additionally, no significant difference was found between the middle- and high-dose SeMet + T-2 toxin–treated groups. The above results indicate that 0.2 mg/kg SeMet may have protective effects against kidney damage caused by T-2 toxin but that the protective effect of SeMet may not be dose-dependent manner.
The maintenance redox homeostasis in the body mainly depends on the dynamic balance between the oxidation and the antioxidant system in the body [9]. When the body is stimulated, excessive ROS are produced, and the body’s antioxidant level is reduced. This causes oxidative stress in the body. Excessive ROS cause oxidative damage to cells, such as DNA damage and protein oxidation [15]. An increasing number of studies have proven that oxidative stress is an important mechanism underlying the toxicity of T-2 toxin. One of the toxic mechanisms of T-2 toxin is increased ROS levels in cells and tissues, which induces oxidative stress. Wu et al. found that this toxin causes cells to produce free radicals, including ROS, that cause lipid peroxidation, leading to changes in membrane integrity, cell redox signals, and cell antioxidant status [16]. Tian et al. found that T-2 toxin disrupts mast chondrocyte homeostasis by activating ROS and then activates the NF-κB pathway to induce the expression of HIF-2α, leading to abnormal expression of target catabolism genes [17]. Our findings in this study are consistent with previous studies. T-2 toxin induces an increase in ROS, which may lead to oxidative stress in the kidney. To maintain oxidative balance, the body uses several antioxidants to remove oxidation products; thus, antioxidant enzymes play an important role in this process. SOD is a key enzyme that detoxifies free radicals in cells. It converts oxygen (O2) to hydrogen peroxide (H2O2), after which catalase (CAT) or glutathione peroxidase (GSH-PX) reduces H2O2 to H2O to eliminate the products of oxidative stress. GSH-PX is an important peroxide-decomposing enzyme in the body. It can reduce toxic peroxides to nontoxic hydroxyl compounds. Our research shows that in the kidneys of rabbits treated with 0.4 mg/kg T-2 toxin, the levels of T-AOC, SOD, and GSH-PX are significantly decreased, and MDA levels are increased. This indicates that T-2 toxin causes oxidative damage in the kidneys of rabbits and weakens the antioxidant capacity of the kidneys. The levels of SOD, GSH-PX, and T-AOC in the kidneys of rabbits in the low-dose SeMet + T-2 toxin–treated group were significantly increased compared with those in kidneys of rabbits in the T-2 toxin–treated group, and the MDA levels were decreased. There was no significant difference between the other two groups. This indicates that a low dose (0.2 mg/kg) of SeMet effectively reverses T-2 toxin–induced oxidative damage in the rabbit kidney. This may be attributed to the strong antioxidant effects of selenium. Selenium is a component of several key antioxidants, and the unique redox properties of selenocysteine play an important role in protection against antioxidant stress [18]. Selenocysteine can reduce hydroperoxide (Rooh) and peroxynitrite (ONOO) to selenic acid (R-Seoh) so that Rooh and ONOO are reduced to alcohol and nitrite, respectively. Several low molecular weight selenium compounds (such as SeMet) have the same antioxidant properties as selenocysteine. In addition, selenium is one of the important constituents of GSH-PX, and its active center is selenocysteine [19, 20]. Therefore, selenium may protect against renal oxidative damage induced by T-2 toxin through the above mechanisms, but the specific mechanism requires further study.
A large number of studies have shown that T-2 toxin causes structural changes and pathological damage to a variety of cells, tissues, and organs, and stimulates inflammatory reactions. Li et al. [21] found that treatment of rat chondrocytes with low concentrations of T-2 toxin causes cytoplasmic condensation, nuclear chromatin plaques, nuclear condensation, and nuclear membrane thickening. In addition, as the concentration of T-2 toxin increases, the amount of intracellular rough endoplasmic reticulum increases, and mitochondrial vacuole degeneration occurs. Previous studies have shown that T-2 toxin can induce inflammatory reactions in mice. Pathological observation of the intestinal tissues of mice has shown that the intestinal wall of the colon becomes thinner and congested that inflammatory cells infiltrate the ileum [22]. Some studies have found that in the kidney, T-2 toxin induces apoptosis of human renal cells. Necrosis of proximal tubule epithelial cells, nuclear sequestration, a decrease in the number of organelles, and significant thickening of the basement membrane have also been found in the kidneys of rabbits treated with T-2 toxin.
In our study, we found that low-dose SeMet alleviated T-2 toxin–induced renal structural damage, including intracavitary congestion, cytoplasmic vacuolar changes, and tubular interstitial edema. T-2 toxin may cause morphological structural changes and increase the mRNA levels of several inflammatory factors in tissues to activate the inflammatory response [23]. T-2 toxin can induce an increase in interleukin 1-β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α) in various cells and tissues. Studies have shown that T-2 toxin can induce the expression of IL-1β and TNF-α in mouse macrophages and activate the inflammatory response [24]. T-2 toxin can also alter the permeability of the blood-brain barrier by activating the transcriptional activities of matrix metalloproteinase 9 (MMP 9), IL-1β, IL-6, and TNF-α in the mouse brain [25]. Our study is consistent with previous studies; we found that T-2 toxin induced an inflammatory response in the kidney and that low-dose SeMet effectively reversed the levels of inflammatory factors (IL-1β, IL-6, and TNF-α) in the kidney. Selenium regulates immune inflammation in many ways. On the one hand, selenium can regulate the immune mechanism by regulating antioxidant enzymes. Recent studies have suggested that under certain conditions, oxidized GPX-Px can promote the formation of disulfide bonds in regulatory proteins. As a whole, this complex mechanism regulates the activation or inactivation of important signaling proteins involved in the immune response, such as protein tyrosine phosphatases (PTPs). In addition, TrxR mediates the reduction of disulfide bonds in T cells through Trx. GSH-Px and TrxR play complementary roles. Their balance is a key factor in regulating the immune response. A lack of selenium or these antioxidants in immune cells can lead to the production of inflammatory factors and inflammatory reactions. On the other hand, selenoprotein S also acts to mediate immune responses and reduce endoplasmic reticulum stress in peripheral macrophages. In addition, selenium can regulate the activity of the eicosanoid synthesis pathway, leading to the synthesis of leukotriene and prostacyclin, and downregulation of the expression of cytokines and adhesion molecules [26]. These aspects may be related to the anti-inflammatory effects of selenium.
In addition, the absorption and action of inorganic selenium and organic selenium in animals are not exactly the same. In animals, SeMet is absorbed in the small intestine through the sodium-dependent neutral amino acid transport system [27]. However, inorganic selenium is absorbed through simple diffusion absorption, and its absorption efficiency is relatively low. More importantly, SeMet can be degraded into selenocysteine through the transsulfur pathway after entering the body, resulting in the promotion of selenoprotein synthesis through biological activity by the donor, or cleared through liver or kidney metabolism. Studies have reported that the degradation rate of selenide methionine is faster than that of inorganic selenium [28]. This suggests that SeMet has less bioavailability and toxicity.
In summary, 0.2 mg/kg SeMet potentially protects against oxidative damage and inflammatory reactions induced by T-2 toxin in the rabbit kidney, and this study provides a new strategy for protecting against T-2 toxin–induced renal injury (Fig. 6).
Data Availability
This published paper includes all data generated or analyzed during this work.
Abbreviations
- WHO:
-
World Health Organization
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- BBB:
-
Blood-brain barrier
- GSH-Px:
-
Glutathione peroxidase
- UREA:
-
Urea nitrogen
- Crea:
-
Creatinine
- H&E:
-
Hematoxylin & eosin
- PAS:
-
Periodic acid-Schiff
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-α
- SeMet:
-
Selenomethionine
References
Escriva L, Font G, Manyes L (2015) In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem Toxicol 78:185–206. https://doi.org/10.1016/j.fct.2015.02.005
Organization WHJWHOTR (2002) Evaluation of certain food additives. Seventy-first report of the Joint FAO/WHO Expert Committee on Food Additives 891 (956):1–80
Makowska K, Obremski K, Gonkowski S (2018) The impact of T-2 toxin on vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nerve structures in the wall of the porcine stomach and duodenum. Toxins (Basel) 10(4). https://doi.org/10.3390/toxins10040138
Manish Adhikari AA-K, Negi B, Kaushik N, Kaushik NK, Adhikari A, Choi EH T-2 mycotoxin: toxicological effects and decontamination strategies. Oncotarget 8:33933–33952
Wu J, Yang C, Liu J, Chen J, Huang C, Wang J, Liang Z, Wen L, Yi JE, Yuan Z (2019) Betulinic acid attenuates T-2-toxin-induced testis oxidative damage through regulation of the JAK2/STAT3 signaling pathway in mice. Biomolecules 9(12). https://doi.org/10.3390/biom9120787
Adhikari M, Negi B, Kaushik N, Adhikari A, Al-Khedhairy AA, Kaushik NK, Choi EH (2017) T-2 mycotoxin: toxicological effects and decontamination strategies. Oncotarget 8(20):33933–33952. https://doi.org/10.18632/oncotarget.15422
He SJ, Hou JF, Dai YY, Zhou ZL, Deng YF (2012) N-acetyl-cysteine protects chicken growth plate chondrocytes from T-2 toxin-induced oxidative stress. J Appl Toxicol 32(12):980–985. https://doi.org/10.1002/jat.1697
Ravindran J, Agrawal M, Gupta N, Rao PV (2011) Alteration of blood brain barrier permeability by T-2 toxin: role of MMP-9 and inflammatory cytokines. Toxicology 280(1–2):44–52. https://doi.org/10.1016/j.tox.2010.11.006
Shanu A, Groebler L, Kim HB, Wood S, Weekley CM, Aitken JB, Harris HH, Witting PK (2013) Selenium inhibits renal oxidation and inflammation but not acute kidney injury in an animal model of rhabdomyolysis. Antioxid Redox Signal 18(7):756–769. https://doi.org/10.1089/ars.2012.4591
Dvorska JE, Pappas AC, Karadas F, Speake BK, Surai PF (2007) Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp Biochem Physiol C Toxicol Pharmacol 145(4):582–587. https://doi.org/10.1016/j.cbpc.2007.02.005
Guerre P, Eeckhoutte C, Burgat V, Galtier P (2000) The effects of T-2 toxin exposure on liver drug metabolizing enzymes in rabbit. Food Addit Contam 17(12):1019–1026. https://doi.org/10.1080/02652030050207819
Qu J, Wang W, Zhang Q, Li S (2020) Inhibition of lipopolysaccharide-induced inflammation of chicken liver tissue by selenomethionine via TLR4-NF-kappa B-NLRP3 signaling pathway. Biol Trace Elem Res 195(1):205–214. https://doi.org/10.1007/s12011-019-01841-0
Chan PK, Gentry PAJT, Pharmacology A (1984) LD50 values and serum biochemical changes induced by. T-2 toxin in rats and rabbits 73(3):402–410
Glávits R, Ványi A, Fekete S, Tamás JJAVH (1989) Acute toxicological experiment of. T-2 toxin in rabbits 37(1–2):75–79
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Doi K, Uetsuka K (2011) Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 12(8):5213–5237. https://doi.org/10.3390/ijms12085213
Tian J, Yan J, Wang W, Zhong N, Tian L, Sun J, Min Z, Ma J, Lu SJ T-2 toxin enhances catabolic activity of hypertrophic chondrocytes through ROS-NF-kappa B-HIF-2 alpha pathway
Wu Q-H, Wang X, Yang W, Nüssler AK, Xiong L-Y, Kuča K, Dohnal V, Zhang X-J, Yuan Z-H (2014) Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch Toxicol 88(7):1309–1326. https://doi.org/10.1007/s00204-014-1280-0
Wang N, Tan H-Y, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxidative Med Cell Longev. https://doi.org/10.1155/2017/7478523
Brenneisen P, Steinbrenner H, Sies H Selenium, oxidative stress, and health aspects. 26 (4–5):0–267
Lin RQ, Sun Y, Ye WC, Zheng T, Wen JK, Deng YQ (2019) T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology 424:11. https://doi.org/10.1016/j.tox.2019.06.001
Li Y, Zou N, Wang J, Wang KW, Li FY, Chen FX, Sun BY, Sun DJ (2017) TGF-beta1/Smad3 signaling pathway mediates T-2 toxin-induced decrease of type II collagen in cultured rat chondrocytes. Toxins (Basel) 9 (11). doi:https://doi.org/10.3390/toxins9110359
Lin R, Sun Y, Ye W, Zheng T, Wen J, Deng Y (2019) T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology 424:152230. https://doi.org/10.1016/j.tox.2019.06.001
Wang X, Liu Q, Ihsan A, Huang L, Dai M, Hao H, Cheng G, Liu Z, Wang Y, Yuan ZJ JAK/STAT Pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 Toxin. 127 (2):412–424
Ravindran J, Agrawal M, Gupta N, Rao PVLJT (2011) Alteration of blood brain barrier permeability by. T-2 toxin: Role of MMP-9 and inflammatory cytokines 280(1–2):44–52
Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, Hermann MJ Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case–control study in women undergoing thyroidectomy for benign goiter. 21 (2):161–167
Schrauzer GN (2003) The nutritional significance, metabolism and toxicology of selenomethionine. Adv Food Nutr Res 47:73–112. https://doi.org/10.1016/s1043-4526(03)47002-2
Patterson BH, Levander OA, Helzlsouer K, Mcadam PA, Lewis SA, Taylor PR, Veillon C, Zech LA (1989) Human selenite metabolism: a kinetic model. Am J Physiol 257(2):556–567
Acknowledgments
We thank the American Journal Experts (AJE) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Henan Provincial Key Research and Development and Promotion Project (192102110077 and 202102110093), the Key Research Project of Henan Province Colleges and Universities (19B230005), the Key Research Project of Henan Province Colleges and Universities (19B230005), and the Young Backbone Teachers Assistance Scheme of Henan Province Colleges and Universities (2019GGJS080).
Author information
Authors and Affiliations
Contributions
Yumei Liu, Ruiqi Dong, and Ziqiang Zhang conceived and designed the study. Yuxiang Yang, Hui Xie, Yufeng Huang, Xiaoguang Chen, and Dongmei Wang performed the experiments. This paper was written by Yumei Liu and Ruiqi Dong. The manuscript was reviewed and edited by Ziqiang Zhang. All authors have read and approved this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All animal care and experimental protocols were conducted according to the University Policies on the Use and Care of Animals and were approved by the Institutional Animal Experiment Committee of Henan University of Science and Technology, China.
Consent to Participate
Written informed consent for publication was obtained from all participants.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Dong, R., Yang, Y. et al. Protective Effect of Organic Selenium on Oxidative Damage and Inflammatory Reaction of Rabbit Kidney Induced by T-2 Toxin. Biol Trace Elem Res 199, 1833–1842 (2021). https://doi.org/10.1007/s12011-020-02279-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02279-5