Abstract
T-2 toxin is produced by the Fusarium genus. Ingestion of food or feed contaminated by T-2 toxin will cause damage to kidney. Selenium (Se), an essential trace element, showed the significant protective effects against kidney and renal cell damage induced by toxic substances. To explore the protective effects and mechanisms of Se against T-2-induced renal lesions, forty-eight male Kunming mice were exposed to T-2 toxin (1.0 mg/kg) and/or Se (0.2 mg/kg) for 28 days. In this study, we found that Se alleviated T-2-induced nephrotoxicity, presenting as increasing the body weight and kidney coefficient, relieving the renal structure injury, decreasing the contents of renal function-related biomarkers, decreasing the levels of reactive oxygen species (ROS), and increasing the mitochondrial membrane potential in T-2 toxin-treated mice. In addition, inhibition of renal cell apoptosis by Se was associated with blocking the mitochondrial pathway in T-2 toxin-treated mice, presenting as decreasing the protein expression of cytochrome-c, activities of caspase-3/9, as well as regulating the protein and mRNA expressions of Bax and Bcl-2. These results documented that the alleviating effect of Se on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated renal apoptosis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxin contamination is a worldwide food safety problem. According to the Food and Agriculture Organization of the United Nations (FAO), about 25% of the world’s grains and 1 billion metric tons of food may be contaminated by mycotoxins, causing serious economic losses [1]. As a type A trichothecene mycotoxin, T-2 toxin is one of the most harmful toxins to the health of humans and animals [2]. T-2 toxin can be transmitted through food chain and is a risk factor for human fatal alimentary tract toxic Aleutian and Kashin Beck disease [3]. The kidney is one of the target organs of T-2 toxin [4,5,6,7,8]. The toxic effect of T-2 toxin is associated with the production of reactive oxygen species (ROS). T-2 toxin can cause excessive ROS production and disturb the cellular redox balance and result in kidney damage [9]. Mitochondria is the target of ROS, and the high level of ROS in mitochondria can attack the phospholipids in the cell membrane, then lead to the depolarization of mitochondrial membrane, cause caspases cascade, and finally trigger mitochondrial apoptosis [10]. Excessive apoptosis can destroy the structure of normal cells and lead to dysfunction [8, 11, 12]. T-2 toxin can induce the apoptosis in kidney and renal cell lines [8, 12, 13]. Thus, excessive ROS and apoptosis are the pathological basis of renal injury induced by T-2 toxin.
As a human body essential trace element, selenium (Se) is a functional component of several enzymes (glutathione peroxidases, selenoprotein P, and thioredoxin reductases) with physiological antioxidant properties [14], which can effectively remove oxygen free radicals, protect organs and tissues from oxidative damage and apoptosis [15]. Se can play a protective role in kidney and renal cell damage induced by various poisons by increasing the expression of selenase in organism, clearing ROS, and inhibiting cell apoptosis [10, 16,17,18]. However, there are few studies on Se alleviating the renal damage caused by T-2 toxin. The only study showed that feeding the diet containing 0.2 mg/kg organic Se for 21 days can significantly alleviate the oxidative stress, the inflammatory response, and pathological damage of rabbit kidney caused by 0.4 mg/kg T-2 toxin, which proves that Se has protective effect on oxidative damage and inflammation of rabbit kidney caused by T-2 toxin; meanwhile, the kidney injury was alleviated [9]. Moreover, inflammation can induce apoptosis, when inflammation occurs, the increase of pro-inflammatory cytokines will induce apoptosis and further aggravate the development of the disease [19,20,21,22]. While it is not clear whether Se can alleviate the apoptosis induced by T-2 toxin. This research aimed to explore whether the protective effect of Se on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated apoptosis. It is hoped to provide theoretical basis and new approaches for the prevention and treatment of T-2 nephrotoxicity.
Materials and Methods
Animals and Experimental Design
All mice were housed in the Biomedical Research Center, and the procedures were approved by the Animal Ethics Committee (Northeast Agricultural University, China). The house has a 22–24 °C temperature, 50–60% relative humidity, and a 12/12-h light/dark cycle. All animals were provided standard pellet diet (Xietong Organism, China) and distilled water ad libitum. The composition of the diet is shown in the text of the supplementary information TEXT S9.
After 7 days of acclimatization, forty-eight male Kunming mice (6 weeks old) were divided into four groups (n = 12): control group (CG, 0.0 mg/kg/day T-2 toxin and 0.0 mg/kg/day Se), T group (TG, 1.0 mg/kg/day T-2 toxin and 0.0 mg/kg/day Se), Se + T-2 group (STG, 1.0 mg/kg/day T-2 toxin and 0.2 mg/kg/day Se), and Se group (SG, 0.0 mg/kg/day T-2 toxin and 0.2 mg/kg/day Se). The STG were treated with Se at 0.2 mg/kg 2 h prior to T-2 toxin administration, and then given T-2 toxin at 1.0 mg/kg. T-2 toxin (≥ 99.8%, Pribolab, China) was dissolved in distilled water containing 4% v/v ethanol, Na2SeO3 (≥ 98%, Sigma, USA) in the STG was dissolved in distilled water, and SG was dissolved in distilled water containing 4% v/v ethanol [23]. The CG was gavage daily with distilled water containing 4% v/v ethanol. The mice were treated by oral gavage for 28 days and sacrificed on the 29th day [24]. To maintain a constant intake of T-2 toxin and Se, the body weight (BW) of mice was measured every day and adjusted the dose accordingly. The T-2 toxin dose in this study was in accordance with that of oral LD50 in mice (10.0 mg/kg BW) [25], and the supplementation of Se (Na2SeO3) is based on the Chinese Dietary Reference Intakes (DRIs) and non-toxic in rodents [26]. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals [27] and approved by the Animal Ethics Committee of Northeast Agricultural University, China. During the experimental period, the health status of mice was monitored daily and no animal died in all the treatment groups.
Sample Preparation
At the end of this study, the mice BW was recorded first. The mice were sacrificed after anesthetization by intraperitoneal injection with 1% pentobarbital (5 mL/kg) and tissue samples retrieved. Next, the serum was obtained from the whole blood (700 μL) by centrifugation at 3000 rpm for 10 min to detect the contents of biochemical indexes of renal function. Kidney tissues were excised immediately and washed in ice-cold saline. Parts of the kidneys were taken out for ultrastructure observation. The rest of kidneys were weighted and stored at − 80 °C for measuring other parameters.
The Histopathological Observation of Kidney
The histopathological lesions of kidney were observed according to the previous study [28]. The specific steps are shown in the text of the supplementary information TEXT S1.
The Ultrastructure Observation of Kidney
The ultrastructure of kidney was observed by transmission electron microscope (TEM) according to the previous study [29]. The specific steps are shown in the text of the supplementary information TEXT S2.
The Analysis of BUN, SCR, Cys-C, and β2-Mg Contents
The renal function biomarkers were detected by automatic blood analyzer (AU680 Beckman Coulter, Inc., USA) as markers of renal dysfunction.
Determination of Oxidative Stress Markers in the Kidney
The ROS and glutathione peroxidase (GSH-Px) of kidney were observed according to the research of Zhou et al. [10]. The specific steps are shown in the text of the supplementary information TEXT S3.
Detection of Kidney Apoptosis
The kidney apoptosis was observed by TUNEL staining according to the previous study [29]. Positive staining was detected by Image-Pro Plus 6.0 software. The specific steps are shown in the text of the supplementary information TEXT S4.
Determination of MMP in the Kidney
The MMP of kidney was observed according to the research of Yu et al. [30]. The specific steps are shown in the text of the supplementary information TEXT S5.
Measurement of Activities of Caspase-3 and Caspase-9 of Kidney
The activities of caspase-3 and caspase-9 observation were measured using kits (C1116, C1158, Beyotime, China). The specific steps are shown in the text of the supplementary information TEXT S6.
Determination of the Protein Expression in Kidney
Protein was extracted by kit, and the concentration was determined by BCA method (P0010S, Beyotime, China). The specific steps are shown in the text of the supplementary information TEXT S7.
Determination of Gene Expression in the Kidney
The mRNA expression of Bcl-2, Bax, and cytochrome-c (Cyt-c) were detected by qRT-PCR (Table 1). The specific steps are shown in the text of the supplementary information TEXT S8.
Statistical Analysis
All the data were presented as mean ± standard deviation (mean ± SD). The data were analyzed by one-way ANOVA and LSD test was used as the post hoc test (SPSS 22.0 software; SPSS Inc., Chicago, IL, USA). In all statistical comparisons, *P < 0.05 and **P < 0.01 symbols indicated significant differences compared with the CG. #P < 0.05 and ##P < 0.01 symbols indicated significant differences compared with the TG.
Results
Se Protects Against the T-2-Induced Reduction of BW and Kidney Coefficient
Compared to the CG, the BW and kidney coefficient were decreased in the TG (P < 0.05, P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.05). There were no significant difference between SG and CG (Fig. 1).
The effects of Se on BW and kidney coefficient from mice exposed to T-2 toxin. a BW. b Kidney coefficient. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 indicate the significance of differences versus the CG. #P < 0.05 and ##P < 0.01 indicate the significance of differences versus the TG. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
Se Alleviates T-2-Induced Renal Structural Damage
In H&E staining, the intact glomeruli and well-arranged, compact tubules were observed in the CG. The destruction of renal tubular structure and the detachment of renal tubular nuclei were observed in the TG. However, these changes were significantly alleviated in the STG as compared to the TG. In addition, treatment with Se along did not cause damage to renal structure, which was similar to the CG in morphology (Fig. 2).
The effects of Se on renal structural damage in kidney from mice exposed to T-2 toxin. H&E staining of kidney in mice (magnification, × 400). The “black arrow” showed the destruction of renal tubular structure and the detachment of renal tubular nuclei. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
Se Alleviates T-2-Induced Ultrastructure in Kidney
TEM examination indicated that normal ultrastructure of renal epithelial cells was found in the CG, with the normal mitochondria and intact cell membrane and nuclear membrane. Swelling of mitochondria, loss of mitochondrial cristae, and vacuolation of mitochondria were observed in the TG. However, these changes were significantly alleviated in the STG as compared to the TG. There were no significant difference between SG and CG (Fig. 3).
The effects of Se on renal ultrastructure and function damage in kidney from mice exposed to T-2 toxin. Representative photomicrographs of renal tubular cells ultrastructure (magnification, 8000×). The “black arrow” showed the mitochondria lesions. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
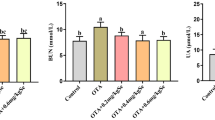
Se alleviates T-2-Induced Renal Dysfunction in Kidney
Compared to the CG, the contents of BUN, SCR, Cys-C, and β2-Mg were increased in the TG (P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.01). There were no significant difference between SG and CG (Fig. 4).
The effects of Se on renal dysfunction in kidney from mice exposed to T-2 toxin. a BUN. b SCR. c Cys-C. d β2-Mg. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 indicate the significance of differences versus the CG. #P < 0.05 and ##P < 0.01 indicate the significance of differences versus the TG. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
Se Alleviates T-2-Induced the Oxidative Stress in Kidney
The contents of ROS were increased in the TG compared to the CG (P < 0.05). The activity of GSH-Px was decreased in the TG compared to the CG (P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.05, P < 0.01). There were no significant difference between SG and CG (Fig. 5).
The effects of Se on ROS and GSH-Px in kidney from mice exposed to T-2 toxin. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 indicate the significance of differences versus the CG. #P < 0.05 and ##P < 0.01 indicate the significance of differences versus the TG. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
Se Alleviates T-2-Induced Apoptosis
TUNEL Staining Assay
The TUNEL-positive staining nuclei were not found in the CG. The percentage of TUNEL-positive nuclei significantly increased in the TG as compared to the CG (P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.01). There were no significant difference between SG and CG (Fig. 6a, b).
The effects of Se on apoptosis in kidney from mice exposed to T-2 toxin. a The percentage of TUNEL-positive cells. b TUNEL staining (magnification, × 400). c Representative photomicrographs of renal tubular cell nucleus ultrastructure (magnification, × 20,000). d Relative MMP level. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 indicate the significance of differences versus the CG. #P < 0.05 and ##P < 0.01 indicate the significance of differences versus the TG. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
TEM Observation of Apoptosis
The normal nuclear structure and chromatin distribution were found in the CG. The nuclear membrane shrunk, and chromatin condensed under the nuclear membrane was observed in the TG compared to the CG. However, these changes were significantly alleviated in the STG as compared to the TG. There were no significant difference between SG and CG (Fig. 6c).
Measurement of MMP
The MMP levels were decreased in the TG as compared to the CG (P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.05). There were no significant difference between SG and CG (Fig. 6d).
Se Alleviates T-2-Induced Apoptosis via Mitochondria Pathways in Kidney
Se Alleviates the T-2-Caused Induction of Activities of Caspase-3 and Caspase-9 in Kidney
T-2 toxin treatment significantly increased the activities of caspase-3 and caspase-9 (P < 0.01). However, these changes were significantly alleviated in the STG as compared to the TG (P < 0.01). There were no significant difference between SG and CG (Fig. 7a, b).
The effects of Se on mitochondrial apoptosis pathways in kidney from mice exposed to T-2 toxin. a, b The activities of caspase-3 and caspase-9. c–e The mRNA expressions of Bax, Bcl-2, and Cyt-c. f Representative image of Bax, Bcl-2, and Cyt-c. g–i The protein expressions of Cyt-c, Bax, and Bcl-2. All data were expressed as mean ± SD. *P < 0.05 and **P < 0.01 indicate the significance of differences versus the CG. #P < 0.05 and ##P < 0.01 indicate the significance of differences versus the TG. CG control group, TG T-2 group, STG Se + T-2 group, SG Se group
Effects of Se on mRNA and Protein Expressions of Bax, Bcl-2, and Cyt-c in Kidney
The mRNA and protein expressions of Bax were significantly increased in the TG as compared to the CG (P < 0.01), and the mRNA and protein expressions of Cyt-c were significantly increased in the TG and STG as compared to the CG (P < 0.05, P < 0.01), whereas they were markedly decreased in the STG as compared to the TG (P < 0.05, P < 0.01); there were no significant difference between SG and CG. The mRNA and protein expressions of Bcl-2 were significantly decreased in the TG and STG as compared to the CG (P < 0.05, P < 0.01), whereas it was markedly decreased in the STG as compared to the TG (P < 0.01); there were no significant difference between SG and CG (Fig. 7c–i).
Discussion
Numerous studies have reported that the T-2-induced toxicity was related to oxidative stress [10, 15]. Antioxidant therapy is considered to be an important way to interfere with T-2 toxicity. Se, well known as an essential trace element, has been reported to provide protection from ROS-induced cell damage [15, 31,32,33]. Se pretreatment can diminish ROS and cell apoptosis induced by various toxic substances [18, 32, 34]. In this study, we aim to clarify whether the protective effect of Se on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated apoptosis.
The kidney is essential for maintaining normal body function, which can regulate the liquid electrolyte and acid-base balance and create a stable environment for the metabolism of tissues and cells [35]. Thus, kidney injury is often related to renal dysfunction and increased risk of death. Se exerted the positive effect on BW reduction induced by streptozotocin in male Wistar rats [36], and kidney coefficient reduction induced by cadmium in ICR male mice [15]. We found Se remarkably ameliorated T-2 toxin induced reduction in the BW and kidney coefficient. Se positively affects feed utilization through participation in the metabolism of carbohydrates, lipids, and proteins [37], which play an important role in improving the BW and organ coefficient of mice. By histopathological examination, Se pretreatment diminished T-2-induced tubular lesions. To further evaluate the ameliorative effect of Se on T-2-induced structure lesion of kidney, the ultrastructure of kidney was observed by TEM, we found that pretreatment with Se markedly antagonized T-2-induced swelling of mitochondria, loss of mitochondrial cristae, and vacuolation of mitochondria. The possible reason is that Se is a component of many selenoproteins with redox function [38], which plays an important role in redox regulation, thus protecting the integrity of cell membrane [39], participating in the repair of damaged kidney tissue and protecting the structure of kidney [40]. These results indicated that Se can alleviate the damage of renal structure and the kidney development inhibition caused by T-2 toxin. BUN and SCR can be used as indicators of acute renal tubular lesions, and serum Cys-C and β2-Mg can be used as indicators of glomerular filtration function [28, 41]. Pretreatment with Se significantly diminished the renal dysfunction of streptozotocin-exposed rats and cadmium-exposed mice [15, 36]. We found that Se pretreatment can reduce the T-2-induced increase of renal function biomarkers, suggesting that Se can antagonize the renal dysfunction induced by T-2 toxin.
Apoptosis is called programmed cell death, which can remove defective cells from the body to ensure the health of multicellular organisms. However, excessive apoptosis will cause cell death and nephrotoxicity [29, 42, 43]. In our study, the results of TUNEL staining and the TEM observation proved that T-2 toxin induced the excessive apoptosis in the kidney, which is similar to the results of heavy metal-exposed mice and chicken [15, 16], while pretreatment with Se attenuated the apoptosis. ROS is mainly produced in mitochondria, and elevated ROS levels will destroy the normal structure of cells, leading to dysfunction. Moreover, ROS is closely related to mitochondrial membrane damage, which can lead to cell apoptosis [44]. Se is involved in the formation of GSH-Px enzyme, which plays an important role in the elimination of ROS [45]. Se attenuated the production of ROS and the decrease of activity of GSH-Px in ochratoxin A-treated porcine kidney epithelial cells and fluorine-treated broiler kidney [18, 32]. In this study, Se administration dramatically attenuated T-2-induced production of ROS and the decrease of activity of GSH-Px. Se plays an important role in human and animal, which neutralizes, eliminates, and blocks the synthesis of ROS, and Se could eliminate ROS through antioxidant GSH-Px [32, 46, 47]. Therefore, we speculate that ROS production may be involved in renal dysfunction and excessive apoptosis caused by T-2 toxin, while Se improves normal renal structure and function and prevents apoptosis by eliminating excessive ROS. Mitochondria are the major source of ROS production in cells, in turn, the most adversely affected organelles; excessive ROS can result in mitochondrial dysfunction [48, 49]. MMP plays a key role in mitochondrial homeostasis by selectively eliminating dysfunctional mitochondria. It is also the driving force for the transport of ions and proteins, which are essential for the healthy function of mitochondria. The continuous decrease or increase of MMP may lead to unnecessary loss of cell viability and various pathological changes [50]. Apoptosis is closely related to the stability of MMP, when MMP collapses, apoptosis is irreversible [51, 52]. The collapse of MMP can lead to apoptosis of normal cells through mitochondrial pathway [53]. We found pretreatment with Se ameliorated T-2-induced MMP collapse. A similar antagonistic effect of Se has been found in the NaF-treated rat kidney cells (NRK-52E) and cadmium-treated ICR male mice kidney [15, 54]. Therefore, it is suggested that Se may protect the kidney by restoring the normal level of MMP and then reducing abnormal apoptosis. When oxidative stress occurs, ROS is produced in large quantities and then attack of membrane phospholipids, the decrease of MMP leads to biochemical changes inside and outside of the mitochondrial membrane, which makes Cyt-c release into the cytoplasm and binds to apoptotic protease activating factor-1 (Apaf-1). When activated Apaf-1 forms apoptosome, it binds and cleaves initiator procaspase-9, and converts it to an active form (caspase-9), then turns the caspase-3 into active cleaved form (cleaved caspase-3) and triggers mitochondrial apoptosis [55]. We found T-2 toxin exposure caused the increase of protein expression of Cyt-c, activities of caspase-3 and caspase-9, while pretreatment with Se alleviated the above changes. Our study confirmed that T-2 toxin decreased MMP and led to the release of Cyt-c into the cytoplasm, which upregulated the activities of caspase-3/9 and promoted apoptosis. Se indirectly regulated the Cyt-c and caspase-3/9 by alleviating the decline of MMP, and finally alleviated apoptosis. Bcl-2 is the first member identified as an antiapoptotic protein. Bax is known as the proapoptotic protein in Bcl-2 family proteins. In the presence of apoptotic stimuli, the expression of Bax increased, and then combined with Bcl-2 to activate caspase cascade to induce apoptosis [56]. Se has a protective effect on oxidative stress and mitochondrial apoptosis induced by cadmium in mice kidney, presenting as blocking ROS production, inhibiting MMP collapse, the Cyt-c release, and caspase activation, and changing the levels of Bcl-2 and Bax [15]. We found T-2 exposure caused the increase of protein and gene expression of Bax, and the decrease of protein and gene expression of Bcl-2, while pretreatment with Se alleviated the above changes. Bcl-2 family members also act as central executers of apoptotic pathways by controlling the release of Cyt-c from mitochondria into the cytosol and activating caspase-3 [57,58,59]. Therefore, we speculate that Se may regulate the MMP and protein expressions of Bcl-2 family, so as to control the release of Cyt-c and regulate the activities of caspase-3/9, and finally alleviate the apoptosis induced by T-2 toxin. These results displayed that the mitigation of Se on T-2-induced excessive apoptosis of kidney is related to the mitochondrial apoptosis pathway. The above results showed that antiapoptosis may be one of the beneficial mechanisms of Se against T-2 nephrotoxicity.
Conclusion
In summary, the alleviating effect of Se on T-2-induced renal lesion is related to the ROS-mediated apoptosis. These results suggest that Se can be used as a dietary additive against the toxicity of T-2 toxin.
Abbreviations
- Se:
-
Selenium
- BW:
-
Body weight
- ROS:
-
Reactive oxygen species
- MMP:
-
Mitochondrial membrane potential
- Cyt-c:
-
Cytochrome-c
- TEM:
-
Transmission electron microscope
- GSG-Px:
-
Glutathione peroxidase
References
Wild CP, Gong YY (2010) Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 31:71–82
Mclachlan A, Shaw KJ, Hocking AD, Pitt JI, Nguyen THL (1992) Production of trichothecene mycotoxins by Australian Fusarium species. Food Addit Contam 9:631–638
Lei R, Jiang N, Zhang Q, Hu S, Dennis BS, He S, Guo X (2016) Prevalence of selenium, T-2 Toxin, and deoxynivalenol in Kashin–Beck disease areas in Qinghai Province, Northwest China. Biol Trace Elem Res 171:34–40
Wan Q, He Q, Deng X, Hao F, Tang H, Wang Y (2015) Systemic metabolic responses of broiler chickens and piglets to acute T-2 Toxin intravenous exposure. J Agric Food Chem 64:714–723
Taylor MJ, Reddy RV, Sharma RP (1985) Immunotoxicity of repeated low level exposure to T-2 toxin, a trichothecene mycotoxin, in CD-1 mice. Mycotoxin Res 1:57–64
Rahman S, Sharma AK, Singh ND, Prawez S (2016) T-2 toxin induced nephrotoxicity in Wistar rats. Indian J Vet Pathol 40:320–326
Matejova I, Faldyna M, Modra H, Blahova J, Svobodova Z (2016) Effect of T-2 toxin-contaminated diet on common carp (Cyprinus carpio L.). Fish Shellfish Immunol 60:458–465
Knigs M, Mulac D, Schwerdt G, Gekle M, Humpf HU (2009) Metabolism and cytotoxic effects of T-2 toxin and its metabolites on human cells in primary culture. Toxicology. 258:106–115
Liu Y, Dong R, Yang Y, Xie H, Zhang Z (2020) Protective effect of organic selenium on oxidative damage and inflammatory reaction of rabbit kidney induced by T-2 Toxin. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02279-5
Zhou YJ, Zhang SP, Liu CW, Cai YQ (2009) The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol in Vitro 23:288–294
Chaudhari M, Jayaraj R, Bhaskar ASB, Rao PVL (2009) Oxidative stress induction by T-2 toxin causes DNA damage and triggers apoptosis via caspase pathway in human cervical cancer cells. Toxicology. 262:153–161
Weidner M, Welsch T, Hübner F, Schwerdt G, Gekle M, Humpf HU (2012) Identification and apoptotic potential of T-2 Toxin metabolites in human cells. J Agric Food Chem 60:5676–5684
Manish A, Bhawana N, Neha K, Anupriya A, Al-Khedhairy AA, Kumar KN, Ha CE (2017) T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget. 8:33933–33952
Xia L, Nordman T, Olsson JM, Damdimopoulos A, Björnstedt M (2003) The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. J Biol Chem 278:2141–2146
Wang Y, Wu Y, Luo K, Liu Y, Cai Y (2013) The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol 58:61–67
Liu L, Yang B, Cheng Y, Lin H (2015) Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol Trace Elem Res 167:308–319
Zhang Y, Hu B, Wang M, Tong J, Long M (2020) Selenium protects against zearalenone-induced oxidative stress and apoptosis in the mouse kidney by inhibiting endoplasmic reticulum stress. Oxidative Med Cell Longev 2020:1–10
Long J, Liu Y, Zhou X, He L (2021) Dietary serine supplementation regulates selenoprotein transcription and selenoenzyme activity in pigs. Biol Trace Elem Res 199:148–153. https://doi.org/10.1007/s12011-020-02117-8
Idriss HT, Naismith JH (2000) TNF-α and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech 50:184–195
Wang T, He C (2018) Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev 44:38–50
Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD (2007) Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71:619–628
Ortiz A, Cuadrado SG, Lorz C, Egido J (1996) Apoptosis in renal diseases. Front Bioence A J Virtual Libr 1:30–47
Wan Q, Wu G, He Q, Tang H, Wang Y (2015) The toxicity of acute exposure to T-2 toxin evaluated by the metabonomics technique. Mol BioSyst 11:882–891
Yang X, Zhang X, Zhang J, Ji Q, Huang W, Zhang X, Li Y (2019) Spermatogenesis disorder caused by T-2 toxin is associated with germ cell apoptosis mediated by oxidative stress. Environ Pollut 251:372–379
Li Y, Wang Z, Beier RC, Shen J, Smet DD, De Saeger S, Zhang S (2011) T-2 Toxin, a trichothecene mycotoxin: review of toxicity, metabolism, and analytical methods. J Agric Food Chem 59:3441–3453
And LBN, Bansal MP (1997) Effect of selenium supplementation on the glutathione redox system in the kidney of mice after chronic cadmium exposures. J Appl Toxicol 17:81–84
N. R. C. C. f. t. U. o. t. G. f. t. Care and U. o. L. Animals (2011) Guide for the care and use of laboratory animals. Publication. 327:963–965
Yu K, Zhang J, Cao Z, Ji Q, Han Y, Song M, Shao B, Li Y (2018) Lycopene attenuates AFB1-induced renal injury with the activation of the Nrf2 antioxidant signaling pathway in mice. Food Funct 9:6427–6434
Wu J, Zhou Y, Yuan Z, Yi J, Tian Y (2019) Autophagy and apoptosis interact to modulate T-2 toxin-induced toxicity in liver cells. Toxins. 11:45
Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Cao Z, Zhang X, Li Y (2019) Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf 173:131–141
Handy DE, Lubos E, Yang Y, Galbraith JD, Loscalzo J (2009) Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem 284:11913–11921
Wang YX, Xiao X, Zhan XA (2018) Antagonistic effects of different selenium sources on growth inhibition, oxidative damage, and apoptosis induced by fluorine in broilers. Poult Sci 97:3207–3217
Baş E, Naziroğlu M (2019) Selenium attenuates docetaxel-induced apoptosis and mitochondrial oxidative stress in kidney cells. Anti-Cancer Drugs 30:339–346
Jin X, Xu Z, Zhao X, Chen M, Xu S (2017) The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere. 180:259–266
Wallace MA (1998) Anatomy and physiology of the kidney. AORN J 68(800):803–816
Roy S, Dontamalla SK, Mondru AK, Sannigrahi S, Veerareddy PR (2011) Downregulation of apoptosis and modulation of TGF-β1 by sodium selenate prevents streptozotocin-induced diabetic rat renal impairment. Biol Trace Elem Res 139:55–71
Stapleton SR (2000) Selenium: an insulin mimetic. Cell Mol Life Sci 57:1874–1879
Zhou J, Huang K, Lei XG (2013) Selenium and diabetes—evidence from animal studies. Free Radic Biol Med 65:1548–1556
Muecke R, Schomburg L, Buentzel J, Kisters K, Micke O (2010) Selenium or no selenium--that is the question in tumor patients: a new controversy. Integr Cancer Ther 9:136–141
Nazıroğlu M, Yıldız K, Tamtürk B, Erturan İ, Arce MF (2012) Selenium and psoriasis. Biol Trace Elem Res 150:3–9
Ruifeng Z, Jiangang MA, Xiaoxiao L (2017) The test of KIM-1, Cys C and β2-Mg to assess the early renal damage in OSAHS patients and its clinical significance. J Clin Otorhinolaryngol Head Neck Surg (China) 31:174–179
Yun L, Ran Z, Yan S, Ying L, Fei L, Ni Y, Ting Z, Kai L, Chun L, Xiu L, Lei Q, Fu X, Zhou J, Liang S (2018) Renal inhibition of miR-181a ameliorates 5-fluorouracil-induced mesangial cell apoptosis and nephrotoxicity. Cell Death Dis 9:610
Edrees NE, Galal AAA, Abdel MAR, Beheiry RR, Metwally MMM (2018) Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem Biol Interact 294:56–64
K. Tarun, C. Santanu, M. Soma and B. Arindam. (2014) Palladium(II) complex induces apoptosis through ROS-mediated mitochondrial pathway in human lung adenocarcinoma cell line (A549). Current Science (00113891). 107: 1711-1719
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 1790:1478–1485
Ruder EH, Hartman TJ, Jeffrey B, Goldman MB (2008) Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 14:345–357
Elizabeth R, Terryl H, Marlene G (2009) Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol 21:219–222
Albers DS, Beal MF (2000) Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 59:133–154
Li X, Fang F, Gao Y, Tang G, Xu W, Wang Y, Kong R, Tuyihong A, Wang Z (2019) ROS induced by killerred targeting mitochondria (mtKR) enhances apoptosis caused by radiation via cyt c/caspase-3 pathway. Oxidative Med Cell Longev 2019:1–11
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M (2018) Mitochondrial membrane potential. Other. 552:50–59
Park C, Cha HJ, Hong SH, Kim GY, Kim S, Kim HS, Kim BW, Jeon YJ, Choi YH (2019) Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Mar Drugs 17:225
Yan X, Wang L, Yang X, Qiu Y, Tian X, Lv Y, Tian F, Song G, Wang T (2017) Fluoride induces apoptosis in H9c2 cardiomyocytes via the mitochondrial pathway. Chemosphere. 182:159–165
Yang Y, Wang G, Wu W, Yao S, Han X, He D, He J, Zheng G, Zhao Y, Cai Z, Yu R (2018) Camalexin induces apoptosis via the ROS-ER stress-mitochondrial apoptosis pathway in AML cells. Oxidative Med Cell Longev 2018:7426950
J. Gao, X. Tian, X. Yan, Y. Wang, J. Wei, X. Wang, X. Yan and G. Song. (2020) Selenium exerts protective effects against fluoride-induced apoptosis and oxidative stress and altered the expression of Bcl-2/Caspase family. Biological Trace Element Research. 1-11
Xiong S, Mu T, Wang G, Jiang X (2014) Mitochondria-mediated apoptosis in mammals. Protein Cell 5:737–749
Hardwick JM, Soane L (2013) Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 5:152–158
Prakash C, Kumar V (2016) Chronic arsenic exposure-induced oxidative stress is mediated by decreased mitochondrial biogenesis in rat liver. Biol Trace Elem Res 173:87–95
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prévost M-C, Alzari PM, Kroemer G (1999) Mitochondrial release of Caspase-2 and -9 during the apoptotic process. J Exp Med 189:381–394
Huang W, Liu M, Xiao B, Zhang J, Cao Z (2021) Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes. Food Chem Toxicol 148:111972
Funding
This study was supported by China Postdoctoral Science Foundation (2020M682296), the Scientific and Technological Project of Henan Province-China (212102110362), and the Heilongjiang provincial natural science foundation of China/C2018020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOC 5978 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, Q., Zhang, J. et al. The Protective Effect of Selenium on T-2-Induced Nephrotoxicity Is Related to the Inhibition of ROS-Mediated Apoptosis in Mice Kidney. Biol Trace Elem Res 200, 206–216 (2022). https://doi.org/10.1007/s12011-021-02614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02614-4