Abstract
The present study investigated the status of calcium and magnesium as well as essential trace elements including iodine, selenium, copper, iron, and zinc in adults residing in the Zhytomyr region of Ukraine. In addition, the relative risk of goiter occurrence was evaluated. In this comparative study, 40 adults without goiter (control group) and 16 adults with diagnosed nodular goiter (NG) were examined. Inductively coupled plasma optical emission spectrometry (ICP-OES) was used for the measurements of Mg, Ca, Se, Zn, Cu, and Fe in serum of patients with NG and control group. Patients with nodular goiter had lower serum values of Ca, Mg, Se, Cu, Fe, and Zn than those in the control group. The presence of mild iodine deficiency was evident in both groups with the median urinary iodine excretion (UIE) 80.5 μg/L in the control group and 64.5 μg/L in goiter group. There was a positive association between goiter presence and low concentration of Ca in serum (odds ratio (OR) = 2.29 (1.26–3.55), p < 0.05) in the NG group. High relative risk of goiter was observed at low concentrations of magnesium (OR = 3.33 (1.39–7.62), p < 0.05) and selenium (OR = 1.63, (1.16–1.78), p < 0.05) in comparison with OR values in the control group. Low concentrations of Ca, Mg, Zn, and Se in serum combined with reduced UIE resulted in the highest risk of goiter (OR = 12.5, (2.15–79.42), p < 0.01). This study proved that Thyroglobulin concentration in serum is the reliable indicator of nodular goiter. We also suggest that a combination of low concentrations of Ca, Mg, Zn, Cu, and Se in blood serum, and reduced iodine concentration in urine resulted in the highest risk of nodular goiter development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Goiter is a worldwide health problem caused by impaired iodine intake. This disease is increasing at an alarming rate and affects up to 10% of the people worldwide [1]. Goiter results in the enlargement of the thyroid gland in the forms of diffuse and nodular growths. Nodular goiter is an excessive growth of thyroid tissue caused by structural and functional changes of several regions within the thyroid gland [2]. The main risk factor of NG is insufficient intake of essential trace elements, especially iodine and selenium [3,4,5]. Iodine deficiency causes TSH elevation and peroxide accumulation in thyroid gland, leading to increased insulin-like growth factor, fibroblast growth factor, and follicular cell mutation [6,7,8]. Follicular cell formation will continue changing, followed by single nodule formation in tissue of thyroid gland.
Imbalanced intake of trace elements, other than iodine, results in higher risks of the thyroid disease [9]. Selenium plays an important role in normal thyroid hormone metabolism [10]. Several selenoproteins, primarily glutathione peroxidase, contribute to thyroid gland functionality acting as antioxidant protector of the thyrocytes against peroxidative damage [10, 11]. Also, selenium-containing enzyme deiodinase is able to remove iodide from thyrosyle ring of the thyroid hormones [10]. Zinc is also involved in the metabolism of thyroid hormones. Zinc is necessary for the proper functionality of the enzyme iodothyronine deiodinase, which is responsible for thyroxine (T4) conversion to the active form of triiodothyronine (T3) [10, 12]. There are limited studies related to calcium’s role in the thyroid hormone metabolism. Increased concentration of TSH may increase concentration of Ca2+ in serum of experimental rats [13]. Copper, iron, and magnesium are indirectly involved in the synthesis of thyroid hormones but the exact mechanisms are unclear. Since the elements mentioned above play significant roles in different physiological processes, their inadequate dietary intake may promote or exacerbate various diseases other than thyroid dysfunction.
There are limited studies about goiter incidence occurrence in Ukraine. Our investigations are focused on the regions mostly affected by the Chernobyl disaster, primarily because people living in the area received high doses of radioactive iodine (131I). The inhabitants of the region are suffered from high rate of thyroid cancer observed in the first year after the disaster [14]. NG is associated with increased prevalence of thyroid cancer. Therefore, associations between NG, thyroid cancer, and other thyroid diseases are attracting considerable attention.
The purpose of this study is to investigate the status of calcium, magnesium, and essential trace elements iodine, selenium, iron, copper, and zinc in individuals with nodular goiter and control group, and to determine associations between concentrations of the trace elements and thyroid hormones.
Materials and Methods
Subjects
In this study, a total of 56 individuals (27 males and 29 females) residing in the Zhytomyr region of Ukraine participated in the medical survey. Nodular goiter was detected among 16 patients (7 males and 9 females) with a mean age of 38.3 ± 1.68 years. Fine needle aspiration biopsy of thyroid nodules was used in this study as a verification method of NG [15]. Cytological diagnoses were made by an experienced pathologist. In this study, single nodules were diagnosed in 60% of participants in NG group, while 20% had nodular goiter with follicular hyperplasia of trabecular hyalinizing adenoma, benign cyst was detected in 14.5% participants of NG group, and only 5.5% individuals of this group had nodular colloid goiter. Individuals with malignant and suspicious nodules were excluded from the study. Also, patients had no history of thyroid surgery. Control group included 40 participants without thyroid diseases with a mean age of 38.9 ± 0.78, and none had thyroid disorders before or at the time of study.
Sampling
The samples of venous blood (10 mL) were taken after 10-h fasting by mobile screening teams of V.P. Komissarenko Institute of Endocrinology and Metabolism with the participants consent during observation of thyroid function. The blood samples were kept for 12 h; sera were centrifuged at 3000 rpm followed by serum transfer into polyethylene tubes (2.5 mL). The samples of serum stored at − 20 °C prior to analysis.
All the samples of morning urine (50–100 mL) were collected in 100-mL polyethylene tubes from individuals followed by transfer of 2 mL of urine in polyethylene tubes (Eppendorf, Germany). These samples kept frozen at − 18 °C before the experiments.
Analysis
Urine samples were digested with hydrochloric acid according to the method, described by Dunn et al. [16] Urinary iodine concentrations were measured by the Sandell–Kolthoff reaction. Besides internal control, our laboratory participated in the external quality control for urinary iodine of EQUIP (Ensuring the Quality of Urinary Iodine Procedures) standardization program of the Centers for Disease Control and Prevention (Atlanta, USA).
Thyroid volume was measured using a real-time ultrasonographic portable scanner (Terason 2000, Burlington, MA, USA) with linear sensor, and frequency of 10 MHz. Details of nodules, echostructure, and echogenicity were recorded. The thyroid volume was calculated based on the volume of ellipsoid as described by Brunn [17]
Thyroid hormone status was determined by concentrations of FT4 (free thyroxine) and TSH (thyroid stimulating hormone) in serum by radioimmunoassay analysis using reagent pack (Amersham, U.K.). Thyroglobulin (TG), Thyroglobulin antibodies (TgAbs), and thyroxine peroxidase (TPOAb) concentrations were measured in serum by LUMitest immunochemiluminescence assays (BRAHMS Diagnostica GMBH, Heningsdorf, Germany) using a Bertholdt 953 luminometer (Pforzheim, Germany). The reference ranges were the following: 10.3–22 pmol/L for FT4, 0.4–4 mIU/L for TSH, < 115 IU/L for TgAbs, 2–70 ng/mL for TG, and < 34 IU/L for TPOAb [18].
Concentrations of calcium, magnesium, zinc, copper, iron, and selenium in serum were determined by optical emission spectrometry coupled by inductively coupled plasma (ICP-OES, Perkin-Elmer, model Optima 2100, USA). Multi-element standard solution (Merck, Germany, catalog number 111355.0100) containing 30 elements was used to build a calibration curve for Ca, Mg, Fe, Se, Cu, and Zn in the ICP-OES analysis, Before analysis, serum samples (0.5 mL) were digested using microwave digestion system (MSW-2, Germany) by 2.5 mL of nitric acid during 30 min. Analysis of Ca and Mg was performed in the radial viewing mode, while determination of Fe, Se, Cu, and Zn was done in the axial viewing mode. The limits of detection (LOD) for calcium, magnesium, copper, iron, selenium, and zinc were 0.06 μg/L, 0.05 μg/L, 0.3 μg/L, 0.3 μg/L, 3 μg/L, and 0.4 μg/L, respectively. In order to provide precision, the measurements were repeated twice. In this respect, experimental value was considered to be accurate if relative standard deviation did not exceed 2%. An external quality control of the laboratory studies of the trace elements content in serum was performed in accordance with the Lead and Multi-Element Proficiency Program (LAMP) of Centers for Disease Control and Prevention and SEQAS programs (“FORTREES,” England). Laboratory reference ranges were as follows: magnesium, 17–28 mg/L; calcium, 90–112 mg/L; zinc, 0.6–1.2 mg/L; iron, 0.6–1.68 mg/L; copper 0.7–1.55 mg/L; selenium 0.046–0.14 mg/L [18].
The study protocol was approved by the Ethics Committee of V.P. Komissarenko Institute of Endocrinology and Metabolism and all participants gave informed consent before blood and urine sampling.
Statistical Analysis
SPSS 21.0 was used for statistical analysis. The experimental data were expressed as median and quartiles due to their non-normal of distribution. The correlation between experimental variables was evaluated using Spearman’s rank correlation coefficients. Mann–Whitney U test was implemented for non-parametric variables. Different logistic regression models were used in order to assess the relative risk of nodular goiter. The results were estimated as crude odds ratio (OR) and adjusted (aOR) and their 95% confidence interval (95 % CI). All tests were repeated three times and considered statistically significant at p < 0.05.
Results
The median UIE level of individuals with nodular goiter was not significantly different from control group (Table 1). We did not observe significant differences in TSH, FT4, TgAbs, and TPOAb in serum between control and NG groups, while the volume of thyroid glands and thyroglobulin level were larger in NG group than those in control group (Table 1). Median size of the thyroid gland (Tvol) in patients with nodular goiter was 23% lower than that in control group.
The median concentrations of serum zinc, selenium, copper, iron, magnesium, and calcium were significantly higher in control group than those in NG group. The median concentrations of selenium and iron in serum reduced dramatically in NG group to 47.5% and 35%, respectively (Table 2). Similar findings were observed for copper and zinc concentrations in serum of both groups. Median concentrations of Zn and Cu in serum of NG group were decreased to 28.8% and 26.6%, respectively. However, median concentrations of Ca and Mg in serum of patients with NG changed only slightly, but were still statistically significant. Concentrations of Fe, Cu, and Se in serum were lower than reference range in 25%, 18.8%, and 60% of the patients with nodular goiter, respectively.
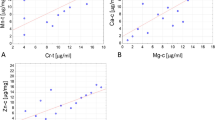
We assessed correlation between concentrations of essential trace elements and parameters of thyroid function in both NG and control groups (Table 3). Strong correlation between Ca and Mg as well as between Ca and Cu was observed in the control group, whereas correlation between Zn and Mg was not statistically different. For the NG group, there was a stronger correlation between Ca and Mg and between Ca and Cu than in the control group. Moreover, positive associations between Ca and Zn, Mg and Zn, Mg and Cu, Zn and Cu, and between Zn and Fe were found in this group (Table 3).
Thyroid status was examined in control and NG groups taking into account correlation between trace elements and thyroid hormone concentrations. Concentration of TSH was positively correlated with selenium concentration in control group (rs = 0.425, p = 0.009, Table 4). Also, correlation between TG and TSH, TSH and TPOAb, and TPOAb and TgAbs was statistically significant in control group. In contrast, in NG group, the following reliable correlation links were observed: TG and UIE, TG and Se level, and Tvol and TPOAb (Table 4).
We evaluated influence of essential trace elements and thyroid hormones on NG development on the basis of OR assessment. In case of deficiency of trace elements, these parameters were analyzed using the χ2 criteria
OR of goiter presence at low concentrations of Ca and Mg in serum was 2.29 (95%, CI 1.26–3.55, p < 0.05) and 3.33 (95%, CI 1.39–7.62, p < 0.05), respectively, in comparison with control group. The risk of nodular goiter was still statistically significant after adjustment by sex, age, and UIE in case of deficiency of calcium (aOR = 3.9, CI 1.08–14.3, p < 0.05) and magnesium (aOR = 4.5, CI 1.16–17.2, p < 0.05) Moreover, OR at low levels of selenium and zinc in the blood serum was found to be 1.63 (95%, CI 1.16–1.785, p < 0.05) and 1.87 (95%, CI 1.13–2.59, p < 0.05), respectively.
The highest OR values were observed with simultaneous deficiency of macro- and trace elements. It was found that the OR was 5.83 (95%, CI 1.87–18.9, p < 0.01) at low concentrations of Ca, Mg, and Zn in serum, whereas at simultaneous decreased concentrations of zinc, calcium, and magnesium in serum together with low UIE, OR increased up to 12.5 (95%, CI 2.15–79.42, p < 0.05).
Discussion
Iodine plays a crucial role in development of NG. The following methods are generally used in the assessment of iodine intake: goiter rate, serum TG, serum TSH concentrations [1, 3], and urinary iodine excretion. UIE is a superior indicator of recent iodine intake because approximately 90% of iodine is released with urine if the individual is iodine replete [1]. Our studies suggest that both control and NG groups were characterized by mild iodine deficiency, but only 18.8% individuals with goiter had normal UIE level, whereas UIE concentration greater than 100 μg/L was detected among 33.3% of participants in the control group. It should be noted that severe iodine deficiency was observed in 5.1% individuals with goiter and 6.25% in control group, respectively. Table 1 summarizes experimental data regarding thyroid status of control and NG groups. Concentrations of TSH, TG, and FT4 in serum are considered alternative indicators of iodine status. Unfortunately, increased iodine consumption not always results in marked decrease of TSH level which still remains within the reference range [1, 19]. Similarly, FT4 level changes very slowly after iodine administration. As a sensitive indicator of iodine status, TG concentration reflects the improved thyroid function after iodine repletion. Median TSH concentration was slightly different in both groups, whereas TG concentration was more than twofold greater than in control group, thus indicating mild iodine deficiency in control and moderate iodine deficiency in the NG group. Therefore, TG concentration in serum was the most reliable parameter of nodular goiter in our investigations. In this study, serum TG concentration was directly correlated with UIE (Table 1), and in the control group, TG was close to normal which is considered to be 10 μg/L [20].
Selenium plays an important role in metabolism of thyroid hormones. A specific selenium-containing enzyme, glutathione peroxidase (GPx), regulates synthesis of thyroid hormones through modulation of H2O2 availability for thyroperoxidase action in follicular lumen [11]. Moreover, another selenoenzyme, iodothyronine deiodinase, can convert thyroxine (T4) to bioactive triiodothyronine (T3) [21]. We observed a marked decrease of median Se concentration in the NG group (21 μg/L) compared with control group (49 μg/L). These concentrations are lower than the minimal limit of recommended daily intake (55 μg/L). Our findings contrast to those obtained by Sakiz et al. [19], who reported similar mean concentration of Se in serum for the patients with single nodules (58.8 ± 15.1 μg/L), multiple nodules (57.3 ± 14.8 μg/L), and control group (57.6 ± 13.3 μg/L). These data can be explained by sufficient dietary iodine of participants. Giray et al. have suggested that low selenium concentration may contribute to goiter among individuals with iodine deficiency [22].
Copper may act as both antioxidant and prooxidant, and it is incorporated into a variety of metabolic enzymes in the human body, including cytochrome C, superoxide dismutase, and lysyl oxidase. Presence of copper is necessary for T4 production [21]. A decrease of Cu concentration in serum was observed among patients with NG, comparing with the control group. These findings are in agreement with the data obtained by Aihara et al. [23] but in contrast with those of Giray et al. [22] and Kazi et al. [24], who reported high Cu concentrations in plasma from patients with NG and in serum from individuals with diffuse goiter, respectively. Thyroid tissue from patients who survived the Chernobyl disaster exhibits decreased concentrations of Cu, Fe, and Zn compared with control group [25]. Mean concentrations of copper, iron, and zinc in patients with NG were 3.35 ± 0.9 μg/g of dry tissue, 201.6 ± 23.4 μg/g, and 41.8 ± 7.2 μg/g, respectively. Concentrations of these trace elements in thyroid tissue were significantly higher in the control group. Mean concentration of Cu, Fe, and Zn in thyroid glands of control group were5.24 ± 0.5 μg/g, 241.4 ± 22.0 μg/g, and 101.3 ± 10.9 μg/g, respectively [25]. These findings correlate with our data, probably due to similarity of procedure of goiter detection. The individuals with NG were euthyroid and did not suffer from hyperthyroidism [25].
Zinc plays significant roles including immune function, growth, sexual maturation, wound healing, and others, and may have an effect on thyroid function [22]. A correlation between the amount of zinc in human diet and goiter development has not been established; however, it is known that zinc deficiency accompanies hypothyroidism [26]. Interestingly, increased level of zinc in serum is associated with decreased FT4 concentration in males, while these observations were not significant in females [27]. Also, the correlation between lower values of Zn level in hair of children with goiter compared with control group [28] has been observed. In our study, we observed a significant decrease of Zn concentration in serum for patients with goiter in comparison with control group, indicating that zinc may act as an additional factor in development of nodular goiter along with deficiency of other essential trace elements, primarily iodine and selenium.
Deficiency of iron affects production of thyroid hormones since heme-dependent TPO decreases [24, 29]. The present study indicates deficiency of iron among individuals, especially in NG group. In other words, almost 25% of patients with goiter had lower than optimal iron concentrations in serum. We observed decreased concentration of iron in serum for the NG group in comparison with controls. These data are similar to results obtained by Kazi et al., who observed significant elevations of iron in serum of control group in comparison with the patients with NG [24].
Currently, limited data exist concerning the role of Mg in thyroid function. Prolonged consumption of Mg supplements may normalize thyroid morphology among patients with thyroid diseases [30]. Also, there is a significant correlation between increased TSH concentration and low Mg concentration in serum in patients with depressive disorders [31]. Administration of Mg has resulted in increased magnesium concentration in serum and decline in Ca concentration [32]. Hyperthyroidism is associated with hypercalcemia, but this correlation is age dependent [33]. Elevated Ca concentration in serum is observed in 18.8% hyperthyroid patients aged 60 years and older, while only 2.3% of hyperthyroid patients under 60 years of age had increased calcium concentration in serum [33]. Our results contrast with the observations mentioned above. Median Ca concentration in serum for NG group was lower than that of the control group. These differences might be due to different ages of participants in our experiment and the survey carried by Szabo et al. [33], which were (38.3 ± 1.68) years and (62 ± 16) years, respectively.
A small number of patients with nodular goiter might be a limited factor in this study. Therefore, a larger group of participants is necessary in order to develop a detailed regression model of for the role of trace elements in NG development.
Conclusions
In conclusion, the UIE measurements revealed mild iodine deficiency among residents of Zhytomyr region in both control and NG groups. Median TG levels also indicated presence of mild iodine deficiency in these groups. This study proved that Thyroglobulin concentration in serum is the most reliable indicator of nodular goiter. Median concentrations of calcium, magnesium, and other trace elements in serum were lower in the NG group comparing with control group. Similar findings of inadequate concentrations of several essential trace elements in serum have been observed among people residing in Kyiv region of Ukraine [34]. Here we suggest that low calcium concentration in serum, but not as much as low concentrations of Mg, Zn, or Se in serum, resulted in increased relative risk of goiter development. The highest risk of goiter occurrence was observed in combination of decreased concentrations of serum calcium, zinc, magnesium, and selenium in serum and reduced concentration of iodine in urine.
References
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30(4):376–408. https://doi.org/10.1210/er.2009-0011
Rocha HS, Lopesa RT, Valiante PM, Tirao G, Mazzarod I, Honnicked MG, Cusatis C, Gilese C (2005) Diagnosis of thyroid nodular goiter using diffraction-enhanced imaging. Nucl Instrum Meth A 548:175–180. https://doi.org/10.1016/j.nima.2005.03.086
Carle A, Krejbjerg A, Laurberg P (2014) Epidemiology of nodular goitre. Influence of iodine intake. Best Pract Res Clin Endocrinol Metab 28(4):465–479. https://doi.org/10.1016/j.beem.2014.01.001
Laurberg P, Nøhr SB, Pedersen KM, Hreidarsson AB, Andersen S, Bülow Pedersen I, Knudsen N, Perrild H, Jørgensen T, Ovesen L (2000) Thyroid disorders in mild iodine deficiency. Thyroid 10(11):951–963. https://doi.org/10.1089/thy.2000.10.951
Liu Y, Huang H, Zeng J, Sun C (2013) Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: a cross-sectional study. BMC Public Health 13:1–7. https://doi.org/10.1186/1471-2458-13-1153
Corvillian B, van Sande J, Laurent E, Dumont JE (1991) The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology 128:779–785. https://doi.org/10.1210/endo-128-2-779
Gydee H, O’Neill JT, Patel A, Bauer AJ, Tuttle RM, Francis GL (2004) Differentiated thyroid carcinomas from children and adolescents express IGF-I and the IGF-I-receptor (IGF-I-R). Pediatr Res 55(4):709–715. https://doi.org/10.1203/01.PDR.0000111282.98401.93
Yeh MW, Rougier JP, Park JW, Duh QY, Wong M, Werb Z, Clark OH (2006) Differentiated thyroid cancer cells invasion is regulated through epidermal growth factor receptor-dependent activation of matrix metalloproteinase (MMP)-2 gelatinase. Endocr Relat Cancer 13(4):1173–1183. https://doi.org/10.1677/erc.1.01226
Przybylik-Mazurek E, Zagrodzki P, Kuźniarz-Rymarz S, Hubalewska-Dydejczyk A (2011) Thyroid disorders-assessments of trace elements, clinical, and laboratory parameters. Biol Trace Elem Res 141(1-3):65–75. https://doi.org/10.1007/s12011-010-8719-9
Köhrle J, Jakob F, Contempre B, Dumont JE (2005) Selenium, the thyroid, and the endocrine system. Endocr Rev 26:944–984. https://doi.org/10.1210/er.2001-0034
Beckett GJ, Arthur JR (2005) Selenium and endocrine systems. J Endocrinol 184:455–465. https://doi.org/10.1089/thy.2005.15.841
Danforth E Jr, Burger AG (1989) The impact of nutrition on thyroid hormone physiology and action. Annu Rev Nutr 9:201–227. https://doi.org/10.1146/annurev.nu.09.070189.001221
MacNeil S, Munro DS, Metcalfe R, Cotterell S, Ruban L, Davies R, Weetman AP (1994) An investigation of the ability of TSH and Graves’ immunoglobulin G to increase intracellular calcium in human thyroid cells, rat FRTL-5 thyroid cells and eukaryotic cells transfected with the human TSH receptor. J Endocrinol 143(3):527–540. https://doi.org/10.1677/joe.0.1430527
Goulko GM, Chepurny NI, Jacob P, Kairo IA et al (1998) Thyroid dose and thyroid cancer incidence after the Chernobyl accident: assessments for the Zhytomyr region (Ukraine). Radiat Environ Biophys 36:261–273
Gharib H (1994) Fine-needle aspiration biopsy of thyroid nodules: advantages, limitations, and effect. Mayo Clin Proc 69:44–49
Dunn JT, Grutchfield HE, Gutekunst R, Dunn AD (1993) Two simple methods for measuring iodine in urine. Thyroid. 3:119–123. https://doi.org/10.1089/thy.1993.3.119
Brunn J, Block U, Ruf et al (1981) Volumetrie der Schilddrüsenlappen mittels Real-time-Sonographie. Dtsch Med Wochenschr 106:1338–1340
Skalnyi AV, Kudrin AV (2000) Radiation, microelements, antioxidants, and immunity (trace elements and antioxidants in health improving). Mir, Moscow (in Russian)
Sakiz D, Kaya A, Kulaksizoglu M (2016) Serum selenium levels in euthyroid nodular thyroid diseases. Biol Trace Elem Res 174(1):21–26. https://doi.org/10.1007/s12011-016-0688-1
Iervasi A, Iervasi G, Carpi A, Zucchelli GC (2006) Serum thyroglobulin measurement: clinical background and main methodological aspects with clinical impact. Biomed Pharmacother 60(8):414–424. https://doi.org/10.1016/j.biopha.2006.07.007
Harris ED (2001) Copper homeostasis: the role of cellular transporters. Nutr Rev 59(9):281–285. https://doi.org/10.1111/j.1753-4887.2001.tb07017.x
Giray B, Arnaud J, Sayek I, Favier A, Hincal F (2010) Trace elements status in multinodular goiter. J Trace Elem Med Biol 24(2):106–110. https://doi.org/10.1016/j.jtemb.2009.11.003
Aihara K, Nishi Y, Hatano S, Kihara M, Yoshimitsu K, Takeichi N, Ito T, Ezaki H, Usui T (1984) Zinc, copper, manganese, and selenium metabolism in thyroid disease. Am J Clin Nutr 40(1):26–35. https://doi.org/10.1093/ajcn/40.1.26
Kazi GL, Kandhro GA, Afridi HA et al (2009) Interaction of copper with iron, iodine, and thyroid hormone status in goitrous patients. Biol Trace Elem Res 134:265–279. https://doi.org/10.1007/s12011-009-8478-7
Błazewicz A, Dolliver W, Sivsammye S, Deol A (2010) Determination of cadmium, cobalt, copper, iron, manganese, and zinc in thyroid glands of patients with diagnosed nodular goitre using ion chromatography. J Chromatogr B 878:34–38. https://doi.org/10.1016/j.jchromb.2009.11.014
Betsy A, Binitha M, Sarita S (2013) Zinc deficiency associated with hypothyroidism: an overlooked cause of severe alopecia. Int J Trichol 5:40–42. https://doi.org/10.4103/0974-7753.114714
Jain RB (2014) Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol Trace Elem Res 159:87–98. https://doi.org/10.1007/s12011-014-9992-9
Kudabayeva K, Koshmaganbetova G, Mickuviene N, Skalnaya M et al (2016) Hair trace elements are associated with increased thyroid volume in schoolchildren with goiter. Biol Trace Elem Res 174:261–266. https://doi.org/10.1007/s12011-016-0711-6
Smith SM, Finley J, Johnson LK, Lukaski HC (1994) Indices of in vivo and in vitro thyroid hormone metabolism in iron-deficient rats. Nutr Res 14:729–739. https://doi.org/10.1016/S0271-5317(05)80208-8
Moncayo R, Moncayo H (2015) Proof of concept of the WOMED model of benign thyroid disease: restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin 3:113–122 https://doi.org/10.1016/j.bbacli.2014.12.005
Hasey GM, D’alssendro E, Cooke RG (1993) The interface between thyroid activity, and depression: a pilot study. Biol Psychiatry 33(2):133–135
(1984) The hypocalcemia associated with magnesium infusion is mediated through parathyroid hormone. Nutr Rev.42(9):315–317. https://doi.org/10.1111/j.1753-4887.1984.tb02374.x
Szabo ZS, Ritzl F (1981) Hypercalcemia in hyperthyroidism. Role of age and goiter type. Klin Wochenschr 59(6):275–279
Kravchenko VI, Luzanchuk IA, Andrusyshyna IM, Polumbryk M (2018) Study of macro- and microelement status in patients with nodular goiter residing in Kyiv region. Galician Med J 25(4). https://doi.org/10.21802/gmj.2018.2.2
Acknowledgments
The authors are grateful to the staff of Laboratory of Analytical Chemistry and Monitoring of Toxic Compounds for their help and instrumental support. Also, the authors are thankful to Prof. Mykola Tronko for his valuable comments and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Ethics Committee of V.P. Komissarenko Institute of Endocrinology and Metabolism and all participants gave informed consent before blood and urine sampling.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kravchenko, V.I., Andrusyshyna, I.M., Luzanchuk, I.A. et al. Association Between Thyroid Hormone Status and Trace Elements in Serum of Patients with Nodular Goiter. Biol Trace Elem Res 196, 393–399 (2020). https://doi.org/10.1007/s12011-019-01943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01943-9