Abstract
The exposure of heavy metals (lead (Pb), cadmium (Cd), copper (Cu), nickel (Ni), and metalloid arsenicals) and their effects on workers’ health from a lead-zinc mine (145 workers) and a steel smelting plant (162 workers) was investigated. Information on subject characteristics was obtained through a questionnaire. We determined the urinary levels of Pb, Cd, Cu, Ni, and arsenicals (including inorganic arsenic (iAs), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA), as were 8-hydroxydeoxyguanosine (8-OHdG) and cystatin C. Lead-zinc mine foundry workers had significantly higher concentrations of urinary Pb, Cd, Cu, Ni, iAs, and MMA than did steel smelting plant workers. Individuals who had consumed seafood in the previous 3 days had higher concentrations of urinary Ni than did individuals who had not consumed seafood. The urinary Cd concentrations in the two groups of factory workers may have been affected by daily smoking. There was no significant difference in urinary 8-OHdG between workers from the lead-zinc mine foundry and the steel smelting plant. Urinary Pb and Cd had significant positive linear dose-dependent effects on 8-OHdG. Urinary cystatin C, a sensitive biological indicator reflecting early renal damage, was found at higher levels in lead-zinc mine workers than in steel smelting plant workers. Binary logistic regression analysis showed that age and urinary Cd were significantly associated with urinary cystatin C. These results indicated that workers from lead-zinc mines may be exposed to higher levels of heavy metals which could lead to greater risk of kidney damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead-zinc mines are rich in mineral metals such as lead (Pb), cadmium (Cd), copper (Cu), and nickel (Ni), which are widely used in the electronics, mechanical, and military industries. The emission of metals caused by mining and smelting in lead-zinc mines pollutes the surrounding atmosphere, water, and soil. This pollution is harmful not only to the occupational population but also to the residents living in the vicinity of the emissions. Environmental and occupational exposure to heavy metals induces several adverse health effects [1]. The production process in lead-zinc mines, including underground ore mining, transportation, crushing, grinding, roughing, and smelting, can release Pb, Cd, Cu, and Ni, as well as metalloid arsenic (As). These heavy metals are not readily degradable in the environment and easily accumulate in human bodies to a very high amount, and beyond a certain limit which can induce toxicity and adverse health effects [2].
Because of the potential risk to the occupational population working in lead-zinc mines, there are growing concerns about the adverse health effects associated with metal exposure encountered in the occupational environment [3, 4]. Several metals, such as Cd, Ni, and As, are known human carcinogens, and the carcinogenic potential of these heavy metals has been widely studied in humans and experimental animals [5, 6]. Lead poisoning can manifest in multiple systems in the body including in the central and peripheral nervous systems, erythropoietin system, cardiovascular system, musculoskeletal system, immune system, and/or reproductive system [7]. Rafal et al. found that workers with occupational exposure to Pb were far more likely to have cardiac injury than those without occupational exposure [8]. Cd was discovered in Germany in 1817 [9]. On the basis of a large number of epidemiological studies, Cd and its compounds were classified as class I carcinogens by the International Agency for Research on Cancer (IARC) in 1993 [10]. Cd causes several chronic toxicity symptoms in human body systems, including in the bones and kidney, as well as the cardiovascular system, respiratory system, reproductive system, and immune system [11]. Moreover, many studies have shown that lung, prostate, kidney, and liver cancers are associated with Cd. Occupational populations with excessive amounts of Cd in their working environment, as compared with individuals with no exposure, present with higher Cd concentrations in the body.
Non-ferrous metal smelting is often accompanied by As release [12]. As is a known human carcinogen that is widely distributed in the environment [13]. Exposure to As can lead not only to pigmentation and hyperkeratosis but also to damage in the liver, kidney, cardiovascular system, and central nervous system. Inorganic As (iAs) undergoes methylation metabolism after absorption into the human body, which is then distributed in various tissues and organs [14]. The metabolites of iAs include monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA); the inorganic species and organic methylated metabolites of MMA and DMA are then excreted in the urine. The toxicities of these arsenicles are different and iAs and MMA are more toxic than DMA [13, 15]. Indeed, current evidence indicates that the unifying factor determining the toxicity and carcinogenicity of heavy metals is the generation of free radicals and oxidative stress, which in turn causes oxidative damage to DNA and enhance lipid peroxidation [16, 17]. Urinary 8-hydroxydeoxyguanosine (8-OHdG) is a widely accepted sensitive biomarker of oxidative DNA damage, whose concentration reflects general oxidative stress levels in the human body [18].
In this study, we conducted a cross-sectional study of an occupational population in a lead-zinc mine foundry in Liaoning Province (China) and one steel smelting plant in the same region. The present study aimed at investigating the levels of urinary Pb, Cd, Cu, Ni, and As in the two groups of foundry workers and to analyze the workers’ living habits and physical characteristics. We examined the influence of age, employment years, body mass index (BMI), smoking, alcohol consumption, and seafood intake on urinary Pb, Cd, Cu, Ni, and As. In addition, we chose cystatin C as an early renal damage biomarker to compare the degree of kidney damage in both groups. Finally, we also evaluated oxidative damage (urinary 8-OHdG) in the study population.
Materials and Methods
Subjects
This study was conducted in two foundries with a total of 307 workers: 145 exposed workers from a lead-zinc mine foundry and 162 workers from a steel smelting plant, both located in the northeastern part of China. All subjects provided informed consent to participate in this study, and the China Medical University Ethics Committee approved the research. A questionnaire was given to all subjects to obtain information about their age, weight, height, occupational history, disease history, dietary status (including consumption of seafood, meat, vegetables, and fruits in the 3 days preceding the start of the study), alcohol consumption, smoking habits, and other lifestyle parameters. Blood pressure was measured after the questionnaire was completed. All subjects with urinary creatinine beyond the range of 0.3–3.0 g/l were excluded according to the World Health Organization criteria for the acceptability of urine samples for biological monitoring [19]. Information on the study subjects is listed in Table 1. The lead-zinc mine foundry workers (average age 46.3 years) were older than the steel smelting plant workers (average age 40.1 years). The smoking rates in the two groups of foundry workers were high, and more than half of the workers were smokers. The mean employment years of the lead-zinc mine foundry workers (16.28 years) were higher than that of the smelting plant workers (7.04 years). There was no difference in BMI between the two groups of foundry workers. The amount of seafood consumption in the previous 3 days was lower in the lead-zinc mine foundry workers (30.69 g) than the smelting plant workers (131.08 g). Moreover, lead-zinc mine foundry workers consumed more alcohol than did smelting plant workers. Spot urine samples from all subjects were collected in PVC bottles and kept on ice; urine samples were stored at − 20 °C, then transported to the China Medical University Laboratory, and stored at − 80 °C before being analyzed.
Determination of Urinary Heavy Metals
Determination of urinary Pb, Cd, Cu, and Ni was performed with a graphite furnace atomic absorption spectrophotometer (Z-2000, Hitachi, Japan). The urine samples and nitric acid were mixed at a ratio of 100:1 (v/v) and then digested. Standard solutions of Pb, Cd, Cu, and Ni (Meilun, Dalian, China) were diluted with normal human urine and used to prepare standard curves. The standard reference materials purchased from Dalian Meilun Biotechnology Corporation (China) was used as quality control. We estimated the accuracy by comparing the difference between the measured values and available certified values with their uncertainty according to the calculation method reported by Linsinger [20]. For measurement of urinary metals, batches of 20 urine samples were analyzed and the standard curves were reanalyzed after each batch to ensure normal performance of the instrument. If the detected values of standard curves were not in agreement with available certified values, the instrument was recalibrated, and the 20 urine samples were re-tested again. The limits of detection (LOD) for these four metals were 0.050 μg/L for Pb, 0.001 μg/L for Cd, 0.009 μg/L for Cu, and 0.01 μg/L for Ni. Samples with concentrations below LOD were set to LOD/2 for each metal.
Determination of Arsenic Species
iAs, MMA, DMA, and trimethylated As (TMA) in urine were analyzed with an atomic absorption spectrophotometer (AA-6800, Shimadzu, Kyoto, Japan) with an As speciation pre-treatment system (ASA-2sp, Shimadzu, Kyoto, Japan). Concentrations of As species were measured as described previously [21]. Quality control for arsenic determinations included the analysis of standard reference material of freeze-dried urine (SRM 2670a). The certified concentration values for arsenic were 480 ± 100 μg/L, and the values measured in this study were 474 ± 20 μg/L. The reliability of arsenic species separation was evaluated by analytical recoveries of added arsenic species. Spiking urine samples with 10 μg/L of iAs, MMA, and DMA resulted in recoveries of 81–92, 88–98, and 89–103% for iAs, MMA, and DMA, respectively. Because TMA was not a urinary iAs metabolite, we calculated total As (TAs) concentrations by summing the concentrations of iAs, MMA, and DMA.
Determination of Urinary Creatinine
The concentrations of urinary creatinine (Cr) were measured through the Jaffe reaction with a method reported previously to remove the influence of the effect of urine dilution on exposure biomarkers measured in spot samples [22]. The concentrations of urinary heavy metals (including urinary Pb, Cd, Cu, Ni, and As) were calibrated on the basis of urinary Cr and were reported as micrograms per gram Cr.
Determination of Urinary 8-OHdG
To determine the concentrations of 8-OHdG, the urine samples were centrifuged at 2000 rpm for 10 min and the supernatants were used for measurement. Urinary concentrations of 8-OHdG were determined using an Abcam ELISA kit (ab201734) according to the manufacturer’s instructions. The concentrations of urinary 8-OHdG were calibrated on the basis of urinary Cr and reported as micrograms per gram Cr.
Determination of Urinary Cystatin C
The urine samples were centrifuged at 2000 rpm for 10 min before tested, and the supernatants were used for measurement with a cystatin C kit (Mlbio, Shanghai, China) according to the manufacturer’s instructions, the levels of urinary cystatin C were further corrected by the concentrations of individual urinary Cr levels and reported as micrograms per gram Cr.
Statistical Analysis
Statistical analysis was performed in the SPSS statistical package (SPSS version 19.0). The distributions of the data were examined with one-sample Kolmogorov-Smirnov normality tests. Nonparametric tests were used if the data were not normally distributed or after transformation. The differences in frequencies were evaluated with chi-square tests. We compared the levels of urinary heavy metals and arsenicals between the two groups of factory workers. The dose-response effects of urinary heavy metals on the oxidative stress biomarker 8-OHdG and kidney damage biomarker cystatin C were separately estimated using bivariate correlation analysis. A binary logistic regression model was used to screen the factors influencing the levels of urinary heavy metals, including age, employment years, factories, BMI, smoking habits, alcohol consumption, and seafood intake in the previous 3 days. In addition, we analyzed factors influencing the levels of urinary 8-OHdG and cystatin C using a binary logistic regression model. Risk factors with significant differences were selected for binary logistic regression. The statistical significance threshold was defined as P < 0.05.
Results
Urinary Heavy Metal Levels
Urinary heavy metal levels of the workers in the two factories are shown in Table 2. Urinary Pb, Cu, and Ni levels of lead-zinc mine foundry workers were significantly higher than those of steel smelting plant workers (P < 0.05). Although there was no significant difference between the levels of urinary Cd of workers in the two factories (P > 0.05), Cd levels of the workers in the lead-zinc mine were higher than those of the steel smelting plant. The lead-zinc mine foundry workers had significantly higher concentrations of iAs and MMA than did the steel smelting plant workers (P < 0.05), and the concentrations of DMA and TAs were significantly lower than those of the steel smelting plant workers (P < 0.05). The steel smelting plant workers also had higher concentrations of DMA and TAs. Furthermore, we found that the steel smelting plant workers had higher amounts of seafood consumption (131.08 g) in the 3 days preceding urine sample collection than did the lead-zinc mine foundry workers (30.69 g). Thus, seafood consumption was probably a major factor that increased DMA and TAs levels in the steel smelting plant workers, similar to many previous reports [23, 24].
Factors Influencing Urinary Heavy Metal Levels
Beyond the analysis comparing foundries, we also analyzed the influences of other potential factors, such as employment years, smoking habits, and seafood consumption in the previous 3 days, on urinary heavy metals. We stratified the employment years into three groups: 10 years or fewer, 10 to 20 years, and 20 years or more. We found that the lead-zinc mine foundry workers with 10 to 20 employment years had the highest urinary Pb. For smelting plant workers, there were no differences in urinary heavy metals across the three employment year groups. In this study, the daily smoking habits of workers in the two factories were also investigated. We divided the subjects into four groups: no smoking, < 10, 10–20, and > 20 branches per day. Urinary Cd levels increased with increasing daily smoking levels. In lead-zinc mine foundry workers, those who had consumed seafood in the previous 3 days had lower levels of urinary Pb than did those who did not consume seafood. In steel smelting plant workers, levels of urinary Ni were higher in those who had consumed seafood than in those who had not consumed seafood (Table 3).
To screen the risk factors affecting the concentrations of urinary heavy metals, we performed binary logistic regression. Age, employment years, factories, BMI, smoking habits, alcohol consumption, and seafood consumption were included. The logistic regression analysis showed that working in lead-zinc mine foundry was a risk factor for urinary levels of Pb and Ni of the workers compared to working in steel smelting plant. In addition, alcohol consumption was another risk factor for urinary levels of Ni. Age and smoking habits were the risk factors for urinary levels of Cd. BMI was a protective factor regarding Cd levels in the urine (Table 4).
Concentrations of 8-OHdG and Cystatin C Biomarkers in the Urine
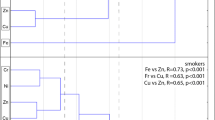
Present evidence indicates that 8-OHdG in urine is a widely accepted sensitive biomarker of oxidative DNA damage. Occupational exposure to heavy metals can trigger oxidative stress responses in the body, which can increase 8-OHdG levels. In this study, there was no significant difference in the concentrations of 8-OHdG between the two groups of factory workers (Table 5). Bivariate correlation analysis showed that 8-OHdG levels of workers were positively correlated with urinary Pb, Cd, iAs, MMA, and DMA concentrations (Spearman correlation coefficients were 0.223, 0.225, 0.267, 0.215, and 0.195, respectively) (P < 0.05) (Table 6). Binary logistic regression was used to screen the risk factors affecting the level of urinary 8-OHdG, and age, employment years, factories, BMI, smoking habits, alcohol consumption, and urinary heavy metals were included in the models. The factors that affected urinary 8-OHdG levels were urinary Pb (OR = 2.502, 95% CI 1.058–5.913) and Cd (OR = 2.473, 95% CI 1.125–5.437) (Table 7).
Cystatin C, a secretory low-molecular-weight protein (13 kDa), is abundant in body fluids and various tissues [25]. The kidney is an important target organ of Cd and Ni. Concentrations of cystatin C increase in urine in cases of renal tubule dysfunction. The preliminary results of this study showed that lead-zinc mine foundry workers might be exposed to Cd and Ni; hence, we selected cystatin C as an early renal damage biomarker, which we used to compare the degree of kidney damage among the two groups of factory workers. The results showed that urinary cystatin C levels in the lead-zinc mine foundry workers were higher than those in the steel smelting plant workers (P < 0.05) (Table 5). Furthermore, to determine the effects of metals on cystatin C, bivariate correlation was performed. Table 6 shows that cystatin C levels in workers was positively correlated with urinary Pb, Cd, Ni, iAs, and MMA (Spearman correlation coefficients were 0.294, 0.176, 0.195, 0.268, and 0.137, respectively) (P < 0.05). Binary logistic regression showed that the risk factors affecting the concentration of urinary cystatin C were urinary Cd (OR = 1.992, 95% CI 1.031–3.849). In addition, urinary cystatin C was associated with age (Table 7).
Discussion
Heavy metal pollution health accidents occur frequently worldwide. Heavy metal pollution has become a major social problem that has received substantial research attention both at home and abroad. Occupational populations contact the heavy metal elements directly, owing to a lack of strict mining production supervision and a lack of basic occupational protection knowledge among most mine workers. Occupational populations are thus likely to encounter occupational disease hazards.
This study included a total of 307 workers from two foundries in Liaoning Province. The five heavy metals analyzed in this study are the most commonly encountered toxic metals detected after occupational and dietary exposure. Urinary concentrations of Pb, Cu, Ni, and As are recognized as short-term indicators of recent exposure, while urinary excretion of Cd is proportional to the body burden and therefore represents an indicator of a lifetime exposure. Our results showed that the lead-zinc mine foundry workers’ urinary Pb, Cd, Cu, and Ni were higher than those of the steel smelting plant workers, indicating that lead-zinc mine foundry workers may have a higher exposure risk of heavy metals. Usually, non-ferrous smelters exposed to inorganic arsenic in the workplace, inorganic As is methylated into MMA and DMA in the liver [12, 26]. The concentrations of urinary iAs and MMA were higher in the lead-zinc mine foundry workers, thus indicating that those workers were probably exposed to As. Many studies have found that seafood products contain a large number of As glucose and As lipids that can be metabolized as DMA [27, 28]. DMA levels in the body significantly increased after seafood consumption [29, 30]. Our study also found that the steel smelting plant workers had higher urinary DMA than those in the lead-zinc mine foundry workers, possibly because the steel smelting plant workers had a higher amount of seafood consumption (131.08 g) in the 3 days preceding urine samples collection than did the lead-zinc mine foundry workers (30.69 g). Furthermore, among the steel smelting plant workers, those who ate seafood exhibited significantly higher urinary Ni levels than those that did not, thus suggesting that seafood products may be enriched in Ni. Usually, seafood is rich in protein, calcium, and zinc which can be replaced and antagonized with Pb after absorption in intestine and excreted with feces. In lead-zinc mine foundry, we observed that workers with no seafood intake have higher levels of urinary Pb than those with seafood intake which indicated that seafood consumption may be an advantageous factor for promoting Pb excretion. However, further investigations are needed to verify these results.
In order to screen for the risk factors affecting the concentrations of urinary heavy metals and arsenic, binary logistic regression analysis of Pb, Cd, Cu, and Ni was carried out. We chose age, employment years, factories, BMI, smoking habits, alcohol consumption, and seafood consumption as influencing factors. In this study, we have found that working in lead-zinc mine foundry was a risk factor for elevating urinary Pb and Ni. In addition, alcohol consumption was another risk factor for elevating urinary Ni. Alcohol consumption can lead to the accumulation of Pb and Cd in the body, but whether it can induce Ni accumulation has not been reported yet. The mechanism of the effect of alcohol consumption on the increase of urinary Ni level needs further confirmation. Age and smoking habits were the risk factors for urinary levels of Cd. BMI was a protective factor regarding Cd levels in the urine.
Smoking is another route of Cd exposure, each cigarette contains approximately 1–2 μg of Cd, and blood cadmium levels in smokers in Europe are three to four times higher than those in non-smokers [31]. In this study, urinary Cd level was elevated with higher daily smoking amounts in the two groups of factory workers, thus indicating that smoking may affect Cd levels in the human body, in agreement with previous reports. Cd can accumulate and remain in the human body for a long time, and can cause serious harm to various organs [32]. Many studies have reported that Cd induces damage to the kidneys, bones, respiratory system, cardiovascular system, reproductive system, and immune system [33,33,35]. The kidney not only is a site of Cd accumulation but also one of the target organs for Cd damage. Cystatin C has recently been used as a sensitive biomarker for early injury of renal function [36]. This study determined the levels of cystatin C in urine to compare the degree of early renal injury among the workers. There were higher levels of cystatin C in the workers in the lead-zinc mine foundry, thus suggesting that lead-zinc mine foundry workers are more likely to have higher renal injury than steel smelting plant workers. Furthermore, we observed a positive linear dose-dependent effect on the urinary levels of cystatin C and urinary Pb, Cd, Ni, iAs, and MMA in the two groups of factory workers. Binary logistic regression analysis of cystatin C also supported the positive association urinary levels of Cd with urinary cystatin C.
Oxidative DNA damage is a widely accepted mechanism of heavy metal-induced carcinogenesis. Although the lead-zinc mine foundry workers had a higher degree of heavy metal exposure than steel smelting plant workers in this study, there was no significant difference in urinary 8-OHdG, thus suggesting that higher doses of heavy metals are required to produce oxidative damage. Oxidative DNA damage is an established mechanism for As-induced carcinogenesis [37]. Many reports carried out in various populations, such as glass production workers, semiconductors, healthy adults, as well as in children, consistently showed that subjects with highly As exposure had elevated the levels of 8-OHdG than those with low As exposure [38,38,39,41]. However, there were no significant associations that have been found between urinary Pb and Cd and 8-OHdG in the present population [42]. In this study, we observed that urinary Pb, Cd, and As had a positive linear dose-dependent effect on oxidative damage to DNA. These results suggested that the occupational exposure to heavy metals may significantly enhanced oxidative damage to DNA.
To our knowledge, there are few studies on the exposure of heavy metals in lead-zinc mine foundry workers. Therefore, this study investigated the levels of heavy metals and arsenide in the urine of two groups of foundry workers. Our results showed that lead-zinc mine foundry workers may have higher exposure to heavy metals, and these results also provide basic data for occupational protection. Further epidemiological studies are needed to develop functional environmental protection measures and to protect occupational populations’ health.
References
Garcia-Nino WR, Pedraza-Chaverri J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69:182–201
Paul D (2017) Research on heavy metal pollution of river ganga: a review. Ann Agric Sci 15:278–286
Pal R, Mahima GA et al (2014) Assessment of heavy metals in suspended particulate matter in Moradabad, India. J Environ Biol 35(2):357–361
Cheng S (2003) Heavy metal pollution in China: origin, pattern and control. Environ Sci Pollut Res Int 10(3):192–198
Abarikwu SO, Essien EB, Iyede OO et al (2017) Biomarkers of oxidative stress and health risk assessment of heavy metal contaminated aquatic and terrestrial organisms by oil extraction industry in Ogale, Nigeria. Chemosphere 185:412–422
Boffetta P, Fontana L, Stewart P et al (2011) Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: a case-control study from Central and Eastern Europe. Occup Environ Med 68(10):723–728
Andrade VM, Aschner M, Marreilha DSA (2017) Neurotoxicity of metal mixtures. Adv Neurobiol 18:227–265
Poreba R, Gac P, Poreba M et al (2012) Assessment of cardiovascular risk in workers occupationally exposed to lead without clinical presentation of cardiac involvement. Environ Toxicol Pharmacol 34(2):351–357
WHO (2004) Cadmium and cadmium compounds. Rep Carcinog 11: I42–I44
Wang Y, Mandal AK, Son Y-OK et al (2018) Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol 353:23–30
Xu MY, Wang P, Sun YJ et al (2017) Joint toxicity of chlorpyrifos and cadmium on the oxidative stress and mitochondrial damage in neuronal cells. Food Chem Toxicol 103:246–252
Lubin JH, Moore LE, Fraumeni JJ et al (2008) Respiratory cancer and inhaled inorganic arsenic in copper smelters workers: a linear relationship with cumulative exposure that increases with concentration. Environ Health Perspect 116(12):1661–1665
Shuhua X, Qingshan S, Fei W et al (2014) The factors influencing urinary arsenic excretion and metabolism of workers in steel and iron smelting foundry. J Expo Sci Environ Epidemiol 24(1):36–41
Ford M (2002) Arsemc. In: Goldfrank LR, Flomenbaum NE, Lewin NA et al (eds) Goldfrank’S Toxicologic Emergencies, 7th edn. McGraw-Hill, New York
Nesnow S, Roop BC, Lambert G et al (2002) DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem Res Toxicol 15(12):1627–1634
Jeng HA, Pan CH, Diawara N et al (2011) Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup Environ Med 68(9):653–658
Junaid M, Hashmi MZ, Malik RN et al (2016) Toxicity and oxidative stress induced by chromium in workers exposed from different occupational settings around the globe: a review. Environ Sci Pollut Res Int 23(20):20151–20167
Wu LL, Chiou CC, Chang PY, Wu JT (2004) Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 339:1–9
WHO (1996) Biological monitoring of chemical exposure in the workplace, vol 1. World Health Organization, Geneva
Linsinger T (2005) Comparison of a measurement result with the certified value. Eur Ref Mater:1–2
Xu Y, Wang Y, Li X, He M, Xue P, Fu J et al (2009) Variations in arsenic methylation capacity and oxidative DNA lesions over a 2-year period in a high arsenic-exposed population. Int Arch Occup Environ Health 82:251–258
Sun G, Xu Y, Li X et al (2007) Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ Health Perspect 115(4):648–652
Ma M, Le XC (1998) Effect of arsenosugar ingestion on urinary arsenic speciation. Clin Chem 44(3):539–550
Francesconi KA, Tanggaar R, Mckenzie CJ et al (2002) Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem 48(1):92–101
Togashi Y, Sakaguchi Y, Miyamoto M et al (2012) Urinary cystatin C as a biomarker for acute kidney injury and its immunohistochemical localization in kidney in the CDDP-treated rats. Exp Toxicol Pathol 64(7–8):797–805
Marcos R, Martinez V, Hernandez A et al (2006) Metabolic profile in workers occupationally exposed to arsenic: role of GST polymorphisms. J Occup Environ Med 48(3):334–341
Raml R, Goessler W, Traar P et al (2005) Novel thioarsenic metabolites in human urine after ingestion of an arsenosugar, 2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinoyl-beta-D-riboside. Chem Res Toxicol 18(9):1444–1450
Schmeisser E, Goessler W, Francesconi KA (2006) Human metabolism of arsenolipids present in cod liver. Anal Bioanal Chem 385(2):367–376
Choi BS, Choi SJ, Kim DW et al (2010) Effects of repeated seafood consumption on urinary excretion of arsenic species by volunteers. Arch Environ Contam Toxicol 58(1):222–229
Xu L, Sun G, Xu Y et al (2008) Effect of seaweed intake on urinary arsenic excretion. Chin J Public Health 24:1093–1094
Nersesyan A, Kundi M, Waldherr M et al (2016) Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium. Mutat Res 770(Pt A):119–139
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79:241–244
Brzoska MM, Moniuszko-Jakoniuk J (2005) Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicol Appl Pharmacol 202(1):68–83
Johri N, Jacquillet G, Unwin R (2010) Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23(5):783–792
El-Boshy M, Ashshi A, Gaith M et al (2017) Studies on the protective effect of the artichoke (Cynarascolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ Sci Pollut Res Int 24(13):12372–12383
Laterza OF, Price CP, Scott MG (2002) Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem 48(5):699–707
Straif K, Benbrahim-Tallaa L, Baan R et al (2009) A review of human carcinogens—part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10:453–454
Hu CW, Pan CH, Huang YL et al (2006) Effects of arsenic exposure among semiconductor workers: a cautionary note on urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Free Radic Biol Med 40:1273–1278
Lin TS, Wu CC, Wu JD et al (2012) Oxidative DNA damage estimated by urinary 8-hydroxy-2′-deoxyguanosine and arsenic in glass production workers. Toxicol Ind Health 28:513–521
Wong RH, Kuo CY, Hsu ML et al (2005) Increased levels of 8-hydroxy-2-deoxyguanosine attributable to carcinogenic metal exposure among school children. Environ Health Perspect 113:1386–1390
Xu Y, Wang Y, Zheng Q et al (2008) Association of oxidative stress with arsenic methylation in chronic arsenic-exposed children and adults. Toxicol Appl Pharmacol 232:142–149
Wang T, Feng W, Kuang D et al (2015) The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers. Environ Res 140:405–413
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (81673207). We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Jin, P., Zhou, Q. et al. Metal Biomonitoring and Comparative Assessment in Urine of Workers in Lead-Zinc and Steel-Iron Mining and Smelting. Biol Trace Elem Res 189, 1–9 (2019). https://doi.org/10.1007/s12011-018-1449-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1449-0