Abstract
Zinc is one of the essential micronutrients that can be obtained via water and diet in aquatic animals to meet their physiological needs. The present study was designed to understand the effect of the supplementation of zinc nanoparticles (Zn-NPs) in mitigating abiotic and biotic stress in Pangasius hypophthalmus. Two zinc nanoparticle-incorporated diets with 10 and 20 mg/kg nanoparticles and a control without zinc nanoparticles were formulated. To study the effect of formulated feeds on stress tolerance, fish were exposed to sublethal dose (4 ppm) of Pb (lead) and temperature at 34 °C. Two hundred and seventy-three fish were randomly distributed into seven treatment groups in triplicates, namely a control group (no Zn-NPs and no Pb and temperature exposure, Ctr/Ctr), control diet fed and exposed to Pb (Ctr/Pb), control diet fed and concurrently exposed to Pb and temperature (Pb-T/Ctr), and Zn-NPs 10 and 20 mg/kg diet with or without stressors (Zn-NPs 10 mg/kg, Zn-NPs 20 mg/kg, Pb-T/Zn-NPs 10 mg/kg, Pb-T/Zn-NPs 20 mg/kg). The effect of Zn-NPs on growth performance, stress biomarkers, biochemical and immunological responses, and survival of P. hypophthalmus following challenge with pathogenic bacteria were evaluated. The growth performance was noticeably (p < 0.01) enhanced, and anti-oxidative stress (catalase, superoxide dismutase, and glutathione-s-transferase) significantly reduced in the Zn-NPs supplemented groups. Similarly, immunological parameters such as total protein, albumin, globulin, and A/G ratio significantly improved, and stress biomarkers such as blood glucose, cortisol, and HSP 70 were reduced in Zn-NPs supplemented groups. Overall, the results suggest that supplementation of dietary Zn-NPs with less concentration in the diet has a definitive role in the mitigation of abiotic and biotic stress in P. hypophthalmus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is an emerging science with proliferated applications not only in medicine, environmental, food, biological, and pharmaceutical science but also in the field of agriculture. The developing countries like India mainly depend on the agriculture including animal husbandry and fisheries to sustain livelihood and to achieve nutritional security [1]; however, to increase the fish production, the aqua-industry requires technologies like nano-based feed formulations to reduce contamination level and the feed cost. Nutrients such as zinc are the best component for nano-based feed formulation as it stands the third position among different nanoparticles produced annually [2]. Zinc nanoparticles (Zn-NPs) are attributed to better antibacterial properties [3] as well as better growth performance and enhanced immune responses in animals [4, 5]. Zn-NPs are one of the most useful metal nanoparticles possessing wider applications in catalysis, energy storage, electronic devices, and several biomedical applications. Generally, zinc compounds and Zn-NPs have similar zinc to oxygen ratio, but at the nano level, atoms are arranged with a wider energy level confinement with small space [6]. Supplementation of dietary Zn-NPs has resulted in enhanced growth performance, improved feed utilization, and economic benefits in the animal feed industries [7, 8]. Among all the trace elements, zinc (Zn) is the second most abundant element found in the animal body, but it cannot be stored in the body [9]. Hence, it is required to be supplemented with regular dietary intake to meet the physiological needs including maintenance of enzymatic processes such as alcohol dehydrogenase, alkaline phosphatase (ALP), aldolase, lactate dehydrogenase (LDH), RNA and DNA polymerases, reverse transcriptase, carboxypeptidase, and superoxide dismutase (SOD) [10]. Zinc is an important component of free radical scavenging system produced during different physiological processes in animals [5]. Numerous studies have demonstrated the beneficial positive role of nano-zinc as compared with conventional Zn [4, 11].
Climate change due to global warming and the resultant food contamination is a major threat to human and animals including fish. Besides exposure to various chemical, physical, and biological factors, aquatic ecosystems are also prone to be severely affected by various anthropogenic and natural disturbances [12,13,14]. Recently, consequences of climate change have attracted attention due to environmental distribution and biological effects of chemical toxicants [15]. The global warming due to climate change and chemical pollutants such as heavy metals adversely affects the environmental distribution and toxicity of numerous chemical toxicants [16]. Lead (Pb) is undoubtedly a dangerous heavy metal responsible for contamination of aquatic ecosystem [17]. Pb occurs mainly in an inorganic form in different oxidation state leading to oxidative stress in aquatic organisms especially fishes and also leads to metal bioaccumulation in different parts of fish tissues [18]. Pb is toxic to the aquatic organisms at a higher level. At lower concentrations, Pb can also induce muscular and neurological degeneration, growth inhibition, mortality, and reproductive impairment [19]. Fish exposed to a higher concentration of heavy metals especially lead (Pb) in water may accumulate substantial quantities in their tissue which may become toxic [20]. The toxicity of metals gets elevated at higher temperature due to enhanced uptake of metals and thus accumulation increases with increasing temperature [21, 22]. The aquatic organism such as fish resident at the edge of their homeostatic or physiological tolerance range may be more vulnerable to the dual stresses of climate change and contaminant exposures [23,24,25].
The present study elucidates the effects of zinc nanoparticles (Zn-NPs) on the alleviations of abiotic stress such as comprehensive stress responses induced by Pb heavy metal and elevated temperature in pathogen-challenged fish.
Materials and Methods
Ethics Statement
The use of fish in this study conforms to the existing laws in India. The care and treatment of fish were in accordance with the guidelines of the CPCSEA [(Committee for the Purpose of Control and Supervision of Experiments on Animals, (Ministry of Environment & Forests (Animal Welfare Division), Government of India]. The study protocol and experimental endpoints were approved by the institute research committee and the authorities of the ICAR-National Institute of Abiotic Stress Management, Baramati, Pune, India.
Experimental Design and Conditions
Pangasius hypophthalmus was obtained from the local fish market (Nil Aquarium, Baramati, Pune, India) and shifted to the NIASM wet laboratory in healthy condition. Fish were quarantined with a prophylactic dip in a salt solution (2%) and then acclimatized to fiberglass reinforced plastic (FRP) tanks (Circular, 500 litre) for 1 month prior to the experiment. Fish were randomly distributed into 21 glass aquaria (60 × 47 × 32 cm) of 60 litre capacity reared for 75 days. Thirteen fish of uniform size (3.67 ± 0.75 g) per glass tank was stocked in seven distinct treatment groups in triplicates following a completely randomized design. The fish were fed with an experimental diet twice daily (10:00 a.m. and 17:00 p.m.) to satiation for 75 days. Round-the-clock aeration was provided with all the glass tanks from a compressed air pump, and manual water exchange (two-thirds) was carried out on every second alternate day. The experimental setup consisted of normal water (without Pb and temperature exposure) and fed with a control diet (control group, Ctr/Ctr), Pb-treated water and fed with the control diet (Pb/Ctr), concurrently exposed to Pb and temperature (34 °C) and fed with control diet (Pb-T/Ctr), normal water (without Pb and temperature exposed) and fed with Zn-NPs-10 and 20 mg/kg (Ctr/Zn-NPs-10 mg, Ctr/Zn-NPs-20 mg), concurrently exposed to Pb and temperature (34 °C) and fed with Zn-NPs @ 10 and 20 mg/kg diet (Pb-T/Zn-NPs 10 mg/kg, Pb-T/Zn-NPs 20 mg/kg). Lead nitrate (Thermo Fisher Scientific India Pvt. Ltd. Mumbai, India) was used for maintaining 1/21 (4 ppm) level of 96 h LC50 (84.93 ppm) as described previously [26]. The temperature was maintained at 34 °C with a thermostatic rod heater. The standard was stored in an airtight container at 4 °C.
Green Synthesis of Zinc Nanoparticles (Zn-NPs)
The fish gill tissue was dissected from live Labeo rohita and washed thoroughly in running water to remove dust and blood, and then tissues were cut in small pieces and mixed with a propriety liquid formulation through mortar pestle. Tissue lysate was centrifuged at 5000 rpm (2935 g), and the supernatant was collected which was further processed for double filtration through Whatman paper to obtain the gill extract [27]. The gill extract was mixed with 200 ml of zinc acetate (200 mM) in distilled water. Thereafter, 200 mM sodium hydroxide solution was added drop by drop in the above solution to bring the pH to 11. A white cloudy precipitate was observed and centrifuged at 8000 rpm (7197 g) for 15 min, followed by washing three times with distilled water. The pellet was dried in the oven at 60 °C until dry and subsequently stored at room temperature. Before using, the dry pellet was crushed in mortar pestle to a fine powder. Similar method was used in our previous work for synthesis of zinc nanopartilces and selenium nanoparticles [27,28,29].
Characterization of Zinc Nanoparticles
The synthesized Zn-NPs were evaluated through an absorption spectrum in the range of 300 to 500 nm in UV-Vis spectrophotometer (UV-1800, Shimadzu, Japan). The peak was observed at 360–380 nm. Synthesized Zn-NPs formulations were diluted with Milli-Q water and processed for particle size and zeta potential determination using a nano size analyzer. [Horiba Scientific Nanoparticles Analyzer nano Partica SZ-100 series (Kyoto, Japan)] at 25 °C. The mean size of Zn-NPs was 206 nm, and a mean zeta potential of − 41 mV was determined (Fig. 1).
Experimental Diet
Three iso-caloric and iso-nitrogenous diets viz. basal diet and two supplemented diets like a zinc free control diet, 10 mg/kg, and 20 mg/kg of zinc nanoparticles diets were prepared. Zinc acetate (HI-MEDIA, Mumbai, India) was used for the formulation of Zn-NPs diet. For the pelleted diet formulation, quality fish meal, soybean meal, sunflower meal, wheat flour, and sunflower oil were procured from the local market. Zinc-free vitamin-mineral mixtures were prepared manually along with ascorbyl phosphate (SD Fine Ltd., Mumbai, India) as the source of vitamin C. The dough was mixed and baked at 60 °C until dry and subsequently stored at 4 °C until required for feeding (Table 1).
Tissue Homogenate Preparation for Enzyme Analysis
Gill, liver, brain, kidney, and muscle tissues of fish from all the groups were dissected, weighed, and homogenized in chilled 0.25 M sucrose solution (5% w/v) in a glass tube using Teflon-coated mechanical tissue homogenizer (Omni Tissue Master Homogenize, Kennesaw, GA). The tubes were kept on ice to avoid denaturation of the enzymes during homogenization. The homogenates were centrifuged at 5000 rpm for 20 min at 4 °C in a cooling centrifuge (Eppendrof, Hauppauge, NY). Protein contents in the supernatants were quantified using the method of Lowry et al. [30]. The supernatants were collected and stored at − 20 °C until analysis.
Sample Preparation for Zinc and Pb Analysis
Water samples (10 ml) were collected from each replicate from all treatments. Fish muscles and feed samples (0.5 g) were taken out for acidic digestion in a microwave digestion system (Microwave Digestion System, Model START-D, SN- 135177, Milestone, USA). HNO3 and H2O2 were added in a 5:1 ratio and kept for digestion. The completely digested samples were allowed to cool to room temperature and filtered with 0.45 μm Whatman paper. The volume was made up to 50 ml and proceed for zinc and lead analysis through inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700 series, Agilent Technologies, USA). The water samples were filtered with 0.45-μm pore size Whatman paper and then acidified using 100 μl of pure HNO3 (69%, Himedia Laboratory Pvt. Ltd. Mumbai, India). Multi-element Calibration Standard (Agilent Technologies, USA) solutions of 10 μg/ml were used to prepare a calibration curve. The calibration curves with R2 > 0.999 were accepted for concentration calculation [12, 13, 27].
Blood Collection
At the end of the 75 days experimental trial, sampling was carried out for the analysis of the different cellular metabolic enzymes and blood-related stress parameters. The sampling was performed 12 h after the last feeding. Three fish from each replicate group were anesthetized with clove oil (50 μl/l), and the blood was collected from the caudal vein using EDTA as anticoagulant, transferred immediately to an Eppendorf tube, and stored at 4 °C. For serum, another two fish from each replicate group were anesthetized, and the blood was collected without anticoagulant and allowed to clot for 2 h followed by a collection of straw-colored serum with a micropipette.
Growth Performance Study
Fish were weighed at 15-day interval till the end of the experiment on day 75. The growth performance of P. hypophthalmus was evaluated in terms of weight gain (%), feed conversion ratio (FCR), and specific growth rate (SGR).
Measurement of Antioxidant Enzymes
Superoxide dismutase (SOD) (EC 1.15.1.1) activity was measured by the method of Misra and Fridovich [31]. The assay was based on the oxidation of epinephrine-adrenochrome transition by the enzyme. Catalase (CAT) (EC 1.11.1.6) activity was measured by the method of Takahara et al. [32]. The reaction mixture consisted of 2.45 ml phosphate buffer (50 mM; pH 7), 50 μl tissue homogenate, and 1 ml of hydrogen peroxide substrate solution (freshly prepared), and the change in absorbance was measured for 3 min at 240 nm. Glutathione-S-transferase (GST) (EC 2.5.1.18) was measured spectrophotometrically (UV-1800, Shimadzu, Japan) by the method of Habing et al. [33]. The S-2,4-dinitrophenyl glutathione (CDNB) was used as a substrate.
Acetylcholine Esterase (AChE)
AChE (EC. 3.1.1.7) activity was measured as the change in OD at 540 nm using the method of Hestrin modified by Augustinsson [34].
Cortisol and HSP-70
Commercially available EIA kit was used for the quantification of serum cortisol (Cayman Chemicals, USA) and HSP-70 (Bioguenix/Enzo Life Science, Mumbai, India) in the gill and the liver according to the manufacturers’ instructions. The absorbance was read in an ELISA plate reader (Clario Star, BMG Labtech, Germany).
Respiratory Burst Activity
The respiratory burst activity assay was determined by the method of Secombes [35] as modified by Stasiack and Baumann [36]. The optical reading for estimation of NBT was observed at 630 nm in an ELISA reader (Clario Star, BMG Labtech, Germany).
Serum Proteins (Total Protein, Albumin, Globulin, and A/G Ratio)
Total plasma protein was quantified colorimetrically by BCA method using protein estimation kit. Albumin was quantified using a bromocresol green binding method by Doumas et al. [37]. Globulin was quantified by subtracting albumin values from total plasma protein. Albumin/globulin ratio (A/G ratio) was determined by dividing albumin values by globulin values.
Blood Glucose
Blood glucose level was estimated by the method of Nelson [38] and Somoyogi [39]. Blood was deproteinized with zinc sulfate and barium hydroxide, and filtered, and the supernatant is used for glucose estimation. The absorbance was recorded at 540 nm against the blank.
Challenge Study with Aeromonas veronii biovar sobria
After 75 days of feeding, eight fishes per group were challenged with a virulent strain of Aeromonas veronii biovar sobria (obtained from Aquatic Animal Health Management Division, Central Institute of Fisheries Education, Mumbai). Initially, the pathogenic isolates of A. veronii biovar sobria were grown on a nutrient broth for 24 h at 37 °C in a BOD incubator and harvested by centrifuging the culture broth at 8000 rpm (7197 g) for 10 min at 4 °C. The cells were then washed thrice with sterile PBS (pH 7.2), and the final concentration was maintained at 108 CFU/ml. The fish in each experimental group were intraperitoneally injected with 0.2 ml of bacterial suspension. Mortality was observed for a week. Tissues were taken from the dead fish for bacteriological culture to confirm A. veronii biovar sobria as the cause of death.
Cumulative mortality (%) and relative percent survival (RPS) in different treatment groups were calculated by the following formula:
Statistical Analysis
The data were statistically analyzed by the Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS, Chicago, IL), in which data were subjected to a one-way ANOVA followed by Duncan’s multiple range tests to determine the significant differences between the means. Comparisons were made at the 5% probability level.
Results
Growth Performance
Growth performance in terms of weight gain (%), FCR, PER, and SGR of P. hypophthalmus fed with Zn-NPs and exposed to Pb and high temperature are presented in Table 2. The maximum weight gain (%), PER, and SGR were observed in the group fed with Zn-NPs @ 10 mg/kg diet with or without stressors (Pb and high temperature) in comparison to other groups. Weight gain (%), PER, and SGR were significantly reduced (p < 0.01) in the group treated with Pb and high temperature and fed with control diet in comparison with control group. In the case of Zn-NPs @ 20 mg/kg diet supplemented group, the weight gain (%), PER, and SGR were found to be noticeably (p < 0.01) less compared to the group fed with Zn-NPs @ 10 mg/kg diet. Further, FCR was significantly (p < 0.01) less in the group fed with Zn-NPs @ 10 mg as compared to other groups.
Effect on Anti-Oxidative Stress Status
Antioxidative status (CAT, SOD, and GST) in the liver, gill, brain, and kidney of P. hypophthalmus fed with Zn-NPs and exposed to Pb and high temperature are presented in Tables 3 and 4. Liver, gill, brain, and kidney CAT, SOD, and GST activities were significantly lowered (p < 0.01) in the group treated with Zn-NPs @ 10 mg/kg diet with or without stressors compared to all other groups. Further, CAT, SOD, and GST activities were remarkably elevated (p < 0.01) in Pb and high-temperature exposure group in comparison to other groups in all tissues. The group treated with Zn-NPs @ 20 mg/kg diet showed significantly (p < 0.01) higher oxidative stress in all four tissues in comparison to the group treated with Zn-NPs @ 10 mg/kg and the control group.
Neurotransmitter Enzymes
Neurotransmitter enzyme activities in the form of acetylcholine esterase (AChE) in the brain, muscle, and liver of P. hypophthalmus fed with Zn-NPs and exposed to Pb and high temperature are presented in the Table 4. AChE activities in the brain, muscle, and liver were significantly (p < 0.01) inhibited in the group exposed to Pb alone or in combination with elevated temperature compared to all other groups (Table 4). The AChE activities were remarkably improved (p < 0.01) in all the tissues studied in the group treated with Zn-NPs @ 10 mg/kg diet. On the contrary, the group treated with 20 mg/kg of Zn-NPs, the AChE activity, was inhibited in all the tissues (Table 4).
Stress Biomarkers
Stress biomarker in terms of cortisol, HSP 70 in liver and gill, and a blood glucose of P. hypophthalmus fed with Zn-NPs and exposed to Pb and high temperature are presented in Table 5. The level of cortisol, blood glucose, and HSP 70 in liver and gill were remarkably elevated (p < 0.01) in concurrent exposure to Pb and high temperature followed by Pb exposure alone. The level of stress biomarkers was significantly reduced (p < 0.01) by supplementation of Zn-NPs @ 10 mg/kg diet, whereas with supplementation of Zn-NPs @ 20 mg/kg diet, a significantly higher level of stress biomarkers in was noticed in treated groups.
Immunological Status
Immunological status in terms of nitroblue tetrazolium (NBT), total protein (TP), albumin, globulin and A/G ratio of P. hypophthalmus fed with Zn-NPs and exposed to Pb and high temperature are presented in Table 5. The NBT activities were significantly higher (p < 0.01) in Zn-NPs @ 10 mg/kg in compared to other groups followed by Zn-NPs @ 10 mg/kg and exposed to Pb and high temperature. Total protein, albumin, globulin, and A/G ratio were significantly altered (p < 0.01) due to Pb and high temperature exposure. Further, with supplementation of Zn-NPs @ 10 mg/kg diet, the immunological status was remarkably improved (p < 0.01). The supplementation of Zn-NPs @ 20 mg/kg diet was responsible (p < 0.01) for reduction in total protein, albumin, and globulin, but the A/G ratio was elevated.
Bacterial Challenged with Aeromonas veronii biovar sobria
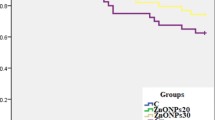
The relative percentage survival (RPS) and cumulative mortality (%) of P. hypophthalmus challenged with A. veronii biovar sobria after the experimental period of 75 days are presented in Fig. 2. After bacterial challenged of P. hypophthalmus, the RPS was − 20, − 26, 26, 0, 20, and − 6% in Pb-exposed group alone, concurrent exposure to Pb and temperature group, Zn-NPs-10 mg/kg diet group, Zn-NPs-20 mg/kg diet group, Zn-NPs-10 mg/kg treated with stressors, and Zn-NPs-20 mg/kg treated with stressor group, respectively. Similarly, high percentage cumulative mortality was observed in Pb and temperature-exposed groups, followed by Pb alone exposed group, whereas the lowest mortality was observed in group treated with Zn-NPs @ 10 mg/kg followed by Zn-NPs @ 10 mg/kg treated with Pb and high temperature. After bacterial challenge, the clinical signs such as hemorrhagia, shallow to deep necrotizing ulcers, and abdominal distension with sero-hemorrhagic fluids exuding from the inflamed vent (Fig. S-1), and also the deformity in vertebral structure such as scoliosis (abnormal lateral curvature) and lordosis (excessive inward curvature) were observed in Pb and high-temperature-exposed groups fed with Zn-free diet (Fig. S-2).
Lead Concentration in Experimental Water and Fish Muscle
Lead (Pb) concentration in experimental water and fish muscle is presented in Fig. 3. Pb concentration in experimental water varies from 2.31, 175.63, 489.53, 5.23, 6.23, 226.25, and 391.23 μg/l in the control group, Pb-exposed group, concurrent exposed to Pb and high temperature, Zn-NPs @ 10 mg/kg, Zn-NPs @ 20 mg/kg, Zn-NPs @ 10 mg/kg and exposed to Pb and high temperature, and Zn-NPs @ 20 mg/kg and exposed to Pb and high temperature, respectively. Similarly, Pb concentration in fish muscle varied from 0.53, 1.43, 1.99, 0.56, 0.47, 0.51, and 1.39 mg/kg in the control group, Pb-exposed group, concurrently exposed to Pb and high temperature, Zn-NPs @ 10 mg/kg, Zn-NPs @ 20 mg/kg, Zn-NPs @ 10 mg/kg and exposed to Pb and high temperature, and Zn-NPs @ 20 mg/kg and exposed to Pb and high temperature, respectively.
Zinc Concentration in Fish Muscle and Experimental Diets
Zn concentrations in the experimental diets were 2.28, 7.69, and 12.64 mg/kg in control diet (Zn 0 mg/kg), Zn-NPs @ 10 mg/kg, and Zn-NPs @ 20 mg/kg, respectively (Fig. 3). On the other hand, the concentrations of Zn in fish muscle were 2.41, 2.19, 2.14, 2.39, 2.51, 3.14, and 2.86 mg/kg in the control group, Pb-exposed group, concurrently exposed to Pb and high temperature, Zn-NPs @ 10 mg/kg, Zn-NPs @ 20 mg/kg, Zn-NPs @ 10 mg/kg and exposed to Pb and high temperature, and Zn-NPs @ 20 mg/kg and exposed to Pb and high temperature, respectively.
Discussion
Dietary zinc has the ability to generate a stable association with macromolecules such as protein, nucleic acid, carbohydrate, lipid, and enzymes and is essential for diverse biological functions. It acts as an electron acceptor, as a cofactor for metalloenzymes such as DNA and RNA polymerases, alcohol dehydrogenase, carbonic anhydrase, and alkaline phosphatase [40]. Zn is also an essential micro-nutrient for the growth of animals including fish. The deficiency of Zn results in reduced appetite, depressed growth, and abnormalities of the skin and appendages [41]. In the present study, Zn-NPs @ 10 mg/kg diet provide better growth performance, whereas a higher concentration @ 20 mg/kg diet inhibited the growth performance of P. hypophthalmus. These results clearly suggest that Zn is necessary for growth and development of freshwater fish at lower concentrations, but higher Zn concentrations are harmful and toxic to fish [42]. Zn-NPs in fish feed have higher intestinal absorption, bioavailability, and catalytic activities [43]. It might be possible that conversion of Zn-NPs increases the efficiency of Zn by enhancing its absorption and bioavailability in the gastrointestinal tract and also has a role in the synthesis of growth hormones [44]. However, the effect of Zn-NPs on growth performance may be attributed to somatic growth by stimulation of DNA and RNA synthesis and cell division [45].
The anti-oxidative status is maintained through CAT, SOD, and GST activities which are responsible for scavenging superoxide radicals and involved in protective mechanism within tissue injury following oxidative process and phagocytosis [46, 47]. Zinc is a key component critical for the function of the transcription factor, antioxidant defense system, and DNA repair. Zn deficiency may lead to increased lipid peroxidation in mitochondrial and microsomal membranes resulting in the osmotic fragility of erythrocyte membranes [48, 49]. The presence of Zn prevents lipid peroxidation and thus plays an important role in protecting the cells from oxidative stresses [50]. Zinc acts as an antioxidant to minimize cell damage due to the formation of free radicals in the cell. Moreover, Zn is an essential component of Cu-Zn-SOD complex involved in the cellular scavenging of free radicals and ROS [10]. In the present study, the Zn-NPs @ 10 mg/kg diet protected the tissues from oxidative damage and this could be attributed to the role of Zn in the form of Cu-Zn-SOD.
AChE activities were inhibited by exposure to Pb and high temperature and improved by supplementation of Zn-NPs @ 10 mg/kg diet. AChE is the most important enzyme for several physiological functions due to its role in the hydrolysis of acetylcholine into cholinergic synopsis [23, 46, 47, 51]. The cholinergic neurotransmission enhances undesirable effects, which have been observed in several neurological disorders due to alteration in AChE. In this study, AChE activities in the brain, muscle, and liver were improved by the application Zn-NPs @ 10 mg/kg diet possibly due to the formation and exocytosis of synaptic vesicles containing neurotransmitters. The activities of AChE are generally decreased by Pb and high temperature. These enzymes have essential roles in the normal sensory and neuromuscular operations [52]. The harmful substances affect fish brain functions through AChE activity and protein carbonylation [53]. The inhibition and overestimation of AChE and acetylcholine receptors can cause miosis, bradycardia, muscle weakness, disorientation, cardiovascular collapse, etc. [54]. In our knowledge, no such reports are available on AChE activities and Zn-NPs application in feed for P. hypophthalmus.
It is well known that stresses due to contaminants and environmental factors result in the elevation of cortisol and HSP 70. Similar results were obtained in the present study. The cortisol and HSP 70 in the liver and gills were elevated in the group exposed to Pb and high temperature (Table 5) Further, supplementation with Zn-NPs @ 10 mg/kg diet reduced cortisol and HSP 70 level which can be an attributed effect of Zn-NPs on the adrenal glands [55] and influence on adrenocorticotropic due to the penetration of Zn-NPs through the blood-brain barrier. In addition, the anti-oxidative properties of zinc on cortisol secretion could also be responsible for the observed effect [56]. The level of HSP affects cell survival with interaction with various elements of programmed cell death machinery, which increase intracellular levels under stress conditions. HSP plays an essential role in maintaining cellular homeostasis by assisting with the correct folding of nascent and stress-accumulated misfolded proteins, preventing protein aggregation or promoting selective degradation of misfolded or denatured proteins [57]. Similarly, in the present study, the blood glucose level increased sharply upon exposure to Pb alone and upon concurrent exposure to Pb and high temperature. Our results are in agreement with Chowdhury et al. [58] and Jiang et al. [59] who reported increase in glucose level in fish under stress condition. It might be due to increase in lactate level due to stress as and the resultant anaerobic metabolism [60]. Supplementation of Zn-NPs @ 10 mg/kg diet had a significant role in the reduction of blood glucose levels. Several mechanisms may be involved in the regulation of blood glucose levels with Zn-NPs. A key regulator of the phosphorylation involved in the insulin receptor called tyrosine phosphatase, a protein which targets the Zn ions [61] and also Zn-improved peripheral insulin sensitivity as it can potentiate insulin-stimulated glucose transport, could possibly be involved in the Zn-NP-mediated alterations in glucose levels [62, 63].
NBT, total protein, albumin, globulin, and A/G ratio were improved by supplementation of Zn-NPs @ 10 mg/kg. Our earlier study also showed that when the fish were exposed to contamination, the innate immunity of the fish diminished considerably [46, 47]. Generally, fish are able to maintain their integrity through an innate immune system based on cell phagocytosis and secretion of soluble antimicrobial molecules. The innate system is characterized by being non-specific and therefore not dependent upon previous recognition of the surface structures of the invader. Our earlier report proved that through nutritional approaches with safe concentration, enhanced immunity of the fish could be achieved [25, 29, 45, 46].
In the present study, Pb and high-temperature-exposed fish were challenged with A. veronii biovar sobria with the resultant of occurrence of severe mortality (Fig. 2). After bacterial challenge, clinical signs such as hemorrhagia, shallow to deep necrotizing ulcers, and abdominal distension with sero-hemorrhagic fluids exuded from the inflamed vent were also observed. The supplementation of Zn-NPs @ 10 mg/kg diet protects against bacterial infection. It is well established that Zn-NPs have antimicrobial activity [64]. The Zn-NPs at lower concentrations are not stable under harsh process conditions but are safe for humans and animals [65]. Zn-NPs possess large surface to volume ratio that helps in interacting with the bacterial cell surface and exhibiting enhanced antibacterial activity as compared to inorganic Zn [66].
The bioaccumulation of Pb in experimental water and fish muscle was elevated, but the application of Zn-NPs @ 10 mg/kg reduced the bioaccumulation level. It might be due to the role of Zn-NPs in the occurrence of fast detoxification in the fish body. The vertebral deformities were also observed such as scoliosis (abnormal lateral curvature) and lordosis (excessive inward curvature) in Pb and high-temperature-exposed fish and fed with Zn-free diet. To the best of our knowledge, the present study is the first report of Zn-NPs as stress mitigators in P. hypophthalmus.
Conclusion
The abiotic and biotic stress response in fish studied in this experiment realistically resonates with the state of aquatic animal health worldwide. We used stresses such as Pb and high temperature and A. veronii biovar sobria as a bacterial agent of infection. To alleviate these multiple stressors in aquatic animals, a nanotechnology-based intervention was attempted. The green synthesis of Zn-NPs from fish wastes will help in the waste management and support the feed industries in nano feed formulation. In the present study, we attempted Zn-NPs @ 10 and 20 mg/kg diet and found that Zn-NPs @ 10 mg/kg diet was suitable for modulating immunity against multiple stressors. Hence, Zn-NPs @ 10 mg/kg are appropriate for feed formulations to alleviate natural and abiotic as well as biotic stresses in cultured fish.
References
Sri Sindhura K, Prasad TNVKV, Selvam PP, Hussain OM (2014) Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl Nanosci 4:819–827. https://doi.org/10.1007/s13204-013-0263-4
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials for Europe and the world. J Nanopart Res 14:1109–1120. https://doi.org/10.1007/s11051-012-1109-9
Padmavathy N, Vijayaraghavan R (2008) Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater 9(3):035004
Sahoo A, Swain RK, Mishra SK, Jena B (2014) Serum biochemical indices of broiler birds fed on inorganic, organic and nano zinc supplemented diets. Int J Recent Scientific Res 5:2078–2081
Zhao CY, Tan SX, Xiao XY, Qiu XS, Pan JQ, Tang ZX (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160:361–367. https://doi.org/10.1007/s12011-014-0052-2
Wang LZ (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter 16(04):58969–58965. https://doi.org/10.1088/0953-8984/16/25/R01
Mishra A, Swain RK, Mishra SK, Panda N, Sethy K (2014) Growth performance and serum biochemical parameters as affected by nano zinc supplementation in layer chicks. Indian J Anim Nutr 31:384–388
Swain PS, Somu Rao BNS, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2:134–141. https://doi.org/10.1016/j.aninu.2016.06.003
Zalewski PD, Ai QT, Dion G, Lata J, Chiara M, Richard ER (2005) Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets: a review. Pharmacol Ther 105:127–149. https://doi.org/10.1016/j.pharmthera.2004.09.004
Prasad AS (1991) Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53:403–412
Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, Zhang F, Zhao YL, Chai ZF (2006) Acute toxicity of nano-and micro-scale zinc powder in healthy adult mice. Toxicol Lett 161:115–123. https://doi.org/10.1016/j.toxlet.2005.08.007
Kumar N, Krishnani KK, Meena KK, Gupta SK, Singh NP (2017) Oxidative and cellular metabolic stress of Oreochromis mossambicus as biomarkers indicators of trace element contaminants. Chemosphere 171:265–274. https://doi.org/10.1016/j.chemosphere.2016.12.066
Kumar N, Krishnani KK, Gupta SK, Singh NP (2017) Cellular stress and histopathological tools used as biomarkers in Oreochromis mossambicus for assessing metal contamination. Environ Toxicol Pharmacol 49:137–147. https://doi.org/10.1016/j.etap.2016.11.017
Kumar N, Krishnani KK, Kumar P, Jha AK, Gupta SK, Singh NP (2017) Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish Shellfish Immunol 62:184–194. https://doi.org/10.1016/j.fsi.2017.01.017
Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, Erwin KN, Levin ED (2009) The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35:971–986. https://doi.org/10.1016/j.envint.2009.02.006
IPCC (2007) Intergovernmental panel on climate change, fourth assessment report. Cambridge University Press, New York
Kumar N, Krishnani KK, Chandan NK, Singh NP (2017) Dietary zinc potentiates thermal tolerance and cellular stress protection of Pangasius hypophthalmusreared under lead and thermal stress. Aquac Res 49:1105–1115. https://doi.org/10.1111/are.13560
Jackson RN, Baird D, Els S (2005) The effect of the heavy metals, lead (Pb 2+) and zinc (Zn 2+) on the brood and larval development of the burrowing crustacean, Callianassa kraussi. Water S A 31(1):107–116
Rabitto IS, Alves JRMC, Silva de Assis HC, Pelletier EE, Akaishi FM, Anjos A, Randi MF, Ribeiro OCA (2005) Effects of dietary Pb (II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotoxicol Environ Saf 60:147–156. https://doi.org/10.1016/j.ecoenv.2004.03.002
Yildirim Y, Gonulalan Z, Narin I, Soylak M (2009) Evaluation of trace heavy metal levels of some fish species sold at retail in Kayseri, Turkey. Environ Monit Asses 149:223–228. https://doi.org/10.1007/s10661-008-0196-7
Hutchins DA, Teyssié JL, Boisson F, Fowler SW, Fisher NS (1996) Temperature effects on uptake and retention of contaminant radionuclides and trace metals by the brittle star Ophiothrix fragilis. Mar Environ Res 41:363–378. https://doi.org/10.1016/0141-1136(95)00026-7
Heugens EHW, Hendriks AJ, Dekker T, van Straalen NM, Admiraal W (2002) A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Crit Rev Toxicol 31(3):247–284. https://doi.org/10.1080/20014091111695
Kumar N, Jadhao SB, Chandan NK, Akhlak MD, Rana RS (2012) Methyl donors potentiates growth, metabolic status and neurotransmitter enzyme in Labeo rohita fingerlings exposed to endosulfan and temperature. Fish Physiol Biochem 38(5):1343–1353. https://doi.org/10.1007/s10695-012-9622-4
Kumar N, Minhas PS, Ambasankar K, Krishnani KK, Rana RS (2014) Dietary lecithin potentiates thermal tolerance and cellular stress protection of milk fish (Chanos chanos) reared under low dose endosulfan induced stress. J Therm Biol 46:40–46. https://doi.org/10.1016/j.jtherbio.2014.10.004
Kumar N, Ambasankar K, Krishnani KK, Kumar P, Akhtar MS, Bhushan S, Minhas PS (2016) Dietary pyridoxine potentiates thermal tolerance, heat shock protein and protect against cellular stress of milk fish (Chanos chanos) under endosulfan-induced stress. Fish Shellfish Immunol 55:407–414. https://doi.org/10.1016/j.fsi.2016.06.011
Kumar N, Krishnani KK, Singh NP (2017) Temperature induces lead toxicity in Pangasius hypophthalmus: an acute test, antioxidative status and cellular metabolic stress. Int J Environ Sci Technol 15:57–68. https://doi.org/10.1007/s13762-017-1364-5
Kumar N, Krishnani KK, Kumar P, Singh NP (2017) Zinc nanoparticles potentiates thermal tolerance and cellular stress protection of Pangasius hypophthalmus reared under multiple stressors. J Therm Biol 70:61–68. https://doi.org/10.1016/j.jtherbio.2017.10.003
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes and histopathological response in fish. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-1165-x
Kumar N, Krishnani KK, Gupta SK, Singh NP (2017) Selenium nanoparticles enhanced thermal tolerance and maintain cellular stress protection of Pangasius hypophthalmus reared under lead and high temperature. Respir Physiol Neurobiol 246:107–116. https://doi.org/10.1016/j.resp.2017.09.006
Lowry OH, Ronebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Takahara S, Hamilton BH, Nell JV, Kobra TY, Ogura Y, Nishimura ET (1960) Hypocatalesemia, a new generis carrier state. J Clin Invest 29:610–619. https://doi.org/10.1172/JCI104075
Habing WH, Pabst MN, Bjacoby W (1947) Glutathione S-transferases. Transferase, the first enzymatic step in mercatpopunc acid formation. J Biol Chem 249:7130–7139
Hestrin S (1949) The reaction of acetyl choline and other carboxylic acid derivatives with hydroxylamine and its analytical application. J Biol Chem 180:249–261
Secombes CJ (1990) Isolation of salmonid macrophage and analysis of their killing activity. In: Stolen JSTC, Fletcher DP, Anderson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS publication, fair haven (NJ), pp 137–152
Stasiack AS, Bauman CP (1996) Neutrophil activity as a potent indicator concomitant analysis. Fish Shellfish Immunol 37:539
Doumas BT, Watson W, Biggs HG (1971) Albumin standards and measurement of serum albumin with bromocresol green. Clin Chim Acta 31:87–96. https://doi.org/10.1016/0009-8981(71)90365-2
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Somoyogi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–68
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Manickam N, Srinivasan V (2014) Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol Trace Elem Res 160:56–66. https://doi.org/10.1007/s12011-014-0026-4
Petrovic V, Nollet L, Kovac G (2010) Effect of dietary supplementation of trace elements on the growth performance and their distribution in the breast and thigh muscles depending on the age of broiler chickens. Acta Vet Brno 79:203–209. https://doi.org/10.2754/avb201079020203
Hao L, Chen L, Hao J, Zhong N (2013) Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): a comparative study with its bulk counterparts. Ecotoxicol Environ Saf 91:52–60. https://doi.org/10.1016/j.ecoenv.2013.01.007
Alishahi A, Mirvaghefi A, Tehrani MR, Farahmand FA, Shojaosadati SA, Dorkoosh FA (2011) Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem 126:935–940. https://doi.org/10.1016/j.foodchem.2010.11.086
Imamoglu S, Bereket A, Turan S, Tagaand Y, Haklar G (2005) Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3,somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J Pediatr Endocrinol Metab 18:69–74. https://doi.org/10.1515/JPEM.2005.18.1.69
Siklar Z, Tuna C, Dallar Y, Tanyer G (2003) Zinc deficiency: a contributing factor of short stature in growth hormone deficient children. J Trop Pediatr 49:187–188
Kumar N, Gupta S, Chandan NK, Aklakur M, Pal AK, Jadhao SB (2014) Lipotropes protect against pathogen-aggravated stress and mortality in low dose pesticide-exposed fish. PLoS One 9(4):e93499. https://doi.org/10.1371/journal.pone.0093499
Kumar N, Ambasankar K, Krishnani KK, Bhushan S, Minhas PS (2016) Dietary pyridoxine protects against stress and maintains immune-hematological status in Chanos Chanos exposed to endosulfan. Basic Clin Pharmacol Toxicol 119:297–308. https://doi.org/10.1111/bcpt.12589
Dhawan DK, Chadha VD (2010) Zinc: a promising agent in dietary chemoprevention of cancer. Indian J Med Res 132:676–682
Verstraeten SV, Zago MP, MacKenzie GG, Keen CL, Oteiza PI (2004) Influence of zinc deficiency on cell-membrane fluidity in Jurkat, 3T3 and IMR-32 cells. Biochem J 378:579–587
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57:399–411. https://doi.org/10.1016/S0753-3322(03)00081-7
Muthappa NA, Gupta S, Yengkokpam S, Debnath D, Kumar N, Pal AK, Jadhao SB (2014) Lipotropes promote immunobiochemical plasticity and protect fish against low-dose pesticide-induced oxidative stress. Cell Stress Chaperons 19(1):61–81. https://doi.org/10.1007/s12192-013-0434-y
Murphy SD (1986) Pesticides. The basic science of poisons. Macmillan, New York, pp 519–581
Miranda RR, Damaso da Silveira AL, de Jesus IP, Grotzner SR, Voigt CL, Campos SX, Garcia JR, Randi MA, Ribeiro CA, Filipak Neto F (2016) Effects of realistic concentrations of TiO2 and ZnO nanoparticles in Prochilodus lineatus juvenile fish. Environ Sci Pollut Res 23(6):5179–5188. https://doi.org/10.1007/s11356-015-5732-8
Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors. Curr Neuropharmacol 11(3):315–335. https://doi.org/10.2174/1570159X11311030006
Espanani HR, Kobra S, Leila S, Vahid YB, Esmaiel A (2014) Investigation of the zinc oxide nanoparticles effect on testosterone, cholesterol and cortisol in rats. Res J Recent Sci 3(4):14–19
Yousefi Babadi V, Amraeai E, Salehh H, Sadeghi L, Najafi L, Fazilati M (2013) Evaluation of iron oxide nanoparticles effects on tissue and enzymes of thyroid in rats. Int Res J Biological Sci 2:67–69
Morimoto RI (1993) Cells in stress: transcriptional activation of heat shock genes. Science 259:1409–1410
Chowdhury MJ, Pane EF, Wood CM (2004) Physiological effects of dietary cadmium acclimation and waterborne cadmium challenge in rainbow trout: respiratory, ionoregulatory, and stress parameters. Comp Biochem Physiol C: Toxicol Pharmacol 139:163–173. https://doi.org/10.1016/j.cca.2004.10.006
Jiang D, Wu Y, Huang D, Ren X, Wang Y (2017) Effect of blood glucose level on acute stress response of grass carp Ctenopharyngodon idella. Fish Physiol Biochem 43:1433–1442. https://doi.org/10.1007/s10695-017-0383-y
Pottinger TG (1998) Changes in blood cortisol, glucose and lactate in carp retained in anglers’ keepnets. J Fish Biol 53:728–742. https://doi.org/10.1111/j.1095-8649.1998.tb01828.x
Haase H, Maret W (2005) Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals 18:333–338. https://doi.org/10.1007/s10534-005-3707-9
Tang X, Shay NF (2001) Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr 131:1414–1420
Wijesekara N, Chimienti F, Wheeler MB (2009) Zinc, a regulator of islet function and glucose homeostasis. Diabetes Obes Metab 11(Suppl 4):202–214. https://doi.org/10.1111/j.1463-1326.2009.01110.x
Valencia PM, Farokhzad OC, Karnik R, Langer R (2012) Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol 7:623–629. https://doi.org/10.1038/nnano.2012.168
Espitia P, Soares ND, Coimbra JD, de Andrade N, Cruz R, Medeiros E (2012) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol 5:1447–1464. https://doi.org/10.1007/s11947-012-0797-6
Sarwar S, Chakraborti S, Bera S, Sheikh IA, Hoque KM, Chakrabarti P (2016) The antimicrobial activity of ZnO nanoparticles against Vibrio cholerae: variation in response depends on biotype. Nanomedicine 12:1499–1509. https://doi.org/10.1016/j.nano.2016.02.006
Acknowledgements
The authors express sincere gratitude to the Director, ICAR-National Institute of Abiotic Stress Management, Baramati, Pune, for providing all the facilities to conduct the present work. The financial assistance has been provided by Indian Council of Agricultural Research (ICAR), New Delhi, India, as an institutional project (no. IXX09673) is gratefully acknowledged. Authors are grateful to Dr. Veera M. Boddu, Research Leader, National Centre for Agriculture Utilization Research (NCAUR) Agricultural Research Service, US Department of Agriculture, ARS/USDA USA, Dr. Sanath H Kumar, Senior Scientist, Central Institute of Fisheries Education, Mumbai India, and Dr. Sib Sankar Giri, Laboratory of Aquatic Biomedicine, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, South Korea, for English editing and correction. I also place a record of thanks to Mrs. Yogita and Mr. Yuvraj Sanas for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 847 kb)
Rights and permissions
About this article
Cite this article
Kumar, N., Krishnani, K.K. & Singh, N.P. Effect of Dietary Zinc-Nanoparticles on Growth Performance, Anti-Oxidative and Immunological Status of Fish Reared Under Multiple Stressors. Biol Trace Elem Res 186, 267–278 (2018). https://doi.org/10.1007/s12011-018-1285-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1285-2