Abstract
A 2-month preliminary study was conducted to delineate the effect of dietary methyl donors (choline, betaine, and lecithin) on the growth performance and metabolic status of Labeo rohita fingerlings subjected to endosulfan alone and in combination with elevated temperature. Four iso-caloric and iso-nitrogenous diets viz. basal diet, betaine-supplemented diet, choline–supplemented diet and lecithin-supplemented diet were prepared and fed to the different experimental groups throughout the experimental period as per the design. Two hundred and seventy fingerlings (average weight 7.95 ± 0.04 g) were randomly distributed in six treatment groups each having three replicates. The experimental groups were as follows: fish subjected to normal water (without endosulfan) and fed with control diet (control group T0), fish subjected to endosulfan-treated water and fed with control diet (T1), fish subjected to concurrent exposure of endosulfan and elevated temperature and fed with control diet (T2), fish subjected to endosulfan and elevated temperature and fed with choline-supplemented diet (T3), fish subjected to endosulfan and temperature and fed with betaine-supplemented feed (T4), and fish subjected to endosulfan and temperature and fed with lecithin-supplemented feed (T5). The result shows that in both the groups, that is, endosulfan exposed and concurrent exposure to endosulfan and elevated temperature group of L. rohita the growth performance like percentage weight gain, feed conversion ratio and specific growth rates were significantly different (P < 0.01) when fed with supplemented diet compared with control fed group. The liver LDH and MDH activity were significantly lower in lecithin, betaine, and choline fed groups. The muscle AST as well as G6PDH, AST, and ALT did not vary but liver ALT, gill and liver ATPase, intestine ALP, muscle and liver glycogen varied significantly with dietary supplementation. The liver and gill glutathione-S-transferase (GST) activities were significantly lower in methyl donors-supplemented groups and brain AChE activity showed lower inhibition in supplemented groups in both endosulfan alone and concurrently exposed endosulfan and temperature groups. The result obtained in this study concludes that inclusion of methyl donors, particularly lecithin and betaine in feed as nutritional supplements have potential to improve growth and stress mitigating effect in L. rohita fingerlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Labeo rohita (Hamilton), one of the Indian major carps, is considered to be the major aquaculture candidate species in India as well as in South East Asian countries (FAO 2001) and provides livelihood to millions of people. But, global warming along with indiscriminate use of antibiotics, synthetic growth promoters, and pesticides like endosulfan is likely to potentially affect its productivity in wild fish populations as well as in aquaculture systems globally (Ficke et al. 2007). As fish is an ectothermal organism, any alterations in the water temperature also have a marked and direct effect on many of the key physiological processes and behavioral activities (Jonassen et al. 1999). Each species has a range of temperature over which it survives, and a narrower range where optimum growth occurs (Katersky and Carter 2007). However, temperature beyond optimum limits of a particular species adversely affects the health of aquatic animal due to metabolic stress and increases oxygen demand leading to susceptibility to diseases (Wedemeyer et al. 1999). Hence, thermal stress studies have gained significant attention among scientists to understand the impact of global warming on animals, including fish, as well.
Methyl groups are of vital importance for all animals, both terrestrial and aquatic. Moreover, animals cannot synthesize methyl groups and thus need to receive them in the diets (Kidd et al. 1997). The methyl group is used in methylation reactions to formulate useful compounds such as methionine, creatine, and phosphatidylcholine (PC) through the S-adenosyl methionine pathway (Bender 1992; NRC 1993). Metabolically, the major sources of methyl groups for practical diets, which support optimal animal performance are betaine, choline, methionine and lecithin, which is a nutritionally superior source of choline.
Lecithin, a source of phospholipids (PL) in the diet acts as a non-protein energy source, feed attractants beside this, it increases resistance to stress and can reduce the oxidation (antioxidant) of vitamin A, C, and E, and hence enhance the utilization of these vitamins in aquacultural species (ADM 2003). The benefits of lecithin are especially pronounced in the diets of young aquatic species as in early life stages, the digestive tracts have a very limited ability to synthesize adequate quantities of phospholipid to enhance growth and survival. Betaine has two major metabolic functions, as methyl donors and as an osmoprotectant. Betaine, being a compatible osmolyte, increases the water retention of cells, replaces inorganic salts, and protects intracellular enzymes against osmotically or temperature induced inactivation (Yancey et al. 1982). Choline is a precursor of betaine, acetylcholine (neurotransmitter), and phosphatidylcholine (PC) and also an important component of some plasmalogens, sphingomyelins, and lecithin. It acts as a source of methyl groups, via betaine, for the synthesis of various methylated metabolites. Choline deficiency has been shown to result in poor growth and fatty liver (Ketola 1976), hepatic lipidosis and renal hemorrhage (Griffin et al. 1994) anorexia and hemorrhagic areas in kidney, liver, and intestine (Wilson and Poe 1988). As a part of our experiment, we studied the effects of these methyl donors under conditions of environmental stress, which we believe will protect fish, we have evaluated the growth performance and metabolic status of L. rohita in response to supplementation of these compounds under stressed husbandry conditions.
Materials and methods
Fish and experimental design
Fingerlings of L. rohita (7.95 ± 0.02 g, average weight ± SE) were procured from Prem Fisheries Consultancy, Gujarat, India and transported in a circular container (150 L) with sufficient aeration to the experimental facilities at Central Institute of Fisheries Education, Mumbai and were acclimatized to the experimental rearing conditions for 15 days. After acclimatization, fish were transferred to 18 uniform size experimental plastic tanks of 150 L capacity and reared for 65 days. Fifteen fish of uniform size (initial weight 7.95 ± 0.02 g, average weight ± SE) per container were stocked in six distinct groups with three replicates for each treatment in plastic containers (80 × 57 × 42 cm) of 150 L capacity each, following a completely randomized design. The fish were fed with the experimental diet twice daily (09:00 and 17:00 h) to approximate satiation throughout the experimental period. Round-the-clock aeration was provided to all the containers from a compressed air pump and manual water exchange (two-third) was carried out at every two alternate day. The experimental groups were as follows: fish subjected to normal water (without endosulfan) and fed with control diet (control group T0), fish subjected to endosulfan-treated water and fed with control diet (T1), fish subjected to concurrent exposure of endosulfan and elevated temperature (34°C) and fed with control diet (T2), fish subjected to endosulfan and elevated temperature and fed with choline-supplemented diet (T3), fish subjected to endosulfan and temperature and fed with betaine-supplemented feed (T4), and fish subjected to endosulfan and temperature and fed with lecithin-supplemented feed (T5). The endosulfan treatment was made at level of 1/10 of LC50 (0.2 ppb) for all the treatment groups using technical grade endosulfan (99%; a:b ratio of 7:3) purchased from Excel Crop Care Limited, Hubli, Karnataka, India) and 34°C temperature was maintained throughout the experiment using thermostatic water heaters (range up to 50°C from normal) with specification DTC-PID—50L × 51B × 52H were procured from General Trading Corporation, Mumbai, India). Water quality parameters were checked every week using the methods of APHA (1998) and were found to be within the recommended range for carp rearing.
Experimental diet
Four iso-caloric and iso-nitrogenous diets viz. basal diet and three supplemented diets as choline, betaine, and lecithin diet were prepared using choline chloride, betaine hydrochloride and soy lecithin as a source of and choline, betaine, and lecithin, respectively. Soy lecithin, betaine hydrochloride, and choline chloride were procured from HIMEDIA (JTJ Enterprises, Mumbai, India) and SD Fine chemical Ltd India. Betaine and choline were first dissolved in water and incorporated along with vitamin mineral premix, whereas lecithin was mixed in oil. For the formulation of pelleted diet, good quality fish meal, soybean meal, sunflower meal, wheat flour, wheat bran, and sunflower oil were procured from local market. Manually prepared vitamin and mineral mixture choline free along with ascorbyl phosphate (SRL Ltd., Mumbai, India) as the source of vitamin C was used. The dough was mixed properly and was pelleted, air-dried and kept in hot air oven at 60°C until dry and was subsequently stored at 4°C until required for feeding.
Tissue homogenate preparation
The muscle, liver, gill, and intestine of the fishes from all the exposed groups were dissected carefully and weighed. Tissues were homogenized separately to get 5% homogenate with chilled sucrose solution (0.25 M) in a glass tube using Teflon-coated mechanical tissue homogenizer (MICCRA D-9, Digitronic, Germany). The tube was continuously kept in an ice to avoid denaturation of the enzymes during the homogenization. The homogenate was centrifuged at 5,000 rpm for 20 min at 4°C in a cooling centrifuge machine and the separated supernatant stored at −80°C until further use.
Growth study
Fishes were weighed at the start and every 15-day interval thereafter till 65th day. At the end of the experiment, fishes were anesthetized with clove oil (50 μL/L) and weighed individually. The growth performance of fingerlings was evaluated in terms of weight gain (%), feed conversion ratio (FCR) and specific growth rate (SGR).
Proximate analysis of feed
The proximate composition of the experimental diets was determined as per the standard methods of AOAC (1995) and presented in Table 1. Samples were analyzed for crude protein (CP), ether extract (EE), ash and total carbohydrate (TC). The digestible energy value of experimental diets was determined by following the method of Halver (1976).
Enzyme assays
Aspartate aminotransaminase (AST; EC.2.6.1.1) and alanine amino transaminase (ALT; EC.2.6.1.2) activities were measured by the estimation of oxaloacetate and pyruvate released, respectively, after incubating the reaction mixture at 37°C for 60 min (Wooten 1964). Lactate dehydrogenase (LDH; l-lactate NAD1 oxidoreductase; EC.1.1.1.27) was assayed using 0.1 M phosphate buffer (pH 7.5), 0.2 mM NADH solution in 0.1 M phosphate buffer. The reaction was initiated by adding 0.2 mM sodium pyruvate as the substrate and optical density (OD) was recorded at 340 nm (Wroblewski 1955). A similar reaction mixture was used for the estimation of malate dehydrogenase (MDH; l-malate: NAD+oxidoreductase: EC.1.1.1.37) except for the substrate (1 mg oxaloacetate/mL of chilled triple distilled water) (Ochoa 1955). Glucose-6-phosphate dehydrogenase (G6PDH; EC.1.1.1.49) activity was measured by method of De Moss (1955). The reaction mixture of 1.5 mL Tris buffer (0.1 M, pH 7.8), 0.2 mL of 2.7 mM NADP, 0.1 mL of tissue homogenate, 1.05 mL of distilled water, and 0.1 mL of 0.02 M glucose-6-phosphate (G6P) are mixed well and optical density (OD) was recorded at 340 nm. Gluthathione-S-transferase (GST; EC 2.5.1.18) was measured spectrophotometrically by the method of Habing et al. (1974). Acetylcholine esterase (AChE) (EC. 3.1.1.7) activity as measured by the change in OD at 540 nm using the method of Hestrin modified by Augustinsson (1949). Total adenosine triphosphatase (ATPase) (E.C.3.6.1.3) was assayed as per the modified method of Post and Sen (1967). Alkaline phosphatase (ALP; EC.3.1.3.1) was determined by the method of Garen and Levinthal (1960). Glycogen was estimated colorimetrically by the method described by Hassid and Abraham (1957).
Statistics
The data were statistically analyzed by statistical package SPSS version 16, in which data were subjected to one-way ANOVA and Duncan’s multiple range tests was used to determine the significant differences between the means. Comparisons were made at the 5 and/or 1% probability level.
Results and discussion
Water quality parameter
The water quality parameters like temperature, pH, dissolved oxygen (DO), free carbon dioxide (CO2), total alkalinity, total hardness, ammonia (NH3), nitrite, and nitrate are presented in Table 2. Water quality parameters were checked every week and were found to be within the recommended range for carp rearing.
Growth performance
The data pertaining to weight gain (%), feed conversion ratio (FCR), and specific growth rate (SGR) are presented in Table 3. The weight gain (%) and SGR were significantly higher in lecithin, betaine, and choline fed group. The treatment groups exposed to low dose of endosulfan (1/10th of LC 50, 0.2 ppb) and concurrently exposed to endosulfan and elevated temperature (34°C) had significantly lower weight gain percentage and SGR than control group. This was in association with observed FCR trend.
The growth performance of L. rohita fingerlings was significantly lower in groups exposed to endosulfan and concurrently exposed to endosulfan (1/10th of LC 50, 0.2 ppb) and elevated temperature (34°C). Similar effects have been observed previously in L. rohita exposed to endosulfan (Ramaneswari and Rao 2000) and in Nile Tilipia (Oreochromis mossambicus) exposed to sublethal level of two pesticides namely malathion and dimethoate (Sweilum 2006). The decreasing trend in the growth of L. rohita could be a consequence of altered metabolism, resulting from toxic stress (Adeyemo 2005; Petri et al. 2006). However, dietary lecithin, betaine, and choline mitigated the negative effect of endosulfan toxicity and high temperature as evident from the increased growth. Lecithin, betaine, and choline in the diet might have enriched the amino acid pool in the cells and these non-essential amino acids act as substrates for gluconeogenesis, which might aid in combating against stressors. However, mainly dietary lecithin and betaine mitigated the negative effect of endosulfan toxicity and this is evident from increased growth. Lecithin in the diet might have enriched the amino acid pool in the cells, and the non-essential amino acids act to produce substrate for gluconeogenesis, which might aid in combating against stressors (Kumar et al. 2011). Based on primary response parameters like weight gain %, SGR and FCR, it appears that supplementation of lecithin have beneficial effect on the growth of L. rohita fingerlings when fed practical diets. The weight gain % was significantly higher in the lecithin fed groups as compared to other groups. The beneficial effects of dietary lecithin on growth have been reported in larval and juvenile fish (Craig and Gatlin 1997). However, the effects of lecithin supplementation on growth of fish differ depending on the growth stages (Poston 1991). The inclusion of lecithin may increase growth by supplying phosphatidylcholine (PC) to the fish, thereby reducing energy normally expended in biosynthesis of PC (Craig and Gatlin 1997). The addition of betaine resulted in increased feed consumption and growth in rainbow trout fingerlings (Can and Sener 1992). Supplementation of dietary betaine has been found to improve growth in juvenile Penaeus monodon (Penaflorida and Virtanen 1996), Penaeus indicus (Jasmine et al. 1993) and Macrobrachium rosenbergii (Felix and Sudharsan 2004). Betaine (0.1–1%) has been shown to have no stimulating effect on feeding in gibel carp juveniles when fed with diets containing fish meal (Xue and Cui 2001). The growth result was well supported by the FCR and SGR values. The lower FCR in lecithin and betaine fed groups indicates better nutrient utilization in these groups. Lecithin supplementations have been reported to increase the feed efficiency of several fish species (Vijayaraghavan and Rao 1986). Thus, the results indicate the importance of lecithin in practical diet for efficient nutrient utilization in L. rohita fingerlings.
Enzymes assays
Lactate dehydrogenase (LDH) and of malate dehydrogenase (MDH)
Effects of dietary choline, betaine, and lecithin on the muscle and liver LDH and MDH activities of the experimental groups at the end of the experiment are shown in Table 4. Dietary choline, betaine, and lecithin significantly (P < 0.01) effected LDH and MDH activities in liver and muscle of L. rohita fingerlings. In liver, considerably higher activities of LDH and MDH were found in groups exposed to endosulfan and were further increased in the group which was concurrently exposed to endosulfan and elevated temperature, while the reduced activities were recorded in betaine and lecithin fed groups.
The present study demonstrates that the inclusion of dietary lecithin, betaine, and choline has significant effect on various enzymes like LDH, MDH, ATPase, ALP etc. In the present study, LDH and MDH activities increased significantly (P < 0.01) in endosulfan exposed and concurrently exposed to endosulfan and temperature groups, suggesting that the animals were under stress induced by endosulfan and concurrent elevated temperature which is in agreement with previous observation that LDH activity generally increases in stress (Vijayaraghavan and Rao 1986). Higher activity of MDH indicates greater activity of TCA cycle due to increased energy demands during stress. LDH and MDH activities decreased significantly (P < 0.01) in the groups fed with lecithin, betaine, and choline-supplemented diets indicating the stress mitigating effect of these methyl donors. Similar stress mitigating role of methyl donor was reported by Kumar et al. (2011) when L. rohita exposed with endosulfan.
G6PDH (glucose-6- phosphate dehydrogenase)
G6PDH (glucose-6- phosphate dehydrogenase) muscle and liver, AST (aspartate amino transaminase) muscle and liver and ALT (alanine amino transaminase) activities in muscle of L. rohita fingerlings did not differ significantly (P > 0.05, Table 4) among different experimental groups.
Alanine amino transaminase (ALT)
Alanine amino transaminase (ALT) activity in liver of L. rohita fingerlings varied significantly (P < 0.01, Table 4) among different treatment groups and the highest activity of liver ALT was recorded in endosulfan-exposed group and group concurrently exposed to endosulfan and temperature which got reduced when fed with methyl donor-supplemented diet and the lowest ALT activity was observed in lecithin-supplemented group.
The higher activity of ALT in liver of fish exposed to endosulfan stress and combined stress of endosulfan and temperature fed with control diets, indicates the mobilization of aspartate and alanine via gluconeogenesis for glucose production to cope up with stress. Similarly, Chatterjee et al. (2006) reported that elevated level of transaminase activity during stress would lead to increased feeding of keto acids into TCA cycle. In the present study, inclusion of methyl donors reduces the ALT activity in liver which can be inferred that inclusion of lecithin, betaine, and choline in diets reduces the stress born energy demand and stress in L. rohita fingerlings.
Antioxidant enzymes (glutathion-S-transferase)
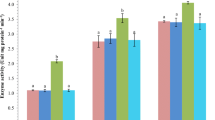
Compared with endosulfan alone and concurrent exposure to endosulfan and temperature glutathione-S-transferase activity in gill and liver exhibited a significant (P < 0.05, Fig. 1a) decreases in betaine- and lecithin-supplemented group in gill as well as in liver.
Impact of dietary choline, betaine, and lecithin on brain AChE (acetyl choline esterase) and GST (glutathion-S-transferase) in gill and liver in response to concurrent exposure to endosulfan (1/10th of LC 50, 0.2 ppb) and elevated temperature (34°C) of L. rohita fingerlings at the experiment period of 65 days (values with different superscript differ significantly (P < 0.01), data expressed as Mean ± SE, (n = 6). (control T0, endosulfan exposed = endo-exp T1, concurrent exposed to endosulfan and temperature = E + T expo T2, choline fed endosulfan and temperature-exposed group T3, betaine fed endosulfan and temperature-exposed group T4, lecithin fed endosulfan and temperature-exposed group T5)
The GST activity in gill and liver of L. rohita fed with methyl donors especially betaine- and lecithin-supplemented group showed decreased in the activity but endosulfan alone and concurrent exposure to endosulfan and temperature-exposed group were found to have higher GST activity. Endosulfan induces oxidative tissue damage resulting from the release of oxygen free radicals (OFRs) Hincal et al. (1995). Due to high reactivity of OFRs, most components of the cellular structure and function may become potential targets of oxidative damage. Reduced activities of the antioxidative enzymes, in supplemented group, indicate stabilization of the cellular structure from such oxidative damage due to supplementation of these methyl donors.
Neuro-transmission enzymes
Data pertaining to the impact of dietary pyridoxine on AChE activity in the brain tissue of L. rohita fingerlings exposed to endosulfan-induced chronic stress is shown in Fig. 1b.
The AChE activity in brain of L. rohita fingerlings was assayed at the end of the experiment. The experimental group fed diet without methyl supplementation showed a decrease in the activity, which indicates stress in animals induced by endosulfan alone and endosulfan- and temperature-exposed group. Similar results were described by Akhtar et al. (2009) who observed inhibition of AChE in L. rohita on exposure to endosulfan. It has also been reported that AChE is inhibited by organophosphorus compounds and some toxins (Gopal et al. 1985). In methyl donors-supplemented group especially betaine and lecithin, the enzyme activity was significantly more. This indicates the stress mitigating effect of dietary betaine and lecithin.
Total adenosine triphosphatase (ATPase)
Data pertaining to the impact of dietary choline, betaine, and lecithin on the ATPase activity of gill and liver of L. rohita fingerlings exposed to endosulfan-induced chronic stress is shown in Table 5. There was a significant (P < 0.01) effect of dietary choline, betaine, and lecithin on ATPase activity of gill and liver. Activity of ATPase was significantly lower (P < 0.01) in endosulfan-exposed group and concurrently exposed to endosulfan and elevated temperature, compared with supplemented group.
Adenosine triphosphatase (ATPase) is a membrane-bound enzyme responsible for the transport of ions through the membrane and immediate release of energy. As this enzyme is related to immediate release of energy, reduction in the activity of this enzyme might have significantly affected the fish in terms of the energy balance and ion transport. This might have considerably affected the glucose metabolism in the hyperglycemia state in L. rohita fingerlings. In present study, liver and gill thereby leading to ATPase activity in liver and gill was reduced significantly in endosulfan-exposed group and concurrently exposed to endosulfan and elevated temperature. The ATPase activity in liver and gill was significantly higher in group fed with lecithin-, betaine-, and choline-supplemented diets. This suggests that lecithin, betaine, and choline may help in providing more energy to L. rohita. Our results are in close agreement with the findings of Sharma (1988), who observed significant reduction in liver ATPase activity in Channa gachua upon endosulfan exposure. In the present study, reduction in liver and gill ATPase activity may be due to alterations in the structure and functions of the liver and gill plasma membrane or may be due to direct inhibition of enzymes by endosulfan. Higher activity of ATPase in liver and gill the treatment groups suggests that the supplementation of dietary lecithin, betaine, and choline may help in reducing energy demands in the L. rohita fingerlings. Similar results were reported by Kumar et al. (2011) who find that lecithin, betaine, and choline enhance ATPase activity during endosulfan exposure to L. rohita.
Alkaline phosphatase (ALP)
The intestinal ALP activity was significantly higher (P < 0.01, Table 5) in endosulfan-exposed group and further elevated when concurrently exposed to endosulfan and elevated temperature. The intestinal ALP activity was normal in betaine- and lecithin-supplemented diet group.
ALP, a zinc-containing metallo-enzyme, plays an important role in phosphorus metabolism. ALP activity was found to be low in the fish exposed to endosulfan alone and was much higher compared with endosulfan in the group exposed concurrently to endosulfan and elevated temperature. These results are consistent with other results previously observed in Channa punctatus (Sharma 1990), and in other species (Verma et al. 2007). Such decreased ALP activity could possibly be an indication of role of endosulfan in inhibiting protein synthesis (Verma et al. 2007). However, when fishes were fed with diet containing lecithin and betaine, ALP activity was normal, which might be due to the easily liberated phosphate ions to combat stressful condition or higher metabolic rate (Kumar et al. 2011).
Glycogen
Glycogen in muscle and liver of the experimental group at the end of experiment are shown in Table 5. No significant difference was observed in muscle and liver glycogen in L. rohita fingerlings. Higher level of muscle and liver glycogen was observed in endosulfan exposed and concurrently exposed to endosulfan and temperature groups as compared to control and choline, betaine, and lecithin fed groups.
Glycogen broke down into glucose in the early stage of stress (Barton and Iwama 1991). In the present study, no significant difference was observed in muscle and liver glycogen level but were higher in endosulfan-exposed group and concurrently exposed to endosulfan and temperature groups. It may be due to utilization of tissue glycogen reserve in initial stage of stress (Vijayan et al. 1997). Similar reports are available in common dentex (Dentex dentex) exposed to handling stress, glycogen reserve were not affected (Morales et al. 2005).
Conclusion
The study concluded that dietary methyl donors particularly lecithin and betaine mitigates endosulfan and temperature induced stress properties in fish. These are revealed by growth performance, LDH, MDH, ALT, ATPase ALP activities, GST, and Acetyl choline esterase. Thus, methyl donors’ supplementation in practical diets is beneficial for achieving a good health status and found to be optimum to reduce stress during culture of L. rohita.
References
Adeyemo OK (2005) Hematological and histopathological effects of cassava mill effluent in Clarias gariepinus. Afr J Biomed Res 8:179–183
ADM (2003) Lecithin in aquaculture. Available at http://www.admworld.com/mktcolpdf/euLecithinsInAquaculture.pdf (retrieved on 1. 6.2007)
Akhtar MS, Pal AK, Sahu NP, Alexander C, Gupta SK, Choudhary AK, Jha AK, Rajan MG (2009) Stress mitigating and immunomodulatory effect of dietary pyridoxine in Labeo rohita (Hamilton) fingerlings. Aquacult Res 1–12. doi:10.1111/j.1365-2109.2009.02383.x
AOAC (1995) Official methods of analysis of the Association of Official Analytical Chemists. In: Cunnif PA (ed) vol 1, 16th edn. AOAC International, Arlington,VA, USA, pp 31–65
APHA-AWWA-WEF (1998) Standard methods for the estimation of water and waste water. In: Clesceri LS, Greenberg AE, Eaton AD (eds), 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC
Augustinsson KB (1957) The reaction of acetyl choline esters and other carboxylic acid derivatives with hydroxylamine and its analytical application. J Biol Chem 180:249–261
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effect of corticosteroids. Annu Rev Fish Dis 1:3–26
Bender DA (1992) Nutritional biochemistry of the vitamins. Cambridge University Press, New York
Can K, Sener K (1992) The effect of betaine—added starter feeds on the growth of rainbow trout (O. mykiss, W. 1792) fry. J Aquat Prod 1:95–104
Chatterjee N, Pal AK, Das T, Manush SM, Sarma K, Venkateshwarlu G, Mukherjee SC (2006) Secondary stress response in Indian major carps Labeo rohita (Ham), Catla catla (Ham) and Cirrhinus mrigala (Ham) fry to increasing packing densities. Aquacult Res 37:472–476
Craig SR, Gatlin DM (1997) Growth and body composition of juvenile red drum (Sciaenops ocellatus) fed diets containing lecithin and supplemental choline. Aquaculture 151:259–267
FAO (2001) Yearbook on fishery statistics. Italy, Rome
Felix N, Sudharsan M (2004) Effect of glycine betaine, a feed attractant affecting growth and feed conversion of juvenile freshwater prawn Macrobrachium rosenbergii. Aquat Nutr 10:193–197
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17:581–613
Garen A, Levinthal CA (1960) Fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli l. Purification and characterization of alkaline phosphatase. Biochem Biophys Acta 38:470
Gopal K, Anand M, Mehrotra S, Ray PK (1985) Neurobehavioural change sin freshwater fish Channa punctatus exposed to endosulfan. J Adv Zool 6:74–80
Griffin ME, Wilson KA, White MR, Brown PB (1994) Dietary choline requirement of juvenile hybrid striped bass. J Nutr 124:1685–1689
Habing WH, Pabst MN, Bjacoby W, Glutathion S (1974) Transferase, the first enzymatic step in mercatpopunc acid formation. J Biol Chem 249:7130–7138
Hassid W, Abraham S (1957) Chemical procedures for analysis of polysaccharides. In Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 34–37
Halver JE (1976) The nutritional requirements of cultivated warm water and cold water fish species. In: Report of the FAO technical conference on aquaculture, Kyoto, Japan, 26 May–2 June 1976. FAO Fisheries Report No. 188 FI/R188 (En), p 9
Hestrin S (1949) The reaction of acetyl choline esters and other carboxylic acid derivatives with hydroxyline and its analytical application. J Biol Chem 180:249–261 Modified by Augustinsson (1957)
Hincal F, Gurbay A, Giray B (1995) Induction of lipid peroxidation and alteration of glutathione redox status by endosulfan. Biol Trace Elem Res 47:321–326
Jasmine GI, Pillai SP, Athithan S, (1993) Effect of feeding stimulants on the biochemical composition and growth of Indian white prawn Penaeus indicus. In: Carrillo M, Dahle L, Morales J, Sorgeloos P, Svennevig N, Wyban J (eds) From discovery to commercialization, vol 19. Oostende Beligium European–Aquaculture Society, Torremolinos, Spain, p 139
Jonassen TM, Imsland AK, Stefansson SO (1999) The interaction of temperature and fish size on growth of juvenile halibut. J Fish Biol 54:556–572
Katersky RS, Carter CG (2007) High growth efficiency occurs over a wide temperature range for juvenile barramundi Lates calcarifer fed a balanced diet. Aquaculture 272:444–450
Ketola HG (1976) Choline metabolism and nutritional requirement of lake trout (Salvelinus namaycush). J Anim Sci 43:474–477
Kidd MT, Ferket PR, Garlich JD (1997) Nutritional and osmoregulatory functions of betaine. Poultry Sci 53:125–139
Kumar N, Jadhao SB, Chandan NK, Kumar K, Jha AK, Bhushan S, Kumar S, Rana RS (2011) Dietary choline, betaine and lecithin mitigates endosulfan induced stress in Labeo rohita fingerlings, fish physiology and biochemistry. doi: 10.1007/s10695-011-9584-y
Morales AE, Cardenete G, Abellan EL, Gracia-Rejon L (2005) Stress related physiological responses to handling in common dentex (Dentex dentex Linnaeus, 1758). Aquacult Res 36:33–40
Moss RDD (1955) Glucose-6-phosphate and 6-phosphogluconic dehydrogenase from leuconostoc mesenteroides. In: Colowick SP, Kalpan NO (eds) Methods in enzymology, vol I. Academic Press Inc., New York, pp 328–332
NRC (National Research Council) (1993) Nutrient requirements of fish. National Academy Press, Washington
Ochoa S (1955) Malic dehydrogenase and ‘malic’enzyme. In: Coloric SP, Kaplan N (ed) Methods of enzymology, vol I. Academic Press, NewYork, NY, USA, pp 735–745
Penaflorida DV, Virtanen E (1996) Growth, survival and feed conversion of juvenile shrimp (Penaeus monodon) fed a betaine/amino acid additive. Isr J Aquacult Bamidgeh 48:3–9
Petri D, Glover CN, Ylving S, Kolas K, Fremmersvik G, Waagbo R (2006) Sensitivity of Atlantic salmon (Salmo salar) to dietary endosulfan as assessed by haematology, blood biochemistry, and growth parameters. Aquat Toxicol 80:207–216
Post RL, Sen AK (1967) Methods in enzymology. In: Colowick SP, Kaplan NO (eds) vol 10. Academic Press Inc., New York, p 762
Poston HA (1991) Response of rainbow trout to soy lecithin, choline, and autoclaved isolated soy protein. Prog Fish Cult 53:85–90
Ramaneswari K, Rao LM (2000) Bioconcentration of endosulfan and monocrotophos by Labeo rohita and Channa punctatus. Bull Environ Contam Toxicol 65:618–623
Sharma RM (1988) Effect of endosulfan on adenosine triphosphatase (ATPase) activity in liver, kidney and muscles of Channa gachua. Bull Environ Contam Toxicol 41:317–323. doi:10.1007/BF01688873
Sharma RM (1990) Effect of endosulfan on acid and alkaline phosphatase activity in liver, kidney and muscle of Channa gachua. Bull Environ Contam Toxicol 44:443–448
Sweilum MA (2006) Effect of sublethal toxicity of some pesticides on growth parameters, haematological properties and total production of Nile tilapia (Oreochromis niloticus L.) and water quality of ponds. Aquacult Res 37:1079–1089
Verma AK, Pal AK, Manush SM, Das T, Dalvi RS, Chandrachoodan PP (2007) Persistent sub-lethal chlorine exposure elicits the temperature induced stress responses in Cyprinus carpio early fingerlings. Pest Biochem Physiol 87:229–237
Vijayan MM, Pereira C, Grau EG, Iwama GK (1997) Metabolic responses associated with confinements stress in tilapia; the role of cortisol. Comp Biochem Physiol 116 C:89–95
Vijayaraghavan S, Rao SJVR (1986) Starvational stress effects on tissue lactate and lactate dehydrogenase activity in Anabas scandens (Cuvier). Comp Physiol Ecol 11:233–236
Wedemeyer GA, Meyer FP, Smith L (1999) Environmental stress and fish diseases. Narendra Publication House, Delhi
Wilson RP, Poe WE (1988) Choline nutrition of fingerling channel catfish. Aquaculture 68:65–71
Wooten IDP (1964) Microanalysis. In: Churchill J, Churchill A (eds) Medical biochemistry, 4th edn. Churchill, London, pp 101–107
Wroblewski Ladue (1955) LDH activity in blood. Proc Soc Exp Biol Med 90:210–213
Xue M, Cui Y (2001) Effect of several feeding stimulants on diet preference by juvenile gibel carp (Carassius auratus gibelio), fed diets with or without partial replacement of fish meal by meat and bone meal. Aquaculture 198:281–292
Yancey PH, Clar ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte system. Science 217:1214–1223
Acknowledgments
Authors are thankful to the Vice-Chancellor/Director, Dr W. S. Lakra Central Institute of Fisheries Education, Mumbai, India for providing all the facilities required for the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, N., Jadhao, S.B., Jha, A.K. et al. Methyl donors potentiates growth, metabolic status and neurotransmitter enzyme in Labeo rohita fingerlings exposed to endosulfan and temperature. Fish Physiol Biochem 38, 1343–1353 (2012). https://doi.org/10.1007/s10695-012-9622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9622-4