Abstract
There were many studies about the effect of excess manganese (Mn) on nervous system apoptosis; however, Mn-induced apoptosis in chicken cerebrums and embryonic neurocytes was unclear. The purpose of this study was to investigate the effect of excess Mn on chicken cerebrum and embryonic neurocyte apoptosis. Seven-day-old Hyline male chickens were fed either a commercial diet or three levels of manganese chloride (MnCl2)-added commercial diets containing 600-, 900-, and 1800-mg/kg-Mn diet, respectively. On the 30th, 60th, and 90th days, cerebrums were collected. Fertilized Hyline chicken eggs were hatched for 6–8 days and were selected. Embryonic neurocytes with 0, 0.5, 1, 1.5, 2, 2.5, and 3 mM Mn were collected and were cultured for 12, 24, 36, and 48 h, respectively. The following research contents were performed: superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) activities; tumor protein p53 (p53), B cell lymphoma-2 (Bcl-2), B cell lymphoma extra large (Bcl-x), Bcl-2-associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak), fas, and caspase-3 messenger RNA (mRNA) expression; and morphologic observation. The results indicated that excess Mn inhibited SOD and T-AOC activities; induced p53, Bax, Bak, fas, and caspase-3 mRNA expression; and inhibited Bcl-2 and Bcl-x mRNA expression in chicken cerebrums and embryonic neurocytes. There were dose-dependent manners on all the above factors at all the time points and time-dependent manners on SOD activity of 1800-mg/kg-Mn group, T-AOC activity, and apoptosis-related gene mRNA expression in all the treatment groups in chicken cerebrums. Excess Mn induced chicken cerebrum and embryonic neurocyte apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the wide application of manganese (Mn), Mn caused environmental pollution. Mn dust pollution impaired the lung function of male workers in a ferromanganese refinery in Guangxi Province, China [1]. High Mn concentrations of tree sparrow livers and hearts were found in polluted mining areas in Jixi, Heilongjiang Province, China [2]. Cerebrum is an important organ of the nervous system and develops from embryonic neurocytes. A study found that excess Mn caused Mn deposition and neurotoxicity in adult mice brains [3]. Excess Mn caused chicken brain damage [4] and induced apoptosis in rat striatal neurocytes [5]. Therefore, the chicken model of Mn poisoning was established in vivo and in vitro to investigate toxic effect of excess Mn on chicken cerebrums and embryonic neurocytes.
Excess Mn can result in oxidative stress. Liu et al. [6] found that excess Mn decreased glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities, increased malondialdehyde (MDA) content, and caused oxidative stress in chicken immune organs and serum. Oxidative stress can induce apoptosis. Oxidative stress increased tumor protein p53 (p53) [7], Bcl-2 associated X protein (Bax) [8, 9], Bcl-2 homologous antagonist/killer (Bak) [10], fas [11], and caspase-3 [8] expressions; decreased B cell lymphoma-2 (Bcl-2) [8, 9] and B cell lymphoma extra large (Bcl-x) [10] expressions; and induced apoptosis. Some studies found that excess heavy metals caused oxidative stress and induced apoptosis. Lead and cadmium (Cd) caused oxidative stress and induced apoptosis in rat proximal tubular cells [12]. Molybdenum (Mo) and Cd inhibited SOD and total antioxidant capacity (T-AOC) activities, resulted in oxidative stress, and induced apoptosis in duck ovaries [13]. Cd decreased SOD activity, caused oxidative stress, and induced apoptosis in mouse N2A neuroblastoma cells [14]. Therefore, we detected SOD and T-AOC activities in chicken cerebrums and embryonic neurocytes.
Studies on the effect of excess Mn were carried out, including immune system [6, 15–18], circulatory system [19], reproductive system [20], and nervous system [4] in chickens. However, the effect of excess Mn on apoptosis in chicken cerebrums and embryonic neurocytes was still unclear. Therefore, oxidative stress biomarker (SOD and T-AOC) activities and messenger RNA (mRNA) expression of apoptosis-related genes (p53, Bcl-2, Bcl-x, Bax, Bak, fas, and caspase-3) were detected to investigate the toxicity of excess Mn on chicken cerebrum and embryonic neurocyte apoptosis.
Materials and Methods
Animal Model and Tissue Samples
One-day-old Hyline male chickens were acclimated for 1 week prior to experiment. One-hundred-and-eighty chickens were randomly divided into four groups of 45 chickens each group. The feeding program consisted of a commercial diet (containing 127.88 mg/kg Mn), and three levels of MnCl2·4H2O (analytical reagent grade, Tianjin, China) added the commercial diet at the levels of 600-, 900-, and 1800-mg/kg-Mn diet, respectively, according to median lethal dose (LD50) of Mn being 1122.35-mg/kg body weight for chickens [19] and the need of the chicken experiment in toxicology [21]. Feed and water were offered ad libitum throughout the experiment. All the chickens were housed in the Laboratory Animal Center, College of Veterinary Medicine, Northeast Agricultural University (Harbin, China). All procedures used in this experiment were approved by the Northeast Agricultural University’s Institutional Animal Care and Use Committee under the approved protocol number SRM-06.
On the 30th, 60th, and 90th days of the experiment, 15 chickens from each group were randomly selected and euthanized. The cerebrums were immediately excised, washed using ice-cold sterile deionized water. Each sample was divided into three portions. First portion was immediately homogenized using an electric homogenizer (Huarui, China) to detect SOD and T-AOC activities. Second portion was put in plastic vials and immediately frozen in liquid nitrogen and then stored in ultralow-temperature freezer at approximately −80 °C for quantitative real-time polymerase chain reaction (PCR). Third portion was kept in glutaraldehyde for ultrastructural observation.
Chicken Embryonic Neurocyte Model
Fertilized eggs of Hyline chicken were collected and hatched in the incubator (38 °C, 50% humidity) for 6–8 days. Embryos were carefully removed from eggs under aseptic condition and collected in phosphate-buffered saline (PBS; 0.1 M phosphate buffer with 0.85% sodium chloride (NaCl), pH 7.2). The cells were transferred into a plate containing PBS and washed two times using PBS. Then, the cells were cut into small pieces mechanically and washed two times using PBS. The cells were digested with trypsin (pH 7.2; Sigma-Aldrich, USA) at 37 °C for 6–8 min. Equal numbers of complete Dulbecco’s modified Eagle’s medium (DMEM) culture solution (Gibco, USA) containing 50 mM sodium bicarbonate (NaHCO3), supplemented with 10% fetal bovine serum (FBS; CAMS, China) and 1% antibiotic-antimycotic solution (Sigma, USA), were added to the plate to stop the enzymolysis of trypsin. The cells were washed two times using PBS, were washed one time using DMEM, and were repeatedly stirred and spread using a pipettor until without any flocculated precipitation. The cells were allowed to stand for 2 min at room temperature and were filtered using a sterile stainless steel mesh with a pore size of 400 μm. The cells were counted and diluted to 1 × 105–1 × 106 cells/mL and were put into six-hole plates (three holes per sample) treated with lysine. MnCl2 at the levels of 0.5, 1, 1.5, 2, 2.5, and 3 mM Mn were immediately added in the treatment groups, respectively. After being cultured for 12, 24, 36, and 48 h, respectively, DMEM was sucked out. The cells were washed using PBS one time. The cells were harvested to detect cell viability, oxidative stress, mRNA expression, and morphological changes.
SOD and T-AOC Activities
SOD and T-AOC activities were measured using diagnostic kits produced by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Relative mRNA Expression of Apoptosis-Related Genes
Gene-Specific Primers for Quantitative Real-Time PCR
Gene-specific primers for p53 (NM_205264.1), Bcl-2 (NM_205339.1), Bcl-x (Z23110.1), Bax (FJ977571.1), Bak (NM_001030920.1), fas (XM421659), caspase-3 (NM_204725.1), and β-actin (L08165) were designed using Primer Premier 5.0 (Premier, Canada) software according to GenBank sequence and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), with the following sequences: p53 forward sense GAGATGCTGAAGGAGATCAATGAG, p53 reverse sense GTGGTCAGTCCGAGCCTTTT, Bcl-2 forward sense ATCGTCGCCTTCTTCGGATT, Bcl-2 reverse sense ATCCCATCCTCCGTTGTCCT, Bcl-x forward sense CTTTCAGCGACCTCACCTC, Bcl-x reverse sense ACAATGCGTCCCACCAGT, Bax forward sense GATGAAGCCACCCAGCAGTA, Bax reverse sense TGGATTCTCACAGTAGGAGGATG, Bak forward sense ACCCGGAGATCATGGAGA, Bak reverse sense GATGCCTTGCTGGTAGACG, fas forward sense GCACTCGGTTTGGAGGTTGT, fas reverse sense CGTGGCATTCCTGCTTCTT, caspase-3 forward sense CATCTGCATCCGTGCCTGA, caspase-3 reverse sense CTCTCGGCTGTGGTGGTGAA, β-actin forward sense CCGCTCTATGAAGGCTACGC, and β-actin reverse sense CTCTCGGCTGTGGTGGTGAA. The β-actin was used as a reference gene.
Total RNA Isolation and Complementary DNA Synthesis
The cerebrum tissues of 50–100 mg were collected and were immediately put in a mortar with liquid nitrogen and grinded to powder; meanwhile, liquid nitrogen was continuously added. And then, RNAiso Plus (TaKaRa, Japan) was added into the mortar. The mixture from the mortar was put into 1.5 mL of Eppendorf (EP) tubes, and then, total RNA was extracted according to the manufacturer’s recommendations.
The cell culture plates were taken out from incubator. One milliliter of RNAiso Plus was added into each hole. The cells were stirred and spread using a pipettor. The lysis solution with cells was put into EP tubes. The EP tubes were repeatedly stirred and spread until without any precipitation. The cells were allowed to stand for 5 min at room temperature, and then, total RNA was extracted according to the manufacturer’s recommendations.
The purity and content of total RNA were checked using gel electrophoresis and spectrophotometer, respectively. Total mRNA was converted into complementary DNA (cDNA) using reverse transcriptase (RT) (HaiGene, Harbin, China). The reverse transcription reaction mixture (consisted of 2.5 μL of golden MLV reverse transcriptase, 10 μL of ×10 RT buffer, 5 μL of dNTP mixture (10 mM each), 2.5 μL of RNase inhibitor (40 U/μL), 5 μL of ×20 oligo-dT (25) and random primer (Haigene, China), and 75 μL of H2O) was incubated in a thermostatic water bath. The RT procedure was at 30 °C for 15 min, at 55 °C for 50 min, and at 85 °C for 10 min. The synthesized cDNA was stored in −20 °C freezer for next step.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed using the ABI PRISM 7500 qPCR system (Applied Biosystems, USA) according to the manufacturer’s recommendations. The reaction system contained 5 μL of TransStart top green qPCR SuperMix (TransGen, China), 0.2 μL of passive reference dye II, 1 μL of five times diluted cDNA, 0.4 μL of primer, and 3.4 μL of H2O. The procedure consisted of 1 cycle at 95 °C for 2 min, 40 cycles at 95 °C for 15 s, and at 60 °C for 1 min. Every sample was measured three times. Gel electrophoresis was used to check PCR products. Relative mRNA abundance was calculated using the method of Pfaffl [22].
Tissue Ultrastructure
On the 90th day of the experiment, the cerebrum tissues of the control group and 1800-mg/kg-Mn group were cut into 1.0-mm3 blocks with a scalpel. These blocks were immediately fixed in 2.5% (v/v; pH 7.2) glutaraldehyde phosphate-buffered saline, then fixed in 1% (v/v) osmium tetroxide, and stained using uranyl acetate and dehydrated. The samples were washed in propylene oxide and impregnated with epoxy resins. Three days later, ultrathin sections were made using a microtome (Leica, Germany), stained with uranyl acetate and lead citrate, and then observed using an H-7650 transmission electron microscope (Hitachi, Japan).
Cell Viability
Cell viability of chicken embryonic neurocytes treated with Mn for 48 h was measured using Cell Counting Kit-8 produced by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Cytomorphology
Morphological observation of chicken embryonic neurocytes treated with Mn for 48 h was performed using acridine orange and ethidium bromide (AO/EB) double fluorescent dye staining (Solarbio, Beijing, China) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analyses of SOD and T-AOC activities and mRNA levels of p53, Bcl-2, Bcl-x, Bax, Bak, fas, and caspase-3 were carried out with one-way and two-way analyses of variance (ANOVAs), using GraphPad Prism for Windows (version 5.01; GraphPad software, USA). The comparisons for groups were verified by nonparametric as Kruskal-Wallis ANOVA and Mann-Whitney U tests. Data were expressed as the mean ± standard deviation (SD).
Results
SOD and T-AOC Activities
SOD and T-AOC activities in chicken cerebrums and embryonic neurocytes were shown in Fig. 1. SOD activity decreased significantly (P < 0.05) with the increase of Mn in chicken cerebrums on the 60th and 90th days starting from 600 mg/kg Mn (Fig. 1 (a1)) and in chicken embryonic neurocytes (Fig. 1 (a2)). In 1800-mg/kg-Mn group, SOD activity on the 90th day was significantly lower (P < 0.05) than that on the 30th day in cerebrums. T-AOC activity of the treatment groups was significantly lower (P < 0.05) than that of the control group in chicken cerebrums (Fig. 1 (b1)). In 600- and 900-mg/kg-Mn groups, T-AOC activity on the 90th day was significantly lower (P < 0.05) than that on the 30th day in cerebrums. T-AOC activity decreased significantly (P < 0.05) with the increase of treatment time in 1800-mg/kg-Mn group in cerebrums. T-AOC activity decreased significantly (P < 0.05) with the increase of Mn from 1 to 2 mM Mn in chicken embryonic neurocytes (Fig. 1 (b2)).
SOD and T-AOC activities in chicken cerebrums and embryonic neurocytes. Statistically significant differences: bars with different uppercase letters within the same group at different time points are significantly different (P < 0.05), and bars with different lowercase letters in different groups at the same time point are significantly different (P < 0.05). Bars represented mean ± SD
Apoptosis-Related Gene mRNA Expression
Relative mRNA expressions of p53, Bcl-2, Bcl-x, Bax, Bak, fas, and caspase-3 in chicken cerebrums and embryonic neurocytes were shown in Fig. 2. Relative mRNA expression of p53 of all the treatment groups was significantly higher (P < 0.05) than that of the control group in chicken cerebrums (Fig. 2 (a1)) and embryonic neurocytes (Fig. 2 (a2)) except that of 600-mg/kg-Mn group on the 30th day in cerebrums and that of 0.5-mM group at 24 and 36 h in embryonic neurocytes. There was an upward trend in p53 mRNA expression in cerebrums and embryonic neurocytes.
Relative mRNA expressions of p53, Bcl-2, Bcl-x, Bak, Bax, fas, and caspase-3 in chicken cerebrums and embryonic neurocytes. Statistically significant differences: bars with different uppercase letters within the same group at different time points are significantly different (P < 0.05), and bars with different lowercase letters in different groups at the same time point are significantly different (P < 0.05). Bars represented mean ± SD
Bcl-2 mRNA expression of the treatment groups was significantly lower (P < 0.05) than that of the control group on the 60th and 90th days except that of 600-mg/kg-Mn group on the 60th day in cerebrums (Fig. 2 (b1)). Bcl-2 mRNA expression of 1800-mg/kg-Mn group was significantly lower (P < 0.05) than that of the other groups in cerebrums on the 90th day. Bcl-2 mRNA expression of the treatment groups was significantly lower (P < 0.05) than that of the control group except that of 0.5-mM-Mn group at 36 and 48 h in embryonic neurocytes (Fig. 2 (b2)). There was a downward trend in Bcl-2 mRNA expression in cerebrums and embryonic neurocytes.
Bcl-x mRNA expression of the treatment groups was significantly lower (P < 0.05) than that of the control group except that of 600- and 900-mg/kg-Mn groups on the 30th day in chicken cerebrums (Fig. 2 (c1)). Bcl-x mRNA expression of 1800-mg/kg-Mn group was significantly lower (P < 0.05) than that of the other groups on the 90th day in cerebrums. Bcl-x mRNA expression of the treatment groups was significantly lower (P < 0.05) than that of the control group except that of 0.5-mM-Mn group at 24 and 48 h in embryonic neurocytes (Fig. 2 (c2)). There was a downward trend in Bcl-x mRNA expression in cerebrums and embryonic neurocytes.
Bax mRNA expression of the treatment groups was significantly higher (P < 0.05) than that of the control group except in 600- and 900-mg/kg-Mn groups on the 30th day in chicken cerebrums (Fig. 2 (d1)). Bax mRNA expression of the Mn treatment groups increased significantly (P < 0.05) with the increase of time in chicken cerebrums. Bax mRNA expression of the treatment groups was significantly higher (P < 0.05) than that of the control group except that of 0.5-mM-Mn group at all the time points and in 1-mM-Mn group at 12 h (Fig. 2 (d2)). There was an upward trend in Bax mRNA expression in cerebrums and embryonic neurocytes.
Bak mRNA expression of the treatment groups was significantly higher (P < 0.05) than that of the control group except that of 600- and 900-mg/kg-Mn groups on the 30th day in cerebrums (Fig. 2 (e1)). Bak mRNA expression increased significantly (P < 0.05) with the increase of Mn on the 60th day in cerebrums. Bak mRNA expression of 1800-mg/kg-Mn group was significantly higher (P < 0.05) than that of 600- and 900-mg/kg-Mn groups on the 90th day in cerebrums. Bak mRNA expression increased significantly (P < 0.05) with the increase of treatment time in cerebrums. Bak mRNA expression of the treatment groups was significantly higher (P < 0.05) than that of the control group except that of 0.5-mM-Mn group at 12 and 24 h (Fig. 2 (e2)). There was an upward trend in Bak mRNA expression in cerebrums and embryonic neurocytes.
Relative mRNA expression of fas in the treatment groups was significantly higher (P < 0.05) than that in the control group except that of 600-mg/kg-Mn group on the 30th day in cerebrums (Fig. 2 (f1)). Relative mRNA expression of fas in 1800-mg/kg-Mn group was significantly higher (P < 0.05) than that in 600- and 900-mg/kg-Mn groups on the 60th and 90th days in cerebrums. Relative mRNA expression of fas increased significantly (P < 0.05) with the increase of treatment time in cerebrums. Relative mRNA expression of fas in the treatment groups was significantly higher (P < 0.05) than that in the control group except that in 0.5-mM-Mn group at 12 and 24 h and that in 1-mM-Mn group at 24 h (Fig. 2 (f2)). There was an upward trend in fas mRNA expression in cerebrums and embryonic neurocytes.
Relative mRNA expression of caspase-3 increased significantly (P < 0.05) with the increase of Mn on the 30th day in cerebrums (Fig. 2 (g1)). Relative mRNA expression of caspase-3 in 600- and 900-mg/kg-Mn groups was significantly higher than that in the control group in cerebrums. Relative mRNA expression of caspase-3 in 1800-mg/kg-Mn group was significantly higher than that in 600- and 900-mg/kg-Mn groups in cerebrums. In all the treatment groups, relative mRNA expression of caspase-3 on the 90th day was significantly higher than that on the 30th and 60th days in cerebrums. Relative mRNA expression of caspase-3 in the treatment groups was significantly higher (P < 0.05) than that in the control group except that in 0.5-mM-Mn group at 12, 24, and 36 h and that in 1- and 1.5-mM-Mn groups at 36 h (Fig. 2 (g2)). There was an upward trend in caspase-3 mRNA expression in cerebrums and embryonic neurocytes.
Tissue Ultrastructure
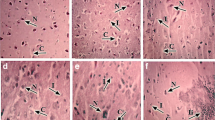
The present study was also designed to observe the ultrastructure of chicken cerebrums in the control group and 1800-mg/kg-Mn group on the 90th day (Fig. 3). In the control group (Fig. 3a), nuclear membrane was clear. Chromatin and endoplasmic reticulum were normal. Mitochondria were full. In 1800-mg/kg-Mn group, chromatin shrinkage occurred. Chromatin marginated to nuclear membrane (Fig. 3b). Endoplasmic reticulum expanded (Fig. 3c). Numbers of mitochondria decreased compared with the control group. Some mitochondrial cristae were fractured. Vacuoles appeared in mitochondria and cytoplasm (Fig. 3d).
Cell Viability
As shown in Fig. 4, cell viabilities of chicken embryonic neurocytes cultured for 48 h with Mn concentrations of 0.5, 1, 1.5, 2, 2.5, and 3 mM Mn were 96.72, 92.44, 88.70, 81.09, 75.91, and 72.25%, respectively. Cell viability (72.25%) in 3-mM-Mn group could meet the requirement of cellular toxicology test.
Cytomorphology
Morphology changes of chicken embryonic neurocytes for 48 h were detected using AO/EB double fluorescent dye staining and shown in Fig. 5. In the control group (Fig. 5a), nuclei were round and green. In 0.5-mM-Mn group (Fig. 5b), chromatin condensation appeared in some nuclei and some yellow irregular nuclei occurred. Numbers of irregular yellow nuclei in 1-mM-Mn group (Fig. 5c) were higher than those in 0.5-mM-Mn group. Orange fragmented chromatin and apoptotic bodies were observed in 1.5-mM-Mn group (Fig. 5d). Numbers of orange fragmented chromatin and apoptotic bodies in 2-mM-Mn group were higher than those in 1.5-mM-Mn group (Fig. 5e). In 2.5- and 3-mM-Mn groups, more orange fragmented chromatin and apoptotic bodies occurred, and a few orange-yellow oval necrotic cells were observed (Fig. 5f, g).
Discussion
Two researches showed that excess Mn decreased GSH-Px and SOD activities and increased MDA content in the immune organs, serum [6], and testis [23] of chickens. We detected SOD and T-AOC activities in chicken cerebrums and embryonic neurocytes. Our results indicated that excess Mn decreased SOD and T-AOC activities in a dose-dependent manner and caused oxidative stress in chicken cerebrums and embryonic neurocytes. Our results were consistent with others. Mo and Cd inhibited SOD and T-AOC activities and caused oxidative stress in duck ovaries [13]. Mo inhibited SOD and T-AOC activities and caused oxidative stress in goat livers [24]. Lead inhibited SOD and T-AOC activities and caused oxidative stress in chicken bursa of Fabricius [25]. Jin et al. [26] found that cypermethrin decreased T-AOC activity in a dose-dependent manner in mice livers. We also found that excess Mn decreased SOD activity of 1800-mg/kg-Mn group and T-AOC activity of 600-, 900-, and 1800-mg/kg-Mn groups in a time-dependent manner in chicken cerebrums.
Oxidative stress increased p53 mRNA expression in human hepatic cells [27]. p53 initiated apoptosis in mice thymocytes [28]. p53 downregulated Bcl-2 protein expression and upregulated Bax protein expression in murine leukemia cell M1 [29], increased Bax/Bcl-2 and Bax/Bcl-x mRNA and protein expression ratio in human chondrocytes [30], and upregulated Bak in mouse JB6 cells [31]. Bcl-2, Bcl-x, Bax, and Bak belonged to Bcl-2 family. Bcl-2 family is a protein family regulating apoptosis. Bcl-2 and Bcl-x inhibited fas mRNA expression and inhibited apoptosis in human breast carcinoma cells [32]. Fas is a receptor on the surface of cells that leads to programmed cell death [33]. Bak and Bax promoted fas and induced apoptosis in mice neutrophils [34]. Increased Bax/Bcl-2 ratio upregulated caspase-3 protein expression and induced apoptosis in human thymus [35]. Caspase-3 is essential for the dismantling of cells and the formation of apoptotic bodies [36]. Our results consisted with above mechanism. In our study, we found that excess Mn upregulated mRNA expressions of p53, Bax, Bak, fas, and caspase-3; downregulated mRNA expressions of Bcl-2 and Bcl-x in a dose-dependent manner; and induced apoptosis in chicken cerebrums and embryonic neurocytes. We also found that the effect of excess Mn on apoptosis-related gene mRNA expression was in a time-dependent manner in chicken cerebrums. Similar results were obtained by other researchers. Excess Mn increased p53, Bax, and caspase-3 mRNA expressions in a dose-dependent manner and induced apoptosis in chicken splenic lynphocytes [16]. Arsenic decreased Bcl-2 protein expression in a dose-dependent manner and induced apoptosis in human umbilical vein endothelial cells [37]. Cobalt downregulated Bcl-x mRNA level in a dose-dependent manner and induced apoptosis in rat PC12 cells [38]. Excess Cd increased mRNA levels of Bak and caspase-3 and induced apoptosis in human peripheral blood lymphocytes [39]. Excess cobalt upregulated protein expression of fas and induced apoptosis in rat PC12 cells [40]. Moreover, our morphological observation indicated that excess Mn caused chicken cerebrum and embryonic neurocyte apoptosis in a dose-dependent manner.
Conclusions
In summary, our results indicated that excess Mn inhibited the activities of SOD and T-AOC; induced mRNA expressions of p53, Bax, Bak, fas, and caspase-3; and inhibited mRNA expressions of Bcl-2 and Bcl-x in chicken cerebrums and embryonic neurocytes. There were dose-dependent manners on all the above factors at all the time points and time-dependent manners on SOD activity of 1800-mg/kg-Mn group, T-AOC activity, and apoptosis-related gene mRNA expression in all the treatment groups in chicken cerebrums. Excess Mn induced chicken cerebrum and embryonic neurocyte apoptosis.
References
Wang FF, Zou YF, Shen YF, Zhong YQ, Lv YN, Huang DM, Chen KC, Li Q, Qing L, Xia B, Su C, Ma SY, Yang XB (2015a) Synergistic impaired effect between smoking and manganese dust exposure on pulmonary ventilation function in Guangxi manganese-exposed workers healthy cohort (GXMEWHC). PLoS One 10(2):e0116558. doi:10.1371/journal.pone.0116558
Gong QQ, Jin ZM, Zou HF (2012) Concentrations of copper, zinc and manganese in tree sparrow (Passer montanus) at Jixi, Heilongjiang Province, China. J For Res 23(2):319–322. doi:10.1007/s11676-012-0259-z
Krishna S, Dodd CA, Hekmatyar SK, Filipov NM (2014) Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol 88(1):47–64. doi:10.1007/s00204-013-1088-3
Liu XF, Zuo N, Guan HN, Han CR, Xu SW (2013a) Manganese-induced effects on cerebral trace element and nitric oxide of Hyline cocks. Biol Trace Elem Res 154(2):202–209. doi:10.1007/s12011-013-9692-x
Wang T, Li XH, Yang DX, Zhang HT, Zhao P, Fu JL, Yao BY, Zhou ZC (2015b) ER stress and ER stress-mediated apoptosis are involved in manganese-induced neurotoxicity in the rat striatum in vivo. NeurotToxicology 48:109–119. doi:10.1016/j.neuro.2015.02.007
Liu XF, Li ZP, Tie F, Liu N, Zhang ZW, Xu SW (2013b) Effects of manganese-toxicity on immune-related organs of cocks. Chemosphere 90(7):2085–2100. doi:10.1016/j.chemosphere.2012.10.072
Chen K, Albano A, Ho A, Keaney JF (2003) Activation of p53 by oxidative stress involves platelet-derived growth factor-β receptor-mediated ataxia telangiectasia mutated (ATM) kinase activation. J Biol Chem 278(41):39527–39533. doi:10.1074/jbc.M304423200
Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, Xu SW (2013a) Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochimica et Biophysica Acta (BBA)-General Subjects 1830(4):3112–3120. doi:10.3945/jn.112.172395
Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, Ren FZ, Xu SW, Wang XL, Lei XG (2013b) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of Se-deficient chick. Biochemical, molecular, and genetic mechanisms. J Nutr 143(5):613–619. doi:10.3945/jn.112.172395
Maroto R, Perez-Polo JR (1997) BCL-2-related protein expression in apoptosis: oxidative stress versus serum deprivation in PC12 cells. J Neurochem 69(2):514–523. doi:10.1046/j.1471-4159.1997.69020514.x
Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB (2002) Oxidative stress induces the expression of fas and fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med 33(12):1641–1650. doi:10.1016/S0891-5849(02)01141-3
Wang L, Wang H, Li JG, Chen DW, Liu ZP (2011) Simultaneous effects of lead and cadmium on primary cultures of rat proximal tubular cells: interaction of apoptosis and oxidative stress. Arch Environ Contam Toxicol 61(3):500–511. doi:10.1007/s00244-011-9644-4
Cao HB, Xia B, Zhang MM, Liao YL, Yang Z, Hu GL, Zhang CY (2015) Changes of antioxidant function and the mRNA expression levels of apoptosis genes in duck ovaries caused by molybdenum or/and cadmium. Biol Trace Elem Res 171(2):410–418. doi:10.1007/s12011-015-0442-0
Huang YH, Shih CM, Huang CJ, Lin CM, Chou CM, Tsai ML, Liu TP, Chiu JF, Chen CT (2006) Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J Cell Biochem 98(3):577–589. doi:10.1002/jcb.20772
Zhu YH, Lu XX, Wu D, Cai SH, Li S, Teng XH (2013) The effect of manganese-induced cytotoxicity on mRNA expressions of HSP27, HSP40, HSP60, HSP70 and HSP90 in chicken spleen lymphocytes in vitro. Biol Trace Elem Res 156(1):144–152. doi:10.1007/s12011-013-9817-2
Zhu YH, Li S, Teng XH (2016) The involvement of the mitochondrial pathway in manganese-induced apoptosis of chicken splenic lymphocytes. Chemosphere 153:462–470. doi:10.1016/j.chemosphere.2016.03.081
Lu XX, Zhu YH, Bai RS, Li S, Teng XH (2015) The effect of manganese-induced toxicity on the cytokine mRNA expression of chicken spleen lymphocytes in vitro. Res Vet Sci 101:165–167. doi:10.1016/j.rvsc.2015.05.009
Liu XF, Li ZP, Han CR, Zhang ZW, Xu SW (2012) Effects of dietary manganese on Cu, Fe, Zn, Ca, Se, IL-1β, and IL-2 changes of immune organs in cocks. Biol Trace Elem Res 148(3):336–344. doi:10.1007/s12011-012-9377-x
Shao JJ, Yao HD, Zhang ZW, Li S, Xu SW (2012) The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol Lett 214(2):99–108. doi:10.1016/j.toxlet.2012.08.011
Du Y, Zhu YH, Teng XJ, Zhang K, Teng XH, Li S (2015) Toxicological effect of manganese on NF-κB/iNOS-COX-2 signaling pathway in chicken testes. Biol Trace Elem Res 168(1):227–234. doi:10.1007/s12011-015-0340-5
Klaassen CD, Amdur MO (2007) Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill Professional/Jaypee Brothers Medical Publishers, New York
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. doi:10.1093/nar/29.9.e45
Liu XF, Zhang LM, Guan HN, Zhang ZW, Xu SW (2013c) Effects of oxidative stress on apoptosis in manganese-induced testicular toxicity in cocks. Food Chem Toxicol 60:168–176. doi:10.1016/j.fct.2013.07.058
Zhuang Y, Liu P, Wang LQ, Luo JR, Zhang CY, Guo XQ, Hu GL, Cao HB (2016) Mitochondrial oxidative stress-induced hepatocyte apoptosis reflects increased molybdenum intake in caprine. Biol Trace Elem Res 170(1):106–114. doi:10.1007/s12011-015-0450-0
Jiao XY, Yang K, An Y, Teng XJ, Teng XH (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res. doi:10.1007/s11356-016-8329-y
Jin YX, Wang LG, Ruan ML, Liu JW, Yang YF, Zhou C, Xu B, Fu ZW (2011) Cypermethrin exposure during puberty induces oxidative stress and endocrine disruption in male mice. Chemosphere 84(1):124–130. doi:10.1016/j.chemosphere.2011.02.034
Ye YY, Liu JW, Xu JH, Sun LJ, Chen MC, Lan MB (2010) Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol in Vitro 24(3):751–758. doi:10.1016/j.tiv.2010.01.001
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH (1993) Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362(6423):849–852. doi:10.1038/362849a0
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC (1994) Tumor suppressor p53 is a regulator of Bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9(6):1799–1805
Chen JH, Cao JL, Chu YL, Wang ZL, Yang ZT, Wang HL (2008) T-2 toxin-induced apoptosis involving fas, p53, Bcl-xL, Bcl-2, Bax and caspase-3 signaling pathways in human chondrocytes. Journal of Zhejiang University SCIENCE B 9(6):455–463. doi:10.1631/jzus.B0820013
Qin JC, Chen HG, Yan Q, Deng M, Liu JP, Doerge S, Ma WY, Dong ZG, Li DW (2008) Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res 68(11):4150–4162. doi:10.1158/0008-5472.CAN-08-0839
Jäättelä M, Benedict M, Tewari M, Shayman JA, Dixit VM (1995) Bcl-x and Bcl-2 inhibit TNF and fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10(12):2297–2305
Wajant H (2002) The fas signaling pathway: more than a paradigm. Science 296(5573):1635–1636. doi:10.1126/science.1071553
Croker BA, O’Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu YF, Smyth GK, Zhang JG, White M, Lackovic K, Cengia LH, O’Reilly LA, Bouillet P, Cory S, Strasser A, Roberts AW (2011) Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. PNAS 108(32):13135–13140. doi:10.1073/pnas.1110358108
Salakou S, Kardamakis D, Samandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papaetropoulos T, Petsas T, Dougenis D (2007) Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In vivo 21(1):123–132
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6(2):99–104. doi:10.1038/sj.cdd.4400476
Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP, Rafii S (2000) Arsenic trioxide induces dose-and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood 96(4):1525–1530
Zou WG, Yan MD, Xu WJ, Huo HR, Sun LY, Zheng ZC, Liu XY (2001) Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res 64(6):646–653. doi:10.1002/jnr.1118
Al-Assaf AH, Alqahtani AM, Alshatwi AA, Syed NA, Shafi G, Hasan TN (2013) Mechanism of cadmium induced apoptosis inhuman peripheral blood lymphocytes: the role of p53, fas and caspase-3. Environ Toxicol Pharmacol 36(3):1033–1039. doi:10.1016/j.etap.2013.09.006
Jung JY, Kim WJ (2004) Involvement of mitochondrial- and fas-mediated dual mechanism in CoCl2-induced apoptosis of rat PC12 cells. Neurosci Lett 371(2–3):85–90. doi:10.1016/j.neulet.2004.06.069
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures used in this experiment were approved by the Northeast Agricultural University’s Institutional Animal Care and Use Committee under the approved protocol number SRM-06. Informed consent was obtained from all individual participants included in the study.
Funding
This work was supported by grants from Hualong Feed Research Collaboration (No. 860035) and the Natural Science Foundation of Heilongjiang Province of China (No. C201420).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
All authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in Biological Trace Element Research.
Rights and permissions
About this article
Cite this article
Zhang, K., Zhu, Y., Wang, X. et al. Excess Manganese-Induced Apoptosis in Chicken Cerebrums and Embryonic Neurocytes. Biol Trace Elem Res 180, 297–305 (2017). https://doi.org/10.1007/s12011-017-0992-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0992-4