Abstract
The possible beneficial role of selenium (Se) in heat shock proteins (HSPs) and inflammation damage induced by lead (Pb) in chickens is unclear. Therefore, the aim of this study was to investigate the effect of Se against Pb on the messenger RNA (mRNA) expression levels of HSPs (HSP 27, 40, 60, 70, and 90); heme oxygenase-1 (HO-1); and the inflammatory cytokines nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) in the peripheral blood lymphocytes of chickens. A total of 360 1-day-old broiler chickens were randomly allocated into four groups (n = 90/group). The control group was fed a basic diet containing 0.2 mg/kg Se and 0.5 mg/kg Pb; the Se supplementation group (+Se group) was fed a Se-adequate (sodium selenite) diet containing 1 mg/kg Se and 0.5 mg/kg Pb; the Pb-supplemented group (+Pb group) was fed a Pb acetate diet containing 0.2 mg/kg Se and 350 mg/kg Pb; and the Se and Pb compound group (Se + Pb group) was fed a diet containing 1 mg/kg Se and 350 mg/kg Pb. The blood was collected and examined for the mRNA levels of HSP and inflammatory cytokine genes at 30 and 60 days old. The results showed that Pb poisoning induced the mRNA expression of HSPs and inflammatory cytokines in the peripheral blood lymphocytes of chickens. In addition, Se alleviated the Pb-induced increase in HSP and inflammatory cytokine mRNA levels in chicken peripheral blood lymphocytes. In conclusion, Se can antagonize the toxic effects of Pb on chickens and protect the chickens’ peripheral blood lymphocytes in normal physiological function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) has long been recognized as a common, nonessential heavy metal toxic to living organisms, with negative effects on general health, reproduction, and behavior, potentially leading to death [1]. Many investigations had indicated that Pb exposure could induce a wide range of biochemical and physiological dysfunctions in humans and laboratory animals [2, 3]. It had also been reported that Pb could induce oxidative stress by generating free radicals and reactive oxygen species (ROS) [4]. However, the toxic role of Pb remained unclear, and the exact mechanism of Pb toxicity still needs further study.
Heat shock proteins (HSPs) are major molecular chaperones that perform important functions in the folding/unfolding and translocation of proteins as well as in the assembly/disassembly of protein complexes [5]. HSPs also possess the ability to modulate the cellular antistress responses and play key roles in protecting organisms from metal stress [6]. Some previous studies have indicated that heavy metal stresses can increase the messenger RNA (mRNA) levels of HSPs in different tissues of western painted turtles [7], and chromium can upregulate the expression of HSP27 and HSP70 in the mouse liver [8]. Heavy metals are well known as potential toxicants, capable of disrupting the activity of a number of prominent proteins as well as altering the expression patterns of numerous genes, thereby interfering with multiple cellular events and leading to increased susceptibility to several diseases [9]. For example, human exposure to Pb increased heme oxygenase-1 (HO-1) gene expression in renal tubule cells [10]. HO is the rate-limiting enzyme in heme catabolism that degrades heme into biliverdin, releasing iron and carbon monoxide. HO is considered to be a stress protein, like HSPs, and is activated by oxidative stress [11].
Inflammation is an important indicator of organism damage due to excess heavy metals. Nuclear factor kappa B (NF-κB) is an inducible transcription factor in lymphocytes and is among the most important transcription factors in terms of inflammatory responses, controlling the expression of numerous genes, such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumor necrosis factor alpha (TNF-α) [12]. TNF-α is a proinflammatory cytokine produced by macrophages [13] and activated NF-κB. Cyclooxygenase (COX) is the key enzyme in the biosynthesis of prostaglandins. COX-2 expression is usually induced by various cytokines, mitogens, and stresses [14]. iNOS could protect to some degree against autoimmunity and function as an intra- and intercellular signaling molecule, shaping the immune response [15]. Some of the interactions between heavy metals and inflammatory factors in chickens have already been reported. Arsenic exposure increased the mRNA level of TNF-α in the mouse liver [16] and of NF-κB, TNF-α, COX-2, PTGEs, and iNOS in the chicken gastrointestinal tract [17]. Excess Mn exposure resulted in inflammatory injury to chicken testis tissue through the NF-κB/iNOS-COX-2 signaling pathway [18]. Selenium (Se) is an essential element in almost all biological systems and a well-established antioxidant [19] that can prevent or decrease the harmful effects of metal on the antioxidant systems in different tissues. The protective effect of Se against the toxicity of different heavy metals in biological systems appears to have been studied [4, 20]. Se can protect against cadmium-induced cytotoxicity in chicken splenic lymphocytes [21], and Se exhibits protective effects against the impairment of spatial learning and memory as well as synaptic structural plasticity induced by Pb exposure in weaned rats [22].
In summary, there has been some research on Pb toxicity and the antagonism of Se against Pb. However, too little is known about the effect of Pb on the mRNA levels of HSPs and on inflammatory injury, as well as about the antagonistic effect of Se against Pb toxicity in chicken peripheral blood lymphocytes. Herein, we established a model of Se and Pb interactions in chicken peripheral blood lymphocytes and detected the effects of Se and Pb on the gene expression levels of HSPs and inflammatory cytokines.
Materials and Methods
Birds and Diets
Three hundred sixty 1-day-old male broiler chickens were purchased from Weiwei Co. Ltd. (Harbin, China) and randomly allocated to four groups (the Se-adequate group, Pb-supplemented group, Se and Pb compound group, and control group). Each treatment was replicated six times with 15 chickens each. The chickens were maintained on a basic diet (control group) containing 0.2 mg/kg Se and 0.5 mg/kg Pb; a Se-adequate (sodium selenite) diet (+Se group) containing 1 mg/kg Se and 0.5 mg/kg Pb; a Pb-supplemented (Pb acetate) diet (+Pb group) containing 0.2 mg/kg Se and 350 mg/kg Pb; or a Se and Pb compound diet (Se + Pb group) containing1 mg/kg Se and 350 mg/kg Pb. The feeding experiment lasted for 60 days, and the experimental chickens were given free access to feed and water. All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. At 30 and 60 days, blood was collected from individual chickens killed with sodium pentobarbital, and the lymphocytes were isolated immediately and stored at −80 °C until analysis.
Quantification of Gene mRNA
The total RNA of chicken lymphocytes (n = 6/diet group) was extracted and isolated by Trizol reagent according to the manufacturer’s instructions (Invitrogen, China). The dried RNA pellets were resuspended in 50 μl of diethyl-pyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand complementary DNA (cDNA) was synthesized from 5 μg total RNA using oligo dT primers and Superscript II Reverse Transcriptase according to the manufacturer’s instructions (Invitrogen, China). Synthesized cDNA was diluted five times with sterile water and stored at −80 °C until use.
After quantification, the expression levels of HSP and inflammatory cytokine genes were determined by real-time quantitative reverse transcription PCR using SYBR Premix ExTaq TM (Takara, China) on an ABI PRISM 7500 real-time PCR system (Applied Biosystems). The PCR primers (Table 1) were designed using the Oligo Primer Analysis software (version 6.0) and synthesized by Invitrogen (Shanghai, China).
Reaction mixtures were as follows: 10 μl of 2× SYBR Green I PCR Master Mix (Takara, China), 0.4 μl of 50× ROX Reference Dye II, 0.4 μl of each primer (10 μM), 2 μl of either diluted cDNA, and 6.8 μl of PCR-grade water. The PCR procedure for HSPs, inflammatory cytokines, and β-actin consisted of 95 °C for 30 s followed by 40 cycles at 95 °C for 15 s and 60 °C for 30 s. The results (fold changes) were expressed as 2−ΔΔCt, in which ΔΔCt = (Ct HSPs − Ct β-actin)B/C/D−(Ct HSPs − Ct β-actin)A, where Ct HSPs and Ct β-actin are the cycle thresholds for the chicken HSP and β-actin genes in the different treated groups, respectively. A is the control group, B is the +Se group, C is the +Pb group, and D is the Se + Pb group.
Statistical Analyses
Data were analyzed using SPSS for Windows (SPSS, Chicago, IL, USA) and are expressed as the mean ± standard deviation. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honestly significant difference test for post hoc multiple comparisons.
Results
The mRNA Expression of HSP27, HSP40, HSP60, HSP70, HSP90, and HO-1 in Chicken Peripheral Blood Lymphocytes
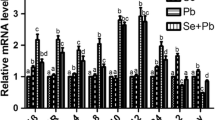
From Fig. 1a–d and Table 2, we can see that there were no significant differences (p > 0.05) in HSP27 (Fig. 1a), HSP40 (Fig. 1b), HSP60 (Fig. 1c), HSP70 (Fig. 1d), and HSP90 (Fig. 1e) mRNA levels between the +Se group and the control group at either time point. The mRNA levels of HSP27, HSP40, HSP60, HSP70, and HSP90 in the +Pb group were significantly higher (p < 0.05) than in the control group and the +Se group at both time points. HSP27, HSP40, HSP60, HSP70, and HSP90 mRNA levels at both time points in the Se + Pb group were significantly higher (p < 0.05) than in the control group and the Se group, except the HSP40 mRNA level at 30 days and the HSP90 mRNA level at 60 days, and the gene expression of HO-1 was similar to the expression of the HSPs. There was no significant difference (p > 0.05) in HO-1 (Fig. 1f) mRNA levels between the +Se group and the control group at 30 days or 60 days. However, in the +Pb group and Se + Pb group, the mRNA level of HO-1 was significantly increased compared with the control or +Se group (p < 0.05).
The mRNA Expression of NF-κB, TNF-α, COX-2, and iNOS in Chicken Peripheral Blood Lymphocytes
The mRNA expression levels of NF-κB, TNF-α, COX-2, and iNOS in chicken peripheral blood lymphocytes are shown in Fig. 2a–d and Table 3. There were no significant differences (p > 0.05) in NF-κB (Fig. 2a), TNF-α (Fig. 2b), COX-2 (Fig. 2c), or iNOS (Fig. 2d) mRNA levels between the +Se group and the control group at the two time points, except that the TNF-α mRNA level at 60 days was significantly decreased (p < 0.05) compared with that in the control group. The mRNA levels of NF-κB, TNF-α, COX-2, and iNOS in the +Pb group were significantly higher than in the control group, the Se group, and the Se + Pb group at 30 and 60 days (p < 0.05). NF-κB, TNF-α, COX-2, and iNOS mRNA levels at the two time points in the Se + Pb group were significantly (p < 0.05) higher than in the control group and the +Se group.
Discussion
Environmental exposure to Pb is considered an important health risk. Most studies have reported that Pb is circulated around the body by blood and accumulated in bones and vital organs (e.g., liver, kidneys), which could therefore be severely harmed [23, 24]. Pb exposure can also cause rat liver inflammatory injury [22]. Pb-induced genotoxicity was found in lymphocytes from peripheral blood samples of humans [25]. In this study, the results showed that Pb affected the mRNA levels of HSPs (HSP27, HSP40, HSP60, HSP70, and HSP90), HO-1, and inflammatory cytokines (NF-κB, TNF-α, COX-2, iNOS) in the peripheral blood lymphocytes of chickens as well as showed the antagonism of Se against Pb.
As molecular chaperones, HSPs are highly conserved cellular stress proteins present in every organism from bacteria to humans. HSPs can protect organisms from a number of stress conditions, including ischemia, metabolic disorders, inflammation and infection, heat stress, ischemic stress, and heavy metal stress [26], and HO-1 has a similar function to HSPs [10]. Some previous studies have shown that the mRNA levels of HSPs (HSP27, 40, 60, 70, and 90) in chicken livers were increased by dietary Se deficiency [27]. LcHSP27 expression showed dramatic upregulation after exposure to the combined stress of temperature and cadmium [28]. The levels of HSP mRNA (HSP90, HSP 70, HSP 60, HSP 40, and HSP 27) and protein (HSP70 and HSP60) were significantly increased (p < 0.05 or p < 0.01) with As2O3 treatment [29]. Continuous Pb treatment elevated the HSP70 level in oribatid mites [30]. In this study, we found that the HSP 27, HSP40, HSP60, HSP 70, and HSP90 mRNA levels of chicken peripheral blood lymphocytes were significantly increased (p < 0.05) in the +Pb group. This result implied that HSPs might be biomarkers of Pb poisoning. In addition, the mRNA level of HO-1 was also significantly increased (p < 0.05) in the +Pb group. This finding was similar to the results of Hilda Vargas’s research, in which increased levels of HO-1 mRNA and HO-1 protein levels were observed as early as 3 h after Pb exposure in the rat kidney cortex [10].
NF-κB is a heterodimeric transcription factor that translocates to the nucleus and mediates the transcription of a vast array of proteins involved in cell survival and proliferation, inflammatory response, and antiapoptotic factors. NF-κB transcription factors regulate genes involved in many aspects of the inflammatory response [31]. NF-κB was involved in the regulation of COX-2 and iNOS expression, important enzymes that mediate inflammatory processes [32]. TNF-α is a proinflammatory cytokine produced by macrophages in response to bacterial endotoxin [13]. Several previous studies have indicated that Pb exposure increases the levels of TNF-α and COX-2 proinflammatory enzymes in a mouse microglial cell line [33]. Pb was responsible for a significant morphological alteration and a decline in cell function and TNF-α release that increased inflammation in mice testicular macrophages [34]. In the current study, we found that in the Pb group, the mRNA levels of NF-κB, TNF-α, COX-2, and iNOS were significantly higher than in the control group. In accordance with the above research results, our results suggested that excess Pb could cause inflammation of chicken peripheral blood lymphocytes.
Se is among the essential micronutrients for living organisms, closely related to human and animal health. Se deficiency mainly influences the expression levels of selenoproteins in chicken muscles [35, 36], as well as downregulates liver selenoproteins and resulting in oxidative stress damage [37–39]. Se also could provide certain protective actions against Pb-induced toxicity in chicken livers [40]. In our study, the mRNA levels of HSP27, HSP40, HSP60, HSP70, HSP90, HO-1, NF-κB, TNF-α, iNOS, and COX-2 decreased in the Se + Pb group compared to those in the Pb group. It was demonstrated that Se exerts a remission effect on the Pb-induced HSPs, HO-1, and inflammatory cytokines. Some studies have reported the antagonistic effect of Se on other heavy metals; for example, Se could protect the nitric oxide and gene expression of inflammatory cytokines induced by cadmium in chicken splenic lymphocytes [41]. Se could also ameliorate cadmium (Cd)-induced brain damage in chickens [42] and exhibited protective effects on the chronic poisoning and decreased HSP70 mRNA level induced by arsenic in rat livers [43].
In conclusion, Pb poisoning induced mRNA expression of HSPs and inflammatory cytokines in chicken peripheral blood lymphocytes. In addition, Se exhibited antagonistic roles against the Pb-induced increase in HSP and inflammatory cytokine mRNA expression in the peripheral blood lymphocytes of chickens.
Reference
Fisher IJ, Pain DJ, Thomas VG (2006) A review of lead poisoning from ammunition sources in terrestrial birds. Biol Conserv 131:421–432
Flora SJ, Pande M, Mehta A (2003) Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 145:267–280
Adonaylo VN, Oteiza PI (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Ates B, Orun I, Talas ZS, Durmaz G, Yilmaz I (2008) Effects of sodium selenite on some biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb2+ and Cu2+. Fish Physiol Biochem 34:53–59
Bernabo P, Rebecchi L, Jousson O, Martinez-Guitarte JL, Lencioni V (2011) Thermotolerance and hsp70 heat shock response in the cold-stenothermal chironomid Pseudodiamesa branickii (NE Italy). Cell Stress Chaperones 16:403–410
Chen H, Xu XL, Li YP, Wu JX (2014) Characterization of heat shock protein 90, 70 and their transcriptional expression patterns on high temperature in adult of Grapholita molesta (Busck). Insect Sci 21:439–448
Ramaglia V, Harapa GM, White N, Buck LT (2004) Bacterial infection and tissue-specific Hsp72, −73 and −90 expression in western painted turtles. Comparative biochemistry and physiology. Toxicol Pharmacol CBP 138:139–148
Lee J, Lim KT (2012) Inhibitory effect of SJSZ glycoprotein (38 kDa) on expression of heat shock protein 27 and 70 in chromium (VI)-treated hepatocytes. Mol Cell Biochem 359:45–57
Ademuyiwa O, Agarwal R, Chandra R, Behari JR (2010) Effects of sub-chronic low-level lead exposure on the homeostasis of copper and zinc in rat tissues. J Trace Elem Med Biol Organ Soc Miner Trace Elem 24:207–211
Vargas H, Castillo C, Posadas F, Escalante B (2003) Acute lead exposure induces renal haeme oxygenase-1 and decreases urinary Na + excretion. Human Exp Toxicol 22:237–244
Maines MD, Kappas A (1976) The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res 8(Suppl 17):39–46
Cogswell JP, Godlevski MM, Wisely GB et al (1994) NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol 153:712–723
Raabe T, Bukrinsky M, Currie RA (1998) Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem 273:974–980
Yuan CJ, Mandal AK, Zhang Z, Mukherjee AB (2000) Transcriptional regulation of cyclooxygenase-2 gene expression: novel effects of nonsteroidal anti-inflammatory drugs. Cancer Res 60:1084–1091
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916
Das S, Santra A, Lahiri S, Guha Mazumder DN (2005) Implications of oxidative stress and hepatic cytokine (TNF-alpha and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol 204:18–26
Xing M, Zhao P, Guo G et al (2015) Inflammatory factor alterations in the gastrointestinal tract of cocks overexposed to arsenic trioxide. Biol Trace Elem Res 167:288–299
Du Y, Zhu Y, Teng X et al (2015) Toxicological effect of manganese on NF-kappaB/iNOS-COX-2 signaling pathway in chicken testes. Biol Trace Elem Res 168:227–234
Klotz LO, Kroncke KD, Buchczyk DP, Sies H (2003) Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr 133:1448S–1451S
Orun I, Talas ZS, Ozdemir I, Alkan A, Erdogan K (2008) Antioxidative role of selenium on some tissues of (Cd2+), Cr3+)-induced rainbow trout. Ecotoxicol Environ Saf 71:71–75
Chen X, Zhu YH, Cheng XY, Zhang ZW, Xu SW (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17:14565–14572
Han XJ, Xiao YM, Ai BM et al (2014) Effects of organic selenium on lead-induced impairments of spatial learning and memory as well as synaptic structural plasticity in rats. Biol Pharm Bull 37:466–474
Hartup BK (1996) Rehabilitation of native reptiles and amphibians in DuPage County, Illinois. J Wildl Dis 32:109–112
Gangoso L, Alvarez-Lloret P, Rodriguez-Navarro AA et al (2009) Long-term effects of lead poisoning on bone mineralization in vultures exposed to ammunition sources. Environ Pollut 157:569–574
Pasha Shaik A, Sankar S, Reddy SC, Das PG, Jamil K (2006) Lead-induced genotoxicity in lymphocytes from peripheral blood samples of humans: in vitro studies. Drug Chem Toxicol 29:111–124
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liu CP, Fu J, Xu FP, Wang XS, Li S (2015) The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers Biometals : an international journal on the role of metal ions in biology. Biochem Med 28:163–173
Yang QL, Yao CL, Wang ZY (2012) Acute temperature and cadmium stress response characterization of small heat shock protein 27 in large yellow croaker, Larimichthys crocea. Comparative biochemistry and physiology. Toxicol Pharmacol CBP 155:190–197
Guo Y, Zhao P, Guo G, et al. (2015) Effects of arsenic trioxide exposure on heat shock protein response in the immune organs of chickens. Biological trace element research
Kohler HR, Alberti G, Seniczak S, Seniczak A (2005) Lead-induced hsp70 and hsp60 pattern transformation and leg malformation during postembryonic development in the oribatid mite, Archegozetes longisetosus Aoki. Comparative biochemistry and physiology. Archegozetes Toxicol Pharmacol CBP 141:398–405
Karin M, Yamamoto Y, Wang QM (2004) The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov 3:17–26
Surh YJ, Chun KS, Cha HH et al (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 480–481:243–268
Kumawat KL, Kaushik DK, Goswami P, Basu A (2014) Acute exposure to lead acetate activates microglia and induces subsequent bystander neuronal death via caspase-3 activation. Neurotoxicology 41:143–153
Barbhuiya SASK, Chakraborty S, Sengupta M (2013) Studies of lead toxicity on inflammatory damage and innate immune functions in testicular macrophages of male Swiss albino mice. Mod Res Inflamm vol 2:75–81
Yao HD, Wu Q, Zhang ZW et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr 143:613–619
Yao H, Liu W, Zhao W et al (2014) Different responses of selenoproteins to the altered expression of selenoprotein W in chicken myoblasts. RSC Adv 4:64032–64042
Liu C, Fu J, Liu C, Li S (2015) The role of nitric oxide and autophagy in liver injuries induced by selenium deficiency in chickens. Rsc Advances 5:50549–50556
Jiang ZH, Khoso PA, Yao HD, et al. (2015) SelW regulates inflammation-related cytokines in response to H2O2 in Se-deficient chicken liver. Rsc Advances 5:
Liu CP, Fu J, Lin SL, Wang XS, Li S (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159:192–198
Xu T, Gao X, Liu G (2015) The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver. Biological trace element research
Liu S, Xu F, Fu J, Li S (2015) Protective roles of selenium on nitric oxide and the gene expression of inflammatory cytokines induced by cadmium in chicken splenic lymphocytes. Biol Trace Elem Res 168:252–260
Liu LL, Zhang JL, Zhang ZW et al (2014) Protective roles of selenium on nitric oxide-mediated apoptosis of immune organs induced by cadmium in chickens. Biol Trace Elem Res 159:199–209
Xu Z, Wang Z, Li JJ et al (2013) Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic food and chemical toxicology. Int J Published British Industrial Biological Res Assoc 58:1–7
Acknowledgments
This work was supported by China Postdoctoral Science Foundation (No. 2012M520702); Startup Foundation for Doctors of Northeast Agricultural University, China (No. 2012RCB92); Heilongjiang Provincial Department of Education Science and Technology research project (No. 12541024); Young Talents Project of Northeast Agricultural University (No. 14QC18); and the International Postdoctoral Exchange Fellowship Program (No. 20130006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
G.X. Sun and Y. Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, G., Chen, Y., Liu, C.P. et al. Effect of Selenium Against Lead-Induced Damage on the Gene Expression of Heat Shock Proteins and Inflammatory Cytokines in Peripheral Blood Lymphocytes of Chickens. Biol Trace Elem Res 172, 474–480 (2016). https://doi.org/10.1007/s12011-015-0602-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0602-2