Abstract
Chromium (VI) is as an extremely toxic chemical substance, and is also an internationally recognized human carcinogen. The principal objective of this study was to determine whether or not Styrax japonica Siebold et al. Zuccarini (SJSZ) glycoprotein prevents hepatocarcinogenesis in chromium-treated BNL CL.2 cells and ICR mice. Firstly, it was evaluated that SJSZ glycoprotein has strong antioxidant character and scavenges radicals. In an effort to assess the chemopreventive effects of SJSZ glycoprotein on hepatocarcinogenesis, ICR mice were intraperitoneally injected with chromium (10 mg/kg, BW) for 8 weeks. After sacrifice, we evaluated indicators of liver tissue damage [the activities of lactate dehydrogenase (LDH) and alanine aminotransferase (ALT), and thiobarbituric acid-reactive substances (TBARS)], antioxidative enzymes [activities of superoxide dismutase (SOD), catalase (CAT) and gluthathione peroxidase (GPx)], and initiating hepatocarcinogenic indicator [heat shock protein (HSP) 27 and 70] and protein kinase C (PKC), p38 MAPK and PCNA via biochemical methods and immunoblot analysis. The results obtained from this study demonstrated that the SJSZ glycoprotein (50 μg/ml) inhibited the production of intracellular ROS in BNL CL.2 cells. In addition, the SJSZ glycoprotein (10 mg/kg, BW) attenuated the levels of LDH, ALT, and TBARS, whereas it increased antioxidative enzymes in mouse serum. SJSZ glycoprotein attenuated the activity of HSP27, HSP70, PKC, MAPKs, and PCNA in BNL CL.2 cells and liver tissue. Taken together, our results indicate that SJSZ glycoprotein might be have a potent preventive effect against hepatocarcinogenesis induced by oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium is an ubiquitous element in nature which exists in primarily two valence states: trivalent [Cr(III)] and hexavalent [Cr(VI)]. However, the effects of these two states on human health differ profoundly. Cr(III) is an essential micronutrient for humans. By way of contrast, Cr(VI) is regarded as one of the most toxic chemical substances known, and is also an internationally recognized heavy metal carcinogen. Cr(VI) is widely used in industries, including the leather, chrome-plating, and dye-producing industries [1]. As a result of the deposition of industrial waste, including chromates, in the soil, drinking water resources might be contaminated, and the concentration of these substances in ambient air might also increase [2]. Chromium is distributed to virtually every organ of the body. Thus, all organs are potentially susceptible to the toxic and carcinogenic effects of chromium (VI). Elevations of chromium levels were observed in each tissue sampled in our study, thereby reflecting oral exposure to different organs, with some of the highest levels detected in the liver, kidney, spleen, and bone. It has also been reported that chromium (VI) induces cytotoxicity and lipid peroxidation in primary-cultured hepatocytes [3].
The production of reactive oxygen species (ROS) is a well-known physiological process. Several studies have provided evidence that free radical-induced oxidative damage of cell membranes, DNA and proteins might cause several diseases, including cancer [4]. Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which consists of tripeptides, together constitute a supportive team of defense against ROS [5]. They perform critical roles as markers for chemoprevention, owing to their antioxidant and detoxification properties.
Chronic tissue inflammation is coupled to increased risk of cancer in a broad variety of organs, including those of the stomach, colon, bladder, and liver [6]. Hepatocellular carcinoma (HCC) occurs principally as the result of inflammation. The process of chronic inflammation results in a stressful condition, and ubiquitous molecules such as heat shock proteins (HSPs) are induced in response to stressful stimuli, contributing to hepatocarcinogenesis [7]. The upregulation of HSPs as observed in various cancers suggests that they might be involved in carcinogenesis. In particular, the enhancement of carcinogenesis via the overexpression of HSP27 and HSP70 has been previously implicated in a rodent model. The activation or phorphorylation of HSPs is regulated by protein kinase C (PKC) activation in various cells.
It is a good strategy to use medical plants for the prevention or healing of several different immune dysfunctional diseases caused by toxic heavy metals such as chromate, since most natural compounds (like phytoglycoprotein) exhibit less cytotoxicity than artificial synthetic compounds such as drugs. Styrax japonica Siebold et al. Zuccarini (Styracaceae, SJSZ) has been previously employed as a folk medicine in Korea, for the treatment of sore throat, cough, odontalgia, and paralysis. In addition, its pericarps are used for producing soap and piscicidal agents, as they contain egosaponin as a major component [8, 9]. It has been previously reported that triterpenoids isolated from the stems and leaves of SJSZ evidence anti-inflammation activity and inhibit the proliferation of HL-60 (human leukemic) cells. Recently, we isolated a glycoprotein from SJSZ with an approximate molecular mass of 38 kDa. The glycoprotein has a carbohydrate and protein content of 52.64 and 47.35%, respectively. Thus, we assume that SJSZ glycoprotein can probably prevent the liver cell damage caused by chromium (VI). However, it has not previously been reported that the SJSZ glycoprotein prevents chromate-induced hepatocarcinogenesis.
Therefore, the principal objective of this study was to determine whether or not the SJSZ glycoprotein can prevent hepatocarcinogenesis via chronic inflammatory factors (Hsp27 and Hsp70) in chromium-treated BNL CL.2 cells and ICR mice.
Materials and methods
Chemicals
All the plastic materials used herein were purchased from Falcon Labware (Becton-Dickinson, Franklin Lakes, NJ). Penicillin G and streptomycin were obtained from Sigma (St Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY, USA). Sodium dichromate [Cr(VI)] and vitamin C were purchased from Sigma (St Louis, MO, USA). All other chemicals and reagents used in this study were of the highest analytical grade available.
Preparation of glycoprotein from Styrax japonica siebold et al. Zuccarini fruit
The SJSZ glycoprotein was isolated and purified from S. japonica Siebold et al. Zuccarini (SJSZ) fruit, as follows. Briefly, SJSZ fruits were harvested in July 2009 from Moodeung mountain in Chonnam province, South Korea. They were identified by Dr. H.T. Lim (Chonnam National University). A voucher specimen (No. LKT0100722) was deposited with the Molecular Biochemistry Laboratory, Chonnam National University, Gwang-ju, Korea. SJSZ (12 kg) was chopped into small pieces and soaked in ethanol (11 l, w/v) for 3 months in a dark basement. The ethanol extract was then passed through Whatman filter paper (No. 2) to remove debris and concentrated with a rotary evaporator (B465; Buchi, Flawil, Switzerland). The concentrated solution was freeze-dried (SFDS06; Samwon, Seoul, Korea). The dried powder (2800 g) was again dissolved in distilled water. The solution was subjected to concanavalin A-Sepharose 4B affinity chromatography (24–45 mm, Sigma, C9017) and eluted with 0.5 M methyl α-d-glucopyranoside containing 0.5 M NaCl at pH 7.4. The eluted solution was then dialyzed through a dialysis membrane (Spectra/por, MWCO 8,000–12,000, CAL., USA) against 20 mM Tris–Cl (pH 7.4) at 4°C overnight and lyophilized. The yield of SJSZ glycoprotein from starting crude materials was 21.0 g (0.75%). To confirm the glycoprotein, we conducted SDS-PAGE on a sample of protein (25–50 mg/ml) containing 0.1% SDS, using an 18% polyacrylamide mini-gel and a Mini-PROTEIN II electrophoresis cell (Bio-Rad) at 110 V and 30 mA for 2.5 h. The SJSZ glycoprotein was confirmed via staining with Schiff’s reagent [10], a specific staining reagent for the glycoprotein detected through a redox reaction. The purity of the SJSZ glycoprotein was more than 98%. The sample of glycoprotein on the gel revealed one band of 38 kDa and consists of carbohydrate (52.64%) [11] and protein (47.36%) [12].
Composition of SJSZ glycoprotein
The carbohydrate content of the SJSZ glycoprotein was determined via the phenol-sulfuric method [11], using glucose as the standard. The proper amount of SJSZ glycoprotein was dissolved in 2 ml dH2O, mixed well with 1 ml 5% phenol, and subsequently added to 5 ml of sulfuric acid. The mixture was then incubated for 10 min at room temperature and transferred to a 25–30°C water bath for 10–20 min. The carbohydrate content was measured at 490 nm. To determine the protein content of the SJSZ glycoprotein, the Lowry method was used [12], with bovine serum albumin as the standard. A reading at 280 nm was also employed to determine the protein content of the SJSZ glycoprotein.
DPPH assay
The 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay was conducted according to the method described by Maffei Facino et al. [13]. A 0.1 mM solution of DPPH radical in 80% methanol was prepared. Then the SJSZ glycoprotein (10–200 μg/ml) was added to a DPPH radical solution. The mixture was shaken vigorously and incubated for 30 min in darkness at 23°C. Then, the absorbance of the resultant solution was spectrophotometrically measured at 517 nm.
Deoxyribose assay
The deoxyribose method (non-site-specific scavenging assay) to determine the hydroxyl radical scavenging effect of the SJSZ glycoprotein was conducted as previously described [14]. The non-site-specific assay reflects the antioxidant efficacy of the SJSZ glycoprotein based on the propensity to compete with deoxyribose for the OH produced by Fe2+-EDTA chelates in the solution via the Fenton reaction. The reaction mixtures contained L-ascorbic acid (100 μM), FeCl3 (100 μM) EDTA (104 μM), H2O2 (1.5 mM), deoxyribose (2.5 mM), and SJSZ glycoprotein (5, 10, 25 and 50 μg/ml) up to a final volume of 1 ml. All components were dissolved in KH2PO4/KOH buffer (10 mM, pH 7.4). After 1 h of incubation at 37°C, 1 ml of 2-TBA (0.5%-2-TBA in 0.025 M NaOH) and 1 ml of 2.8% trichloroacetic acid were added to the mixture solution and heated for 30 min at 80°C in a water bath. The absorbance of the mixture was read spectrophotometrically at 523 nm. This assay was also carried out without ascorbic acid (pro-oxidant assay) or EDTA (site-specific scavenging assay), in order to evaluate the SJSZ glycoprotein’s pro-oxidant and metal chelating potential, respectively.
Cell culture
BNL CL.2 cells (murine embryonic liver cell) were incubated in DMEM containing 10% FBS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in an atmosphere containing 5% CO2. The medium was renewed twice per week. The cells (1 × 106 cells/ml) were dispensed into 96-well flat bottom plates or 6-well plates. Sodium dichromate [Cr(VI)] was dissolved in culture media for BNL CL.2 cell culture in this study.
MTT assay
Cell viability was measured via 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay [15]. The cells (1 × 106 cells/ml) were divided into 96-well plates. The cells were exposed to 30 mU/ml glucose/glucose oxidase (G/GO) in DEME containing 0.5% β-d-glucose for 4 h in the presence or absence of SJSZ glycoprotein. After exposure, 2 μl of the MTT solution (5 mg/ml) was added to each well, and the plates were incubated at 37°C and a 5% CO2 atmosphere for an additional 4 h. After removing the medium, acidic isopropanol (70 μl) was added to each well, and the plates were read on a microplate reader (Dynatech Microlisa Reader, S/N UVT06234, CA, USA) at 570 nm.
Experimental design
Male mice (ICR), aged 5 weeks, were purchased from Daehan Laboratories (Animal Research Center Co., Ltd, DaeJeon, Korea) and housed in accordance with the Guiding Principles in the Use of Animal in Toxicology, adopted by the Society of Toxicology in 1989, in the Experimental Animal Room of Veterinary College of Chonnam National University (CNU). All mice were fed a commercial diet and water ad libitum, and maintained for at least 1 week prior to the experiments. The body weight and food intake of each group were recorded once weekly. The mice were divided into the following five groups:
-
Group 1: Control (n = 6);
-
Group 2: SJSZ glycoprotein (10 mg/kg, BW) (n = 6);
-
Group 3: Chromium (10 mg/kg) (n = 6);
-
Group 4: Chromium (10 mg/kg) + SJSZ glycoprotein (5 mg/kg, BW) (n = 6);
-
Group 5: Chromium (10 mg/kg) + SJSZ glycoprotein (10 mg/kg, BW) (n = 6);
The extract-exposed groups were pretreated with their respective extracts for 1 week and continued for an additional 7 weeks during which the treatment groups were intraperitoneally treated with chromium. The mice were administered with a 5 or 10 mg/kg body weight of SJSZ glycoprotein once per day for 1 week, and the control was treated with 100 μl of phosphate-buffered saline (PBS). Sodium dichromate [Cr(VI)] was dissolved in PBS (pH 7.0) for in vivo in this study. Beginning at 1 week, the mice received intraperitoneal injections of 10 mg/kg chromium dissolved in PBS (pH 7.0) once per day for 7 weeks. At 8 weeks, the blood from mice was collected via cardiac puncture and centrifuged at 10,000 g for 5 min at 4°C. The supernatant was separated and stored at −70°C for later measurement of the activity of alanine transaminase (ALT), lactate dehydrogenase (LDH), and formation of thiobarburic acid reactive substances (TBARS). Mouse plasma was used to measure ALT, LDH, and TBARS after treatment with chromium or chromium with SJSZ glycoprotein pretreatment. Liver tissues were used to assess the activities of antioxidant enzymes, PKC, p38 MAPK, HSP27, and HSP70 in this study.
LDH and ALT assay
The levels of LDH and ALT in mice serum were measured according to the method developed by Bergmeyer and Bernt [16, 17]. The LDH and ALT activities were measured as the rate of loss of β-NADH absorption for 2 min at 340 nm.
Thiobarbituric acid reactive substances (TBARS) assay
Lipid peroxidation was estimated by the amounts of thiobarbituric acid reactive substances (TBARS) in plasma according to the method of Ohkawa et al. [18]. One volume of sample was thoroughly mixed with two volumes of stock solution (15% w/v trichloroacetic acid, 0.375% w/v triobarbituric acid and 0.25 N HCl). The mixture was heated for 30 min in a boiling water bath. After cooling, the flocculate precipitate was removed via 10 min of centrifugation at 1000×g and the absorbance of the sample was used as a standard. Data were expressed as a percentage of the control.
Determination of intracellular ROS
The intracellular ROS was measured using the non-fluorescent dye, 2′,7′-dichlorodihydrofluorescein (H2DCF-DA), which is a membrane-permeable fluorigenic tracer that is oxidized by various ROS species. The dye is deacetylated by intracellular esterases to non-fluorescent 2′,7′-dichlorohydrofluorescein (DCFH), which is oxidized to the fluorescent compound 2′,7′-dichlorofluorescein (DCF) by ROS. The cells were pre-incubated with 10 μM H2DCF-DA for 30 min at 37°C, and then washed twice in PBS to remove the extracellular H2DCF-DA. After that the cells were treated with chromium (15 μM) for the indicated time, or co-treated with SJSZ glycoprotein (10–50 μg/ml) in the presence of 15 μM chromium for 30 min. Finally, the fluorescence intensity was measured at an excitation of 485 nm and an emission of 530 nm using a fluorescence microplate reader (Dual Scanning SPECTRAmax, Molecular Devices Corporation, Sunnyvale, CA, USA). The values were calculated as the relative intensity of DCF fluorescence, compared with the control.
Assay of antioxidant and glutathione-metabolizing enzymes
The hepatic antioxidant enzyme activities were assessed in accordance with the methods of Kakkar et al. [19], Thomson et al. [20], and Rotruck et al. [21]. After the mice were sacrificed, the liver tissues were extracted and homogenized with 1 ml homogenizing buffer [10 mM EDTA, and 250 mM surcrose, pH 7.5]. The homogenates were subsequently centrifuged for 10 min at 600×g at 4°C to remove the nuclear fractions, and the remaining separated supernatant was re-centrifuged at 10,000×g for 20 min at 4°C to collect the mitochondrial fraction (pellet) including peroxisomes for the CAT assay. The supernatant was then ultra-centrifuged for 1 h at 100,000×g at 4°C to isolate the cytosolic fraction for the SOD and GPx assays. The protein quantities were assessed using the method of Lowry et al. [12] and the proteins were stored at -70°C for further experimental use. One unit of antioxidant enzymes was defined as the amount of enzyme required to reduce the NBT (50%) for SOD at 560 nm, to reduce 1 μM H2O2/mine for CAT at 220 nm, and to oxidize 1 μM NADPH/min for GPx at 412 nm, respectively. Values were calculated as a percentage of the control value.
Preparations of membrane, cytosolic, nucleic, and whole protein extracts
To detect the translocation of PKC, the membrane extracts were prepared according to Patton’s method [22]. In brief, the BNL CL.2 cells were rinsed twice in PBS after removing the medium and scraped in 300 μl of buffer A (20 mM Tris–HCl, pH 7.5, 0.25 M sucrose, 2 mM EDTA, and 2 mM EGTA) containing a protease inhibitor cocktail (Boehringer, Mannheim). The cells were sonicated and centrifuged for 1 h at 10,000g to sediment all membranes and the insoluble cytoskeletal components. The membrane proteins in the pellet were extracted with buffer B (20 mM Tris–HCl, pH 7.5, 1% NP-40, 150 mM NaCl, 1 mM EGTA and 1 mM EDTA) containing a protease inhibitor cocktail (Boehringer, Mannheim) on ice for 30 min and then centrifuged for 15 min at 100,000g at 4°C. The supernatant was saved as a detergent-soluble membrane fraction. On the other hand, either nucleic protein extract for proliferating cell nuclear antigen (PCNA) or whole cellular protein extract for the immunoblotting of p38 MAPK, HSP27 and HSP70 was isolated from BNL CL.2 cells as described previously [22]. The amount of protein was measured via the method developed by Lowry et al. [12] and the cellular proteins were stored at −70°C prior to use.
Immunoblot analysis
Livers were homogenized in ice-cold lysis buffer [150 mM NaCl, 0.5% Triton-X 100, 50 mM Trix-HCl (pH 7.4), 20 mM EGTA, 1 mM DTT, 1 mM Na3VO4 and protease inhibitor cocktail tablet]. Lysates were centrifuged at 14,800 g for 30 min at 4°C. The amount of protein was measured via Lowry’s method [12], and the cellular proteins were stored at −70°C prior to use. Cellular proteins were separated in a 10% polyacrylamide mini-gel at 100 V for 2 h at room temperature using a mini-protein II electrophoresis cell (Bio-Rad). After electrophoresis, the proteins were transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were then incubated for 1 h at room temperature in TBS-T solution (10 mM Tris–HCl, pH 7.6, 150 mM NaCl, and 0.1% (v/v) Tween-20) containing 5% non-fat dry milk. The membranes were incubated for an additional 2 h at room temperature with primary antibodies [PKC, total-p38 MAPK, phospho-p38 MAPK, HSP27, HSP70, and α-tubulin] in TBS-T solution. After washing three times with TBS-T, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse IgG and anti-rabbit IgG (1:10,000; Cell Signaling Technology, MA, USA) in TBS-T solution. The resultant protein bands were visualized via enhanced chemiluminescence (Amersham Pharmacia Biotech, England, UK) The results of the immunoblot assay were calculated as relative intensities using Scion imaging software (Scion Image Beta 4.02, Frederick, MD, USA).
[3H]-thymidine incorporation
The experiments to assess the levels of [3H]-thymidine incorporation were conducted as described by Gabelman and Emerman [23]. Following the indicated incubation period, 1 μCi of [methyl-3H] thymidine was added to the cultures. Incubation with [3H]-thymidine continued for an additional 5 h at 37°C. The cells were washed twice with PBS, fixed for 15 min in 10% trichloroacetic acid (TCA) at 23°C, and then washed twice in 5% TCA. The acid-insoluble material was dissolved for 12 h in 2 N NaOH at 23°C. The aliquots were removed to assess the radioactivity using a liquid scintillation counter (LS 6500, Beckman Instruments, Fullerton, CA). All experiments were conducted in triplicate, and the values were converted from absolute counts to a fold of the control in order to compare the results between experiments.
Statistical analysis
All experiments were conducted in triplicate, and the data were expressed as means ± SE. All data were analyzed using a Kruskal–Wallis test (individual difference between treatments being statistically identified using Dunn’s test). Differences were considered significant at P < 0.05.
Results
SJSZ glycoprotein
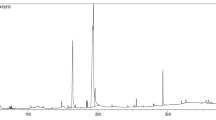
As shown in Fig. 1, the gel was stained with Schiff’s reagent for the glycoprotein isolated from SJSZ fruit in accordance with Nevile and Glossmann’s method [10]. The results of SDS–PAGE were shown that SJSZ glycoprotein has just one band with a molecular weight of 38 kDa on the gel (18%) (Fig. 1). In addition, the total carbohydrate and protein moieties of the SJSZ glycoprotein were 52.64 and 47.35%, respectively.
SDS-PAGE of SJSZ glycoprotein. Electrophoresis was conducted using 18% acrylamide gel containing 0.1% SDS. The gels were stained with Schiff’s reagent for glycoprotein staining in accordance with the method developed by Neville and Glossmann [10]. Lane 1 pre-stained molecular weight as marker; lane 2 75 mg/ml, and lane 3 100 mg/ml SJSZ glycoprotein
Antioxidant properties of SJSZ glycoprotein
The in vitro antioxidant activity of the SJSZ glycoprotein was evaluated via DPPH assay. The results shown in Table 1 illustrated that the SJSZ glycoprotein evidences a significant dose-dependent scavenging effect. The scavenging effects were 3.2, 9.7, 28.2, and 42.1% at 5, 10, 25, and 50 μg/ml of SJSZ glycoprotein, respectively. On the other hand, the scavenging activity of SJSZ glycoprotein on the hydroxyl radical was measured using a deoxyribose assay. The data in Table 2 shows that the SJSZ glycoprotein has a significant inhibitory effect in deoxyribose degradation. The scavenging activities of the SJSZ glycoprotein were 46.8, 54.1, 54.9, and 64.3% at 5, 10, 25, and 50 μg/ml of SJSZ glycoprotein, respectively, in the non-site-specific scavenging assay. The results on scavenging activity of non-specific radical were shown that SJSZ glycoprotein affects two and half times weaker than vitamin C.
Inhibitory effect of SJSZ glycoprotein on G/GO-induced cytotoxicity
As shown in Fig. 2, when cells exposed to G/GO for 4 h, the percentage of cell viability decreased. However, SJSZ glycoprotein increased the percentage of cell viability in the presence of G/GO. For instance, cell viabilities were 47.22, 68.26, 82.91, and 91.87% at 5, 10, 25, and 50 μg/ml SJSZ glycoprotein, respectively. Also, vitamin C increased the cell viabilities in presence of G/GO. The results on scavenging activity of hydroxyl radical were shown that SJSZ glycoprotein affects two and half times weaker than vitamin C.
Antioxidant effect in G/GO-treated BNL CL.2 cells. BNL CL.2 cells were treated with G/GO (30 mU/ml) for 4 h in the absence or presence of SJSZ glycoprotein (5–50 μg/ml) or vitamin C (10 and 20 μg/ml). Viabilities of the cells were expressed as a percentage of the control value. Each bar represents the means ± SE from triplicates (n = 9). #Significant difference between treatments and controls, P < 0.05. *Significant difference between G/GO treatment and SJSZ glycoprotein or vitamin C treatment in the presence of G/GO, P < 0.05
Inhibitory effect of SJSZ glycoprotein on ALT, LDH, and TBARS in chromium-treated ICR mice
The levels of ALT, LDH, and TBARS were measured in the plasma in order to evaluate hepatic tissue damage (Table 3). The results indicated that the SJSZ glycoprotein (10 mg/kg, BW) did not evidence significant hepatotoxicity. Serum ALT, LDH, and TBARS levels in the chromium-treated groups were higher than those in the non-treated groups throughout the experiment. Administrations of SJSZ glycoprotein significantly reduced the increased plasma levels of ALT, LDH, and TBARS.
Inhibitory effect of SJSZ glycoprotein on production of intracellular ROS in chromium-treated BNL CL.2 cells
As shown in Fig. 3, when the BNL CL.2 cells were treated with chromium (15 μM) for 30 min, the relative amounts of intracellular ROS were increased as compared to the controls. However, when the BNL CL.2 cells were treated with SJSZ glycoprotein (10, 25, and 50 μg/ml) in the presence of chromium, the intracellular ROS production was reduced in a dose-dependent manner, as compared to chromium treatment alone. For example, the levels of intracellular ROS were reduced by 0.20- and 0.46-fold at 25 and 50 μg/ml SJSZ glycoprotein in the presence of chromium.
Inhibitory effect of SJSZ glycoprotein on production of intracellular ROS in chromium-treated BNL CL.2 cells. Fluorescence intensity is expressed as relative intensity based on monitoring of dichlorodihydrofluorescein (DCF) fluorescence using a fluorescence microplate reader (Dual Scanning SPECTRAmax, Molecular Devices Corporation,Sunnyvale, CA, USA). Data are expressed as the means ± SE from triplicates (n = 9). #Significant difference between treatments and control, P < 0.05. *, **Significant difference between chromium treatment and SJSZ glycoprotein treatment in the presence of chromium, P < 0.05 and P < 0.01
Effect of SJSZ glycoprotein on anti-oxidative enzymes in chromium-treated BNL CL.2 cells and ICR mice
The hepatic antioxidant enzyme activities (SOD, CAT, and GPx) are shown in Fig. 4. The SOD, CAT, and GPx activities in chromium-treated BNL CL2.cells and ICR mice were reduced as compared to the controls. Interestingly, pretreatment with SJSZ glycoprotein resulted in a significant increase in SOD, CAT and GPx activities in the chromium-treated group. Also, vitamin C significantly increased antioxidant enzymes in the chromium-treated group. The results on the hepatic antioxidant enzyme activities (SOD, CAT, and GPx) were shown that SJSZ glycoprotein affects two and half times weaker than vitamin C.
Effect of SJSZ glycoprotein on antioxidative enzymes in chromium-treated BNL CL.2 cells and ICR mice. The activities of hepatic SOD, GPx, and CAT in BNL CL2 cells and mice liver were measured at 560 and 412 nm, respectively. All data are expressed as the means ± S.E. from triplicate experiments (n = 6), separately. #Significant difference between treatments and the control, P < 0.05. *Significant difference between chromium treatment alone and the SJSZ glycoprotein treatment or vitamin C in the presence of chromium, P < 0.05. BW body weight
Inhibitory effect of SJSZ glycoprotein on PKC activity in chromium-treated BNL CL.2 cells and ICR mice
It was then determined whether the SJSZ glycoprotein inhibits PKC activity in the chromium-treated ICR mice and BNL CL.2 cells. For instance, the relative band-intensity of PKC was enhanced by 1.68- and 1.94-fold after chromate treatment, relative to the controls, in BNL CL.2 cells and ICR mice. However, when the cells were treated with chromate in the presence of SJSZ glycoprotein, PKC activity was reduced 0.75- and 0.35-fold with SJSZ glycoprotein (50 μg/ml) in cells and SJSZ glycoprotein (10 mg/kg, BW) in mice, respectively, relative to the chromium group (Fig. 5).
Inhibitory effect of SJSZ glycoprotein on PKC activity in chromium-treated BNL CL.2 cells and ICR mice. BNL CL.2 cells were treated with chromium (15 μM) for 30 min in the absence or presence of SJSZ glycoprotein (10, 25, and 50 μg/ml). The relative intensities of bands were calculated with Scion Imaging Software (Scion Image Beta 4.02, Frederick, MD, USA). Data were represented as absolute intensities and expressed as means ± SE from triplicates (n = 9). #Significant difference between the treatment and control groups, P < 0.05. *Significant difference between chromium treatment and the SJSZ glycoprotein in the presence of chromium, P < 0.05. Lane 1 control; lane 2 chromium alone; lane 3 10 μg/m1 SJSZ glycoprotein in the presence of chromium; lane 4 25 μg/ml SJSZ glycoprotein in the presence of chromium; lane 5 50 μg/ml SJSZ glycoprotein in the presence of chromium (a). Lane 1 control; lane 2 10 mg/kg SJSZ glycoprotein alone; lane 3 chromium alone; lane 4 5 mg/kg SJSZ glycoprotein in the presence of chromium; lane 5 10 mg/kg SJSZ glycoprotein in the presence of chromium. BW body weight (b)
Inhibitory effect of SJSZ glycoprotein on p38 MAPK phosphorylation in chromium-treated BNL CL.2 cells and ICR mice
We then attempted to determine whether the SJSZ glycoprotein inhibits p38 MAPK activity in the chromium-treated ICR mice and BNL CL.2 cells. For instance, the relative band-intensity of p38 MAPK was enhanced 1.35-.and 1.27-fold after chromate treatment in BNL CL.2 cells and ICR mice, relative to the controls. However, when the cells were treated with chromate in the presence of SJSZ glycoprotein, p38 MAPK activity was reduced by 0.40-and 0.36-fold with SJSZ glycoprotein (at 50 μg/ml) in the cells and with SJSZ glycoprotein (at 10 mg/kg, BW) in the mice, respectively, as compared to the chromium group (Fig. 6).
Inhibitory effect of SJSZ glycoprotein on p38 MAPK phosphorylation in chromium-treated BNL CL.2 cells and ICR mice. BNL CL.2 cells were treated with chromium (15 μM) for 1 h in the absence or presence of SJSZ glycoprotein (10, 25, and 50 μg/ml). The relative intensities of bands were calculated with Scion Imaging Software (Scion Image Beta 4.02, Frederick, MD, USA). Data were represented as absolute intensities and expressed as means ± SE from triplicates (n = 9) #Significant difference between the treatment and control groups, P < 0.05. *Significant difference between chromium treatment and the SJSZ glycoprotein in the presence of chromium, P < 0.05. Lane 1 control; lane 2 chromium alone; lane 3 10 μg/m1 SJSZ glycoprotein in the presence of chromium; lane 4 25 μg/ml SJSZ glycoprotein in the presence of chromium; lane 5 50 μg/ml SJSZ glycoprotein in the presence of chromium (a). Lane 1 control; lane 2 10 mg/kg SJSZ glycoprotein alone; lane 3 chromium alone; lane 4 5 mg/kg SJSZ glycoprotein in the presence of chromium; lane 5 10 mg/kg SJSZ glycoprotein in the presence of chromium. BW body weight (b)
Inhibitory effect of SJSZ glycoprotein on HSP27 and HSP70 activity in chromium-treated BNL CL.2 cells and ICR mice
We assessed the impact of the SJSZ glycoprotein on the expression of the tumor markers HSP27 and HSP70. As is shown in Fig. 7, HSP27 and HSP70 expression were increased markedly by chromium. However, treatment with SJSZ glycoprotein in the presence of chromate induced a significant reduction in HSP27 and HSP70 activity. For instance, the relative intensity of HSP27 and HSP70 increased by 1.47-and 1.50-fold in the presence of chromium in BNL CL.2 cells, as compared to the controls. Additionally, the relative intensities of HSP27 and HSP70 were increased by 2.12 and 1.84-fold in the presence of chromium in liver tissues, as compared to the levels in the controls. However, treatment with SJSZ glycoprotein in the presence of chromate reduced the levels of HSP27 and HSP70 as compared to what was observed with chromate treatment alone (Fig. 7).
Inhibitory effect of SJSZ glycoprotein on HSP27 and HSP70 activity in chromium-treated BNL CL.2 cells and ICR mice. BNL CL.2 cells were treated with chromium (15 μM) for 3 h in the absence or presence of SJSZ glycoprotein (10, 25 and 50 μg/ml). The relative intensities of bands were calculated with Scion Imaging Software (Scion Image Beta 4.02, Frederick, MD, USA). Data were expressed as absolute intensities and expressed as means ± SE from triplicates (n = 9). #Significant difference between the treatment and control groups, P < 0.05. *Significant difference between chromium treatment and the SJSZ glycoprotein in the presence of chromium, P < 0.05. Lane 1 control; lane 2, chromium alone; lane 3 10 μg/m1 SJSZ glycoprotein in the presence of chromium; lane 4 25 μg/ml SJSZ glycoprotein in the presence of chromium; lane 5 50 μg/ml SJSZ glycoprotein in the presence of chromium (a). Lane 1 control; lane 2, 10 mg/kg SJSZ glycoprotein alone; lane 3 chromium alone; lane 4 5 mg/kg SJSZ glycoprotein in the presence of chromium; lane 5 10 mg/kg SJSZ glycoprotein in the presence of chromium. BW body weight (b)
Inhibitory effect of SJSZ glycoprotein on [3H]thymidine-incorporation and PCNA expression in chromium-treated BNL CL.2 cells and ICR mice
As shown in Fig. 8a, when the BNL CL.2 cells were treated with SJSZ glycoprotein (25 and 50 μg/ml) in the presence of chromium, [3H]-thymidine incorporation was reduced in a dose-dependent manner relative to that observed with chromium treatment alone. For instance, the relative values of [3H]-thymidine incorporation were diminished by 0.18- and 1.34-fold as the result of treatment with 50 and 100 μg/ml of SJSZ glycoprotein, respectively, compared to what was observed in the chromium treatment group (Fig. 8a).
Inhibitory effect of SJSZ glycoprotein on and [3H]thymidine-incorporation and expression of PCNA in chromium-treated BNL CL.2 cells and ICR mice. BNL CL.2 cells were treated for 12 h with chromium in the presence or absence of the SJSZ glycoprotein (10, 25, and 50 μg/ml) and pulsed with 1 μCi [3H]-thymidine for 5 h (a). BNL CL.2 cells were treated with chromium (15 μM) for 3 h in the absence or presence of SJSZ glycoprotein (10, 25, and 50 μg/ml). The relative intensities of bands were calculated with Scion Imaging Software (Scion Image Beta 4.02, Frederick, MD, USA). Data were represented as absolute intensities and expressed as means ± SE from triplicates (n = 9). #Represents a significant difference between the treatment and control groups, P < 0.05. *Significant difference between chromium treatment and the SJSZ glycoprotein in the presence of chromium, P < 0.05. Lane 1 control; lane 2 chromium alone; lane 3 10 μg/m1 SJSZ glycoprotein in the presence of chromium; lane 4 25 μg/ml SJSZ glycoprotein in the presence of chromium; lane 5 50 μg/ml SJSZ glycoprotein in the presence of chromium (b). Lane 1 control; lane 2 10 mg/kg SJSZ glycoprotein alone; lane 3 chromium alone; lane 4 5 mg/kg SJSZ glycoprotein in the presence of chromium; lane 5 10 mg/kg SJSZ glycoprotein in the presence of the chromium. BW body weight (c)
We then attempted to determine whether the SJSZ glycoprotein inhibited PCNA activity in the chromium-treated ICR mice and BNL CL.2 cells. For instance, the relative band-intensity of PCNA was enhanced by 3.91- and 1.43-fold following chromate treatment in BNL CL.2 cells and ICR mice, relative to the controls. However, when the cells were treated with chromate in the presence of SJSZ glycoprotein, PCNA activity was reduced by 0.25-and 0.73-fold with SJSZ glycoprotein (at 50 μg/ml) in cells and with SJSZ glycoprotein (at 10 mg/kg, BW) in mice, respectively, as compared to the chromium group (Fig. 8b, c).
Discussion
Carcinogenesis may arise as a result of chemical or biological damage to normal cells via a multistep process involving changes at the initiation level, followed by promotion and progression, leading to malignancy. The promotional stage of cancer is reversible stage and appears to be the most appropriate target stage for chemoprevention interventions [24]. Chromium (VI) is regarded as one of the most toxic chemical substances known, and is also an internationally recognized heavy metal carcinogen. Previous studies have shown that chromium (VI) induces oxidative stress via enhanced ROS production, as well as enhanced oxidative excretion in vitro and in vivo models [25]. In this study, we have investigated the activities of the SJSZ glycoprotein against hepatocarcinogenesis at the initiation stage of chromium induction.
The DPPH assay provides information regarding the reactivity of test compounds with stable free radicals. The reduction in absorbance in the assay indicates the capacity of test compounds to scavenge free radicals independent of any enzyme activity. In addition, as shown by the results of the deoxyribose assay, the SJSZ glycoprotein does possess a scavenging activity capacity, although its ability is two and half times weaker than that of vitamin C in this regard (Tables 1 and 2). We also evaluated the protective effect of SJSZ glycoprotein from oxidative stress in protecting BNL CL.2 cells. In the oxidative stress induced by the G/GO system, H2O2 is generated continuously by glucose oxidase acting as β-d-glucose, which is expressed as G/GO. As shown in Fig. 2, the SJSZ glycoprotein scavenges hydroxyl radical induced by G/GO.
Lactate dehydrogenase is a cytosolic enzyme which catalyzes the reversible oxidation of L-lactate to pyruvate; its increased activity in serum confirms increased hepatocyte membrane permeability and cellular damage. It has been previously reported that its level is correlated strongly with tumor bulk, as a high rate of glycosis has been previously associated with cancerous conditions, and glycosis is the only energy-producing pathway for malignant cells [26]. The activities of serum enzymes such as ALT normally denote liver function. Elevated ALT levels are regarded as the most sensitive markers in the diagnosis of hepatocellular damage and the loss of functional membrane integrity [27]. Elevated serum ALT levels are correlated with a high incidence of HCC development in chronic hepatitis patients. As is shown in Table 3, the SJSZ glycoprotein inhibits elevated serum enzymes (LDH and ALT) in chromium-induced HCC. It has also been noted that elevated levels of lipid peroxidation (TBARS) in the liver are considered one of the basic mechanisms of tissue damage induced by free radicals, and that they function as an important causative factor in carcinogenesis [28]. The results obtained in the present study demonstrated that chromium treatment significantly increased TBARS levels, whereas SJSZ glycoprotein (10 mg/kg, BW) normalized the chromium-induced TBARS levels. One possible explanation for this is that the SJSZ glycoprotein (10 mg/kg, BW) exerts its protective effect against chromium-induced HCC, probably via the prevention of membrane damage and loss of integrity, as well as by repairing the hepatic tissue damage induced by carcinogenesis.
The carcinogenicity of specific chromium compounds is affected by both the valence and the solubility of chromium species. Chromium (VI) has been identified as more toxic and carcinogenic than Cr(III) because the former can pass through the cell membrane more readily than the latter. Once within the cell, chromium (VI) is reduced to its lover oxidation states [Cr(V)] and [Cr(IV)] and then Cr(III) by low molecular weight molecules, enzymatic and non-enzymatic reductants. These reactive chromium intermediates are capable of generating a spectrum of ROS, which is an important characteristic of chromium (VI) metabolism [29]. It has been reported previously that ROS, which is capable of producing lipid peroxidation and oxidation of DNA and other cellular macromolecules, plays a role in the initiation, promotion, and progression of liver cancer [30]. As shown in Fig. 3, the SJSZ glycoprotein prevents ROS production induced by chromium under both in vivo and in vitro conditions. This indicates that the SJSZ glycoprotein exerts a strong antioxidant effect.
Antioxidant enzymes are regarded as a primary defense mechanism that protects biological macromolecules against oxidative damage. Thus, elevated levels of antioxidant enzymes may be involved in increased protection against oxidative stress-induced hepatotoxicity [31]. The principal antioxidant enzymes are SOD, CAT, and GPx. SOD constitutes the first line of defense against superoxide radicals, which dismutate two superoxide radicals to H2O2 and O2. CAT and GPx function as supporting antioxidant enzymes by converting H2O2 to H2O, thereby providing protection against ROS [32]. Therefore, we determined the changes in enzyme activity during the exposure of mice or cells to chromate. As is shown in Fig. 4, chromate significantly reduced the levels of SOD, CAT, and GPx in the mouse liver, whereas pretreatment with the SJSZ glycoprotein gradually normalized the abnormal levels of anti-oxidant enzymes in chromate-treated mouse liver and BNL CL.2 cells.
Free radicals and ROS may be involved in the mediation of signal transduction via interaction with PKC [33]. Recent studies have shown that PKC is activated rapidly in cells after oxidative exposure [8]. It has been established in previous studies that PKC is an upstream regulator of the p38 MAPK cascade for HSP 27 expression. In order to evaluate the carcinogenesis in chromium-treated BNL CL.2 cells and ICR mice, we assessed the activity of PKC. As shown in Fig. 5, the SJSZ glycoprotein inhibits chromium-induced PKC activity.
Mammalian p38 MAPKs are activated by a broad range of cellular stresses, both acute and chronic [34]. As p38 MAPK activity is critical for normal immune and inflammatory responses, different components of this pathway constitute potential targets for the treatment of inflammatory diseases and carcinogenesis [35]. Earlier studies have either observed a lack of association between ROS production and p38 MAPK activation by heat shock, or have linked p38 MAPK activation with ROS production. The results of Western blot analyses demonstrated that the protein levels of p38 MAPK in chromate-treated BNL CL.2 cells and ICR mice were increased relative to the controls. However, the SJSZ glycoprotein inhibits these increases in protein levels (Fig. 6). In this study, we propose that persistent p38 MAPK activation by chromate-treated ROS may contribute to the mechanism of toxicity of this element and its ability to cause cancer.
The process of chronic inflammation results in a stressful condition, and ubiquitous molecules such as HSPs are induced in response to stressful stimuli, thereby contributing to hepatocarcinogenesis. HSP27 belongs to the phylogenically conserved small HSP family, which can function as molecular chaperones and protect cells against heat shock and oxidative stress under in vitro conditions. It has been previously reported that HSP70 is an antiapoptotic protein, and its overexpression allows cells to survive under a variety of adverse conditions, including carcinogenesis [36]. In addition, HSP70 could be a sensitive marker for the differential diagnosis of early HCC from preneoplastic lesions or non-cancerous liver tissue. As is shown in Fig. 7, HSP27 and HSP70 were increased in chromium-treated BNL CL.2 cells and ICR mice, whereas the SJSZ glycoprotein inhibited this increase.
With regard to the effects of the SJSZ glycoprotein on proliferation in chromium (VI)-induced BNL CL.2 cells using [3H]-thymidine incorporation, our data demonstrated that the SJSZ glycoprotein inhibits cell proliferation in the chromium (VI)-induced BNL CL.2 cells (Fig. 8). PCNA has been identified as a cyclin or as an auxiliary protein for DNA polymerase-δ. Our results showed that the SJSZ glycoprotein inhibits the activity of PCNA induced by chromium (VI), which performs a pivotal role in DNA repair, cell proliferation, and cell cycle control (Fig. 8).
In conclusion, the results of this study indicated that SJSZ glycoprotein reduced the levels of intracellular ROS and the activity of PKC, p38 MAPK, HSP27, and HSP70 in chromium-treated BNL CL.2 cells and ICR mice. Collectively, the results obtained from this study demonstrate that SJSZ glycoprotein exerts a modulatory effect on the activity of HSP27 and HSP70, which are known to induce hepatocarcinogenesis via chromium cytotoxicity scavenging.
References
Langard S (1983) The carcinogenicity of chromium compounds in man and animals. In: Burrows D (ed) Chromium D metabolism and toxicity. Boca Raton, CRC Press, pp 13–30
Becker N, Chang-Claude J, Frentzel-Beyme R (1991) Risk of cancer for arc welders in the Federal Republic of Germany: results of a second follow up. Br J Ind Med 48:675–683
Susa N, Ueno S, Furukawa Y (1998) Protective effect of diethyldithiocarbamate pretreatment on chromium (VI)-induced cytotoxicity and lipid peroxidation in primary cultures of rat hepatocytes. J Vet Med Sci 60:71–76
Nishigori C (2006) Cellular aspects of photocarcinogenesis. Photochem Photobiol Sci 5:208–214
Sundaresan S, Subramanian P (2003) S-allylcysteine inhibits circulatory lipid peroxidation and promotes antioxidants in N-nitrosodiethylamine-induced carcinogenesis. Pol J Pharmacol 55:37–42
Hold GL, El-Omar EM (2008) Genetic aspects of inflammation and cancer. Biochem J 410:225–435
Joo M, Chi JG, Lee H (2005) Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci 20:829–834
Kim G, Yurkow EJ (1996) Chromium induces a persistent activation of mitogen-activated protein kinases by a redox-sensitive mechanism in H4 rat hepatoma cells. Cancer Res 56(9):2045–2051
Yoshikawa K, Hirai H, Tanaka M, Arihara S (2000) Antisweet natural products. XV. Structures of Jegosaponins A-D from Styrax japonica Sieb. et Zucc. Chem Pharm Bull 48:1093–1096
Neville DM Jr, Glossmann H (1974) Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol 32:92–102
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339:69–72
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maffei Facino R, Carini M, Aldini G, Berti F, Rossoni G (1999) Panax ginseng administration in the rat prevents myocardial ischemia-reperfusion damage induced by hyperbaric oxygen: evidence for an antioxidant intervention. Planta Med 65:614–619
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 16:215–219
Lee JC, Lim KT (2001) Inhibitory effects of the ethanol extract of Ulmus davidiana on apoptosis induced by glucose–glucose oxidase and cytokine production in cultured mouse primary immune cells. J Biochem Mol Biol 34:463–471
Bergmeyer HU, Bernt E (1974) UV-assay with pyruvate and NADH. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 574–578
Bergmeyer HU, Bernt E (1974) Glutamate-pyruvate transaminase: UV assay, manual method. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 752–758
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Thomson JF, Nance SL, Tollaksen SL (1978) Spectrophotometric assay of catalase with perborate as substrate. Proc Soc Exp Biol Med 157:33–35
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Patton WF, Dhanak MR, Jacobson BS (1989) Differential partitioning of plasma membrane proteins into the triton X-100-insoluble cytoskeleton fraction during concanavalin A-induced receptor redistribution. J Cell Sci 92:85–91
Gabelman BM, Emerman JT (1992) Effects of estrogen, epidermal growth factor, and transforming growth factor-a on the growth of human breast epithelial cells in primary culture. Exp Cell Res 201:113–118
Hong WK, Sporn MB (1997) Recent advances in chemoprevention of cancer. Science 278:1073–1077
Geetha S, Sai Ram M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC (2003) Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium(VI) induced oxidative stress in albino rats. J Ethnopharmacol 87:247–251
Rohr-Udilova NV, Stolze K, Sagmeister S, Nohl H, Schulte-Hermann R, Grasl-Kraupp B (2008) Lipid hydroperoxides from processed dietary oils enhance growth of hepatocarcinoma cells. Mol Nutr Food Res 52:352–359
Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, Totsuka S (1996) Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer 86:589–595
Banakar MC, Paramasivan SK, Chattopadhyay MB, Datta S, Chakraborty P, Chatterjee M, Kannan K, Thygarajan E (2004) 1alpha, 25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J Gastroenterol 10:1268–1275
Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ (1997) Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic Biol Med 22:471–478
Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312
Margaill I, Plotkine M, Lerouet D (2005) Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39:429–443
Vásquez-Garzón VR, Arellanes-Robledo J, García-Román R, Aparicio-Rautista DI, Villa-Treviño S (2009) Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res 43:128–137
Bagchi D, Bagchi M, Tang L, Stohs SJ (1997) Comparative in vitro and in vivo protein kinase C activation by selected pesticides and transition metal salts. Toxicol Lett 91:31–37
Cuenda A, Rousseau S (2007) p38 MAP-Kinases pathway regulation function and role in human diseases. Biochem Biophys Acta 1773:1358–1375
Zhang J, Shen B, Lin A (2007) Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci 28:286–295
Sakamoto M (2009) Early HCC: diagnosis and molecular markers. J Gastroenterol 44:108–111
Acknowledgment
This study was financially supported by Chonnam National University, 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Lim, KT. Inhibitory effect of SJSZ glycoprotein (38 kDa) on expression of heat shock protein 27 and 70 in chromium (VI)-treated hepatocytes. Mol Cell Biochem 359, 45–57 (2012). https://doi.org/10.1007/s11010-011-0998-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0998-8