Abstract
Past and recent applications of 2,4,6-trinitrotoluene (TNT) in military and civilian industries have led to contamination of soil and marine ecosystems. Among various TNT remediation techniques, biological remediation is widely accepted for its sustainability, low cost, and scalable applications. This study was designed to isolate a fungus strain from a TNT-contaminated soil to investigate its tolerance to and potential for removal of TNT. Thus, a soil column with a history of periodic TNT amendment was used to isolate dominant strains of fungi Fusarium solani isolate, which is not commonly reported for TNT mineralization and was found predominant in the subsurface layer of the TNT-amended soil. F. solani was investigated for TNT concentration tolerance at 30, 70, and 100 mg/L on agar plates and for TNT removal in liquid cultures at the same given concentrations. F. solani activity was compared with that of a reference soil-born fungus that has been intensively studied for TNT removal (Phanerochaete chrysosporium) obtained from the American Type Culture Collection. On agar media, F. solani showed a larger colony diameter than P. chrysosporium at similar TNT concentrations, indicating its high potential to tolerate toxic levels of TNT as found in contaminated sites. In the liquid culture medium, F. solani was able to significantly produce higher biomass than P. chrysosporium in all TNT concentrations. The TNT removal percentage from the liquid culture at the highest TNT concentration of 100 mg/L reached about 85% with F. solani, while P. chrysosporium was no better than 25% at the end of an 84-h incubation period. Results indicate a significant potential of using F. solani in the bioremediation of polluted TNT soils that overcome the high concentration barrier in the field. However, further investigation is needed to identify enzymatic potential and the most effective applications and possible limitations of this method on a large scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4,6-Trinitrotoluene (TNT) is a nitroaromatic explosive that has been extensively used for military purposes because of its low melting point, chemical and thermal stability, and low sensitivity to impact and friction [1, 2]. In addition, TNT is used as raw materials for manufacturing pesticides, herbicides, pharmaceutical products, and dyes in civilian industries [3]. Unfortunately, TNT is considered a major anthropogenic contaminant of soils and water that could reach the environment from TNT handling, disposal, and storage activities. Accordingly, countries in the Middle East, Africa, and Eastern Europe that have suffered from military conflicts have critical TNT environmental contamination that can reach up to 200 g/kg and 100 mg/L in soil and water, respectively [4].
Toxic and mutagenic effects of TNT on humans have been described at low concentrations, where its lethal dose is estimated at 14.8 mg/kg of body weight [5]. Exposure to TNT is linked to diseases such as rashes, toxic hepatitis, sneezing, coughing, cyanosis, peripheral neuritis, cataract, muscular pain, aplastic anemia, as well as kidney and liver damage [6, 7]. Moreover, in 2018, TNT was classified by the United States Environmental Protection Agency (USEPA) as a possible human carcinogenic (Class C) material where a lifetime exposure to 0.1 mg/L of TNT in drinking water leads to an additional predictable cancer risk of 1 in 10,000 [8]. The TNT routes of entry to humans can be through ingesting, drinking, and inhaling contaminated sources [4]. It is worth mentioning that direct exposure could rise from using ammunition during military training and weapon testing activities [9].
Besides the costly remediation of contaminated sites by physical, chemical, or physiochemical techniques, bioremediation represents a reasonable and promising alternative for treating such contaminant. In that regard, several genera of bacteria and fungi were proposed to decontaminate TNT from soil and water [10]. Yeast had also been tested for its ability to decontaminate TNT and showed low efficiency compared to other organisms [11]. Among the fungal community, in particular, the white rot fungus P. chrysosporium has been extensively studied since the 1990s [12,13,14,15]. Moreover, Trichoderma viride has been recently reported to degrade TNT at concentrations up to 100 ppm [3].
Although many organisms in the soil are able to mineralize TNT to a certain extent, TNT is often persistent in the environment and hard to degrade. This suggests the presence of metabolic barriers that prevent biodegradation of TNT into its carbon and nitrogen pools [16]. Some studies have outlined the presence of biogeochemical factors in soil environments that have some control over TNT degradation [11]. Overall, the high concentrations of TNT in the environment (> 50 mg/L) have an inhibitory effect on the growth of many organisms [13].
According to the literature, the pathways for TNT biodegradation and biotransformation include the application of several enzymatic machinery to achieve reductive denitration of the aromatic ring in the TNT structure [17]. The reduced TNT derivatives serve as the base for peroxidase-mediated mineralization but also possess a high ability to bind covalently to the humus fraction of soil organic matter [12, 18]. Absorption of secondary metabolites to soil humic acids makes them less mobile and more toxic in the environment but may serve as potential substrates for further reduction or co-metabolic biotransformation [16, 19, 20].
With reference to the biodegradation pathway, many fungi species can biotransform one or more nitro groups of TNT molecules into amino groups producing several intermediates. It has been reported that TNT disappearance coincided with the appearance of 5-(hydroxymethyl)-2-furancarboxaldehyde as the major compound and 4-propyl benzaldehyde as the minor compound [3]. Likewise, Lee et al. [21] reported 4-amino-2,6-dinitrotoluene (4-ADNT) and 2-amino-4,6-dinitrotoluene (2-ADNT) as TNT degradation intermediates by the white-rot fungus Irpex lacteus.
Studies of the biodegradation of TNT using indigenous microbial isolates obtained from contaminated sites have been discussed in many reports [15, 22,23,24]. The long-term exposure of those microbial isolates to contaminants can lead to specific adaptation skills of microflora, as has been suggested [22, 23]. These isolates can provide applicable bioremediation schemes for contaminated sites, either in situ or ex situ, and can be used for biostimulation and bioaugmentation procedures [15].
This study aimed to isolate an acclimatized fungal strain with a high tolerance for TNT, investigate its potential for growth under different concentrations of the contaminant, and examine its tolerance to and potential for removal of TNT from an aqueous solution in comparison to a reference fungal sp. that is well-known to degrade TNT. In this manner, the isolated fungus strain was identified as F. solani, which was not previously reported for this application, and the reference fungal sp. used was P. chrysosporium. Findings from this research can improve the practical application of bioremediation in contaminated soils with high concentrations of TNT.
Materials and Methods
Chemicals and Reference Fungal Strain
Analytical and non-radiolabeled HPLC-grade TNT was used. Culture media reagents such as yeast extract, agar, malt extract, and potato dextrose extract were obtained from Difco. Glucose was obtained from Fisher Scientific. The fungal strain P. chrysosporium BKM-F-1767 was obtained from the American Type Culture Collection (ATCC 24,725).
Media Preparation
Yeast Malt Glucose (YMG) agar media was prepared using 4 g yeast extract, 10 g malt extract, 4 g glucose, and 15 g agar in 1 L of distilled water. Sterilized by autoclave and poured into Petrie dishes. The YMG agar media with different TNT concentrations (30, 70, and 100 mg/L) (referred to as TNT-YMG agar) was prepared by adding proper amount of filtered TNT stock solution (1000 mg/L prepared in acetone) to a sterile YMG media. The YMG broth cultures were prepared using the same recipe of YMG media, excluding the solidifying agent (agar). YMG broth with different TNT concentrations (referred to as TNT-YMG broth) was prepared by mixing proper amounts of filtered aliquots of the TNT stock solution with sterile YMG broth to obtain broth with 30, 70, and 100 mg TNT/L.
Fungal Isolation from Soil
The soil samples used in this study were very sandy silt taken from the Kansas river basin south of Manhattan KS in the USA. Two columns of soil (20 cm diameter and about 50 cm length) were used for fungal isolation; the first column was from soil exposed periodically to high concentrations of TNT for about 24 months to allow artificial selection pressure and acclimatization to attract potential degraders. At the same time, the other column served as a control with the soil of no TNT feeding. Surface and subsurface soil layers (5–10 cm and 10–15 cm, respectively) from the top of each column were used in this study for fungal isolation under aerobic conditions. The four soil samples representing two depths from the two tested soil columns were air-dried and sieved through a 2 mm mesh diameter.
Soil solution stock was prepared for isolating fungi by mixing 10 g of soil with 90 mL of sterile 0.85% normal saline solution and sterilized glass beads. The mixture was vigorously shaken for 1 h to ensure proper dispersal of microbial cells in the soil solution. Then, serial dilution from soil solution stock was prepared. One milliliter of aliquots of different dilutions (10−3, 10−4, and 10−5) were used to inoculate the surface of a general fungal medium, acidified Potato Dextrose Agar (PDA) and incubated at 25 ± 2 ºC for 7 days. Colonies of similar morphology (F1 – F10) were counted and maintained in pure culture on PDA for further use.
Fungal Identification
A pure culture of F1 isolate was inoculated on YMG media for species identification. Both macroscopic and microscopic characteristics such as colony morphology, pigment production, spore shape and size, chlamydospores, and fungal hyphae were used. Glass slides with fungal growth were prepared and stained with cotton blue to allow visualization of microscopic structures that were then compared to a fungal identification manual [25]. For macroscopic observation, the cultural appearances (colony color and pigmentations) were observed on PDA and identified using the Methuen Handbook of Color chart [26].
TNT Tolerance Assay on Agar
All fungal strains isolated from the soil columns were used in this assay in addition to the ATCC reference culture of P. chrysosporium. Pure fungal cultures were grown on YMG media and incubated at 28 ± 2 °C until the colonies covered the plate surface. A 0.5-cm-diameter plug cutter was used to prepare a disk with fungal inoculum prepared previously. One plug of each fungal isolate was placed in the center of each TNT-YMG agar plate containing different TNT concentrations. The colony diameter was measured after incubation for 7 days at 28 ± 2 °C. These concentrations were selected to represent acute and chronic toxicity exposure reported in hot spots contaminated with TNT [27]. Each concentration of TNT-YMG agar cultured with the appropriate fungus was replicated three times. The diameter of the fungal colony was recorded for statistical analysis following the incubation period.
Fungal Growth in YMG Broth Media
YMG agar plates were grown with isolated F. solani or the reference P. chrysosporium until the colonies filled the surface of the plates. Then, 5 agar plugs of active mycelium of each fungal strain were used to inoculate 50 ml of YMG broth. The cultures were incubated at 28 ± 2 °C for 6 days to obtain high mycelial growth. The fungal suspension was then blended with a glass homogenizer for subsequent use.
Fungal Growth in TNT-YMG Broth Media
One milliliter of the previously prepared fungal suspension was used to inoculate 50 mL of TNT-YMG broth culture of 30, 70, and 100 mg/L TNT. Two experiments were conducted separately. One served as a screening study where the culture flasks were incubated at 28 ± 2 ºC for 28 days (long-term monitoring). The second experiment was incubated at 28 ± 2 ºC for 84 h (short-term monitoring) and was consistently checked every 12 h for TNT concentration using high-pressure liquid chromatography (HPLC). At the end of the incubation periods, fungal biomass was measured.
Fungal Biomass Measurement
The dry weight of fungal biomass was measured by filtering the broth culture after the 84-h incubation period through a pre-weighed Whatman filter paper placed in a Buchner funnel. The filter paper and the retained residue on the filter were dried in the oven overnight at 80 ºC. The difference in filter mass was correlated to the fungal biomass.
Analytical Method for HPLC
High-level direct injection EPA Method 8330 A [28] using high-pressure liquid chromatography (HPLC) was used to detect the TNT concentration at initial and incubation intervals with the tested fungal sp. The HPLC was from Beckman, model 110 A with reverse-phase column (20 μm, 100ºA pore size, poly-styrene-divinylbenzene, Hamilton PRP-1). Elution was performed with methanol-water (95:5, vol/vol) at a 1 mL /min flow rate. TNT was determined by monitoring the elution at 238 nm. The elution time was set to be 30 min at a temperature of 30 ºC. A sample of 250 µL from each culture flask was used to inject the HPLC column through a 0.45 μm syringe disk filter (Whatman® ReZist® syringe filter). The short-term experiment was performed in triplicates.
Statistical Analysis
The experiments were designed based on randomized complete block design with three replications for each experimental unit. All statistical significance was defined at α = 0.05 level. The analysis of variance was done by implementing factorial analysis using mixed model (Proc Mixed) and general linear model (Proc GLM). The factors were fungus types, TNT levels, and measurement time. Least squares means were separated using Tukey’s multiple comparison test using statistical analysis software (SAS, V. 9.3).
Results and Discussion
Fungal Isolation from Soil
The total number of fungal isolates obtained in both control and TNT-amended soil columns was ten (F1 - F10). Based on the results in Table 1, there is a noticeable difference in soil fungal composition and count between the surface and subsurface layers for the control and TNT-amended soils. According to [29] and references therein, soil microbial composition as a function of soil depth is related to the levels of organic matter, nutrients, oxygen, and moisture in each soil layer [29]. Thus, the ten folds increment in F1 (later identified as F. solani) counts in the subsurface layer compared to the control could be attributed to the favored conditions in the subsurface layer, such as higher moisture and nutrients.
Moreover, the TNT-amended soil has less fungal diversity than the control. For example, in the surface layer, the control column had seven fungal populations with counts ranging from 17 to 416 CFU/ g of soil, whereas the TNT-amended soil only had three fungal populations with counts ranging from 17 to 117 CFU/g of soil. In addition, some fungal sp. In the control soil, surface and subsurface layers did not appear in the TNT-amended soil, e.g., F4 - F10. It can be concluded that TNT resulted in toxicity to many fungal species in the soil, and only those tolerant and acclimatized to TNT at the given site are expected to dominate the soil and be able to establish themselves in this condition. TNT exerts stress on some microbes by interfering with the expression of some of their genes [20, 25, 30]. This result clearly shows that F1 fungus seems to have the highest potential among naturally existing fungi at this experimental condition to adapt and establish itself in TNT-contaminated soil. It is worth mentioning that other soil types from other locations may support different genera.
Fungal Identification
Both colony morphology and microscopic structures (Fig. 1) were used to identify F1 isolates and the P. chrysosporium used in this study. The distinctive characteristics of Fusarium species identification are based on the shapes and sizes of macro- and microconidia, the presence and absence of chlamydospores, colony appearances, pigmentations, and growth rates on agar media [31]. The colony morphology of F. solani isolated from the TNT-amended soil and dominating the subsurface layer on acidified PDA media showed that colonies were low-floccose, loose, slimy, and sporadic with aerial hyphae that give rise to conidiophores laterally.
TNT Tolerance
Fungal isolates (F1 - F10) were screened for their tolerance on TNT-YMG agar plates containing various levels of TNT (30, 70, and 100 mg/L). Their capacity to biotransform this xenobiotic compound at concentrations similar to those found in contaminated environments was examined. Results showed that all fungal isolates except F. solani failed to support growth at any concentration of TNT level used in this study, indicating intolerance to TNT. These findings were used to compare the TNT tolerance of F. solani isolate with another well studied fungus, P. chrysosporium, that is reported for its capability of degrading TNT [12, 13, 32].
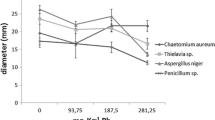
As shown in the image in Fig. 2 and the histogram in Fig. 3, the fungal colony of F. solani isolates at each corresponding TNT level has a bigger diameter than that of P. chrysosporium. The statistical analysis (data not shown) proved that the colonies’ diameters were concentration dependent, with significant differences between these diameters. Moreover, P. chrysosporium was more sensitive to the increase in TNT levels compared to F. solani isolates. For example, increasing the concentration from 70 to 100 mg/L TNT led to 29% and 68% colony diameter reduction for F. solani and P. chrysosporium, respectively. Therefore, mycelial growth inhibition with higher TNT concentrations can be used as a measure of TNT toxicity.
Biodegradation in TNT-YMG Broth
The results of the screening study for 28 days (long-term monitoring) are depicted in Table 2. As can be noticed, F. solani was able to reach 100% TNT removal at all initial concentrations of TNT in 5 days or less. On the other hand, P. chrysosporium achieved 100% TNT removal for only the 30 and 70 mg/L initial TNT concentrations but in a more extended period of incubation (> 18 days). For the 100 mg/L initial TNT concentrations, no complete removal was achieved by P. chrysosporium even after 28 days of incubation, and a plateau of 74.2% TNT removal was observed after 5 days. The reduction of contaminants removal from culture media is generally linked to contaminant toxicity at the cellular level. The TNT toxicity to P. chrysosporium was previously reported even at a low TNT concentration of 20 mg/L, where little fungal growth from spore inoculum was observed when this concentration was used [33, 34]. Similarly, Funder et al. [25] reported about 94% mass recovery of TNT in solutions containing 100 mg/L of TNT after 90 days of incubation with P. chrysosporium.
In a study conducted by Anasonye et al. [35] on the bioremediation of TNT using white-rot Phanerochaete velutina fungus that tolerated high levels of TNT contamination in soil (1000 mg kg−1). TNT degradation was 80% and 70% in lab and pilot scale experiments, respectively, with high manganese peroxidase, but no laccase activity in soil was found during treatment. Our results demonstrate that F. solani seems to have similar tolerance to TNT as other white rot fungi. Therefore, it can be a good candidate for a pilot study for remediating TNT-contaminated sites, especially because F. solani showed a higher percentage of degradation compared with P. velutina.
The screening study for 28 days with prolonged sampling intervals seemed to miss the kinetics in the initial stages of TNT removal. Therefore, another set of experiments with short-term monitoring of the TNT in broth media was conducted for 84 h and was consistently checked every 12 h for TNT concentration. The results of short-term monitoring experiments in Fig. 4a. showed that there was a significant increase in the degradation of TNT using F. solani that spiked after 24 h of incubation. For medium containing 30 mg/L or 70 mg/L TNT, complete removal was almost achieved after less than 60 h of incubation. When 100 mg/L TNT broth was used, F. solani culture showed a general trend of increase in TNT removal, reaching around 70% at 84 h. However, better performance would be achieved if the culture was monitored for longer, as shown in the long-term study.
P. chrysosporium was more successful in removing TNT from a culture medium containing 30 mg/L than those of higher concentrations, reaching around 80% after 72 h (Fig. 4b). In the study of Kim and Song [13], inhibition of mycelial growth and reduction of TNT degradation in concentrations greater than 20 mg/L was reported. In the current study, the results of TNT degradation in liquid cultures verified the lower tolerance of P. chrysosporium to TNT concentrations higher than 30 mg/L.
The reduction in TNT removal at high TNT concentrations could be attributed to growth inhibition by elevated TNT concentrations, as stated by other researchers [13, 36,37,38]. Comparable findings using other microorganisms linked the slower growth of fungal mycelia with lower production of enzymes required for this process [39,40,41]. Others mentioned that nutrient availability in the culture media can have an effect on mycelial growth and degradation efficiency [37, 42, 43].
Fungal Biomass
Following the short-term incubation experiment (Fig. 5) with the corresponding fungal strains, F. solani produced more biomass than P. chrysosporium in all TNT concentrations. However, the biomass reduction was statisticaly significant (data not shown) between 30 and 70 mg/L, but not between 70 and 100 mg/L TNT for both fungi. The reduction in biomass for F. solani between 30 and 70 mg/L was about 66% while that for P. chrysosporium was about 84%.
As indicated in the previous section, the higher removal of TNT from the culture media could be attributed to higher biomass, and the possible increase in extracellular enzyme activity, that is responsible for the TNT mineralization process [43]. P. chrysosporium was found to be more sensitive to the increase in TNT levels compared to F. solani, indicating response to toxic levels of TNT above 30 mg/L. The biomass variation between the two strains could be attributed to the differences in their tolerance and potential for TNT degradation. As described by others, the current study results confirmed the correlation between fungal biomass and pollutant degradation efficiency [13, 36, 38]. Higher mycelial biomass could increase the surface area for TNT metabolic assimilation and physical adsorption [4]. On the other hand, higher TNT concentration leads to cellular toxicity and a reduction in TNT removal [33, 34], which can be used as an indicator of TNT toxicity.
Conclusion
This study represents the first reporting of TNT removal by F. solani isolated from TNT-amended soil. It was found that F. solani dominated the subsurface layer in the TNT-amended soil and was further tested. The results showed that F. solani has high ability to tolerate and degrade TNT. F. solani was better able to tolerate TNT toxicity even at TNT all TNT concentrations in this study (30, 70, and 100 mg/L) compared to P. chrysosporium. The tested concentrations simulate acute and chronic levels of TNT reported in contaminated hot spots. Tolerance of F. solani was demonstrated by bigger colonies and higher mycelial biomass on agar and broth media, respectively.
Removal of TNT from culture media under different TNT concentrations by F. solani was examined and compared to P. chrysosporium. F. solani was able to reach a complete TNT removal at all initial concentrations of TNT in 5 days or less. Oppositely, P. chrysosporium required a longer time to achieve complete removal at low TNT concentrations (> 5 days for 30 and 70 mg/L TNT), and no complete removal at high TNT levels was achieved even at more extended incubation period (74.2% TNT removal at 100 mg/L TNT, and 28 incubation days).
F. solani seems to have a high potential among naturally existing fungi to adapt and establish itself in the TNT contaminated soil. However, investigating the potential use of F. solani fungi in full-scale remediation of TNT-contaminated sites is recommended. Furthermore, Future studies of nitroaromatic metabolism in F. solani should focus on identifying TNT metabolites. It would also be important to purify and characterize the nitroreductase from the membrane extract. That work could enhance the understanding of how nitroreductases function and their role in bioremediation.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Szala, M., & Sabatini, J. J. (2018). 2,4,6-Trinitrotoluene – A useful starting compound in the synthesis of modern energetic compounds. Zeitschrift für Anorganische und Allgemeine Chemie, 644(5), 262–269. https://doi.org/10.1002/zaac.201700414
Khilyas, I. V., Lochnit, G., & Ilinskaya, O. N. (2017). Proteomic analysis of 2,4,6-trinitrotoluene degrading yeast Yarrowia lipolytica. Frontiers in Microbiology, 8, 2600. https://doi.org/10.3389/fmicb.2017.02600
Alothman, Z. A., Bahkali, A. H., Elgorban, A. M., Al-Otaibi, M. S., Ghfar, A. A., Gabr, S. A., Wabaidur, S. M., et al. (2020). Bioremediation of explosive TNT by Trichoderma viride. Molecules, 25(6), 1393. https://doi.org/10.3390/molecules25061393
Serrano-González, M. Y., Chandra, R., Castillo-Zacarias, C., Robledo-Padilla, F., Rostro-Alanis, M. J., & Parra-Saldivar, R. (2018). Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction. Defence Technology, 14(2), 151–164. https://doi.org/10.1016/j.dt.2018.01.004
Schuster, R., Strehse, J. S., Ahvo, A., Turja, R., Maser, E., Bickmeyer, U., Lehtonen, K. K. (2021). Exposure to dissolved TNT causes multilevel biological effects in Baltic mussels (Mytilus spp.). Marine Environmental Research, 167, 105264. https://doi.org/10.1016/j.marenvres.2021.105264
Zuo, J., Zhao, X., Ju, X., Qiu, S., Hu, W., Fan, T., & Zhang, J. (2016). A new molecularly imprinted polymer (MIP)-based electrochemical sensor for monitoring cardiac troponin I (cTnI) in the serum. Electroanalysis (New York), 28(9), 2044–2049. https://doi.org/10.1002/elan.201600059
Gao, W., Wang, T., Zhu, C., Sha, P., Dong, P., & Wu, X. (2022). A ‘sandwich’ structure for highly sensitive detection of TNT based on surface-enhanced Raman scattering. Talanta, 236, 122824. https://doi.org/10.1016/j.talanta.2021.122824
USEPA. (2018). 2018 Edition of the Drinking Water Standards and Health Advisories Tables, in EPA 822-F-18-001. U.S. Environmental Protection Agency.
Spina, F., Cecchi, G., Landinez-Torres, A., Pecoraro, L., Russo, F., Wu, B., Cai, L., et al. (2018). Fungi as a toolbox for sustainable bioremediation of pesticides in soil and water. Plant Biosystems-An International Journal Dealing with All Aspects of Plant Biology, 152(3), 474–488. https://doi.org/10.1080/11263504.2018.1445130
Gupta, S., Goel, S. S., Siebner, H., Ronen, Z., & Ramanathan, G. (2023). Transformation of 2, 4, 6-trinitrotoluene by Stenotrophomonas strain SG1 under aerobic and anaerobic conditions. Chemosphere, 311, 137085. https://doi.org/10.1016/j.chemosphere.2022.137085
Ziganshin, A. M., Gerlach, R., Borch, T., Naumov, A. V., & Naumova, R. P. (2007). Production of eight different hydride complexes and nitrite release from 2,4,6-trinitrotoluene by Yarrowia lipolytica. Applied and Environment Microbiology, 73(24), 7898–7905. https://doi.org/10.1128/AEM.01296-07
Hawari, J., Halasz, A., Beaudet, S., Paquet, L., Ampleman, G., & Thiboutot, S. (1999). Biotransformation of 2, 4, 6-trinitrotoluene with Phanerochaete chrysosporium in agitated cultures at pH 4.5. Applied and Environmental Microbiology, 65(7), 2977–2986. https://doi.org/10.1128/AEM.65.7.2977-2986.1999
Kim, H. Y., & Song, H. G. (2000). Comparison of 2,4,6-trinitrotoluene degradation by seven strains of white rot fungi. Current Microbiology, 41(5), 317–320. https://doi.org/10.1007/s002840010142
Montpas, S., Samson, J., Langlois, É., Lei, J., Piché, Y., & Chênevert, R. (1997). Degradation of 2, 4, 6-trinitrotoluene by Serratia Marcescens. Biotechnology Letters, 19, 291–294. https://doi.org/10.1023/A:1018326228448
Kao, C. M., Lin, B. H., Chen, S. C., Wei, S. F., Chen, C. C., Yao, C. L., & Chien, C. C. (2016). Biodegradation of trinitrotoluene (TNT) by indigenous microorganisms from TNT-contaminated soil, and their application in TNT bioremediation. Bioremediation Journal, 20(3), 165–173. https://doi.org/10.1080/10889868.2016.1148007
Zaripov, S. A., Naumov, A. V., Abdrakhmanova, J. F., Garusov, A. V., & Naumova, R. P. (2002). Models of 2,4,6-trinitrotoluene (TNT) initial conversion by yeasts. Fems Microbiology Letters, 217(2), 213–217. https://doi.org/10.1111/j.1574-6968.2002.tb11477.x
Xu, M., Liu, D., Sun, P., Li, Y., Wu, M., Liu, W., Maser, E. (2021). Degradation of 2,4,6-trinitrotoluene (TNT): Involvement of protocatechuate 3,4-dioxygenase (P34O) in Buttiauxella sp. S19-1. Toxics, 9(10). https://doi.org/10.3390/toxics9100231
Covino, S., Stella, T., & Cajthaml, T. (2016). Fungal applications in sustainable environmental biotechnology. Fungal Biology, vol. Mycoremediation of Organic Pollutants: Principles, Opportunities, and Pitfalls (pp. 185–231). Springer.
Barra Caracciolo, A., & Terenzi, V. (2021). Rhizosphere microbial communities and heavy metals. Microorganisms, 9(7), 1462. https://doi.org/10.3390/microorganisms9071462
Khanna, K., Kohli, S. K., Ohri, P., Bhardwaj, R., & Ahmad, P. (2022). Agroecotoxicological aspect of cd in soil-plant system: Uptake, translocation and amelioration strategies. Environmental Science and Pollution Research International, 29(21), 30908–30934. https://doi.org/10.1007/s11356-021-18232-5
Lee, S., Lee, S. Y., & Shin, K. S. (2009). Biodegradation of 2,4,6-trinitrotoluene by white-rot fungus Irpex lacteus. Mycobiology, 37(1), 17–20. https://doi.org/10.4489/MYCO.2009.37.1.017
Romero-Silva, R., Sánchez-Reyes, A., Díaz-Rodríguez, Y., Batista-García, R. A., Hernández-Hernández, D., & de Robles, J. T. (2019). Bioremediation of soils contaminated with petroleum solid wastes and drill cuttings by Pleurotus sp. under different treatment scales. SN Applied Sciences, 1(1209), 588673. https://doi.org/10.1007/s42452-019-1236-3
Carles, L., Rossi, F., Joly, M., Besse-Hoggan, P., Batisson, I., & Artigas, J. (2017). Biotransformation of herbicides by aquatic microbial communities associated to submerged leaves. Environmental Science and Pollution Research International, 24(4), 3664–3674. https://doi.org/10.1007/s11356-016-8035-9
Gumuscu, B., & Tekinay, T. (2013). Effective biodegradation of 2,4,6-trinitrotoluene using a novel bacterial strain isolated from TNT-contaminated soil. International Biodeterioration & Biodegradation, 85, 35–41. https://doi.org/10.1016/j.ibiod.2013.06.007
Funder, S. (1961). Practical mycology. Manual for identification of fungi. Practical mycology. Manual for identification of fungi. Brøggers Boktr. A/S.
Kornerup, A., & Wanscher, J. H. (1978). Methuen handbook of colour. 3d 1983 reprint ed. E. Methuen.
Ayoub, K., van Hullebusch, E. D., Cassir, M., & Bermond, A. (2010). Application of advanced oxidation processes for TNT removal: A review. Journal of Hazardous Materials, 178(1–3), 10–28. https://doi.org/10.1016/j.jhazmat.2010.02.042
US Environmental Protection Agency. (1994). Nitroaromatics and nitramines by high performance liquid chromatography (HPLC).
Naylor, D., McClure, R., & Jansson, J. (2022). Trends in microbial community composition and function by soil depth. Microorganisms, 10(3). https://doi.org/10.3390/microorganisms10030540
Cabrera, M., Márquez, S. L., & Pérez-Donoso, J. M. (2022). Comparative genomic analysis of Antarctic Pseudomonas isolates with 2, 4, 6-trinitrotoluene transformation capabilities reveals their unique features for xenobiotics degradation. Genes, 13(8), 1354. https://doi.org/10.3390/genes13081354
Leslie, J. F., & Summerell, B. A. (2006). The Fusarium laboratory manual. The Fusarium laboratory manual.
Sehrawat, A., Phour, M., Kumar, R., & Sindhu, S. S. (2021). Microbial Rejuvenation of Polluted Environment. Bioremediation of pesticides: An eco-friendly approach for environment sustainability (25 vol., pp. 23–84). Springer.
Spain, J. C. (1995). Biodegradation of nitroaromatic compounds. Annual Review of Microbiology, 49(1), 523–555. https://doi.org/10.1146/annurev.mi.49.100195.002515
Tiwari, J., Tarale, P., Sivanesan, S., & Bafana, A. (2019). Environmental persistence, hazard, and mitigation challenges of nitroaromatic compounds. Environmental Science and Pollution Research International, 26(28), 28650–28667. https://doi.org/10.1007/s11356-019-06043-8
Anasonye, F., Winquist, E., Räsänen, M., Kontro, J., Björklöf, K., Vasilyeva, G., Jørgensen, K. S., et al. (2015). Bioremediation of TNT contaminated soil with fungi under laboratory and pilot scale conditions. International Biodeterioration & Biodegradation, 105, 7–12. https://doi.org/10.1016/j.ibiod.2015.08.003
Claus, H. (Ed.). (2014). Microbial degradation of 2,4,6-trinitrotoluene in vitro and in natural environments. In: Singh, S. (ed) Biological remediation of explosive residues. Vol. Environmental Science and Engineering (pp. 15–38). Springer.
Muter, O., Potapova, K., Limane, B., Sproge, K., Jakobsone, I., Cepurnieks, G., & Bartkevics, V. (2012). The role of nutrients in the biodegradation of 2,4,6-trinitrotoluene in liquid and soil. Journal of Environmental Management, 98, 51–55. https://doi.org/10.1016/j.jenvman.2011.12.010
Okozide, O. E., Adebusoye, S. A., Obayori, O. S., & Rodrigues, D. F. (2021). Aerobic degradation of 2, 4, 6-trinitrophenol by Proteus sp. strain OSES2 obtained from an explosive contaminated tropical soil. Biodegradation, 32, 643–662. https://doi.org/10.1007/s10532-021-09958-7
Manai, I., Miladi, B., El Mselmi, A., Smaali, I., Ben Hassen, A., Hamdi, M., & Bouallagui, H. (2016). Industrial textile effluent decolourization in stirred and static batch cultures of a new fungal strain Chaetomium globosum IMA1 KJ472923. Journal of Environmental Management, 170, 8–14. https://doi.org/10.1016/j.jenvman.2015.12.038
Sepehri, A., & Sarrafzadeh, M. H. (2018). Effect of nitrifiers community on fouling mitigation and nitrification efficiency in a membrane bioreactor. Chemical Engineering and Processing-Process Intensification, 128, 10–18.
Wahal, S., & Viamajala, S. (2010). Maximizing algal growth in batch reactors using sequential change in light intensity. Applied Biochemistry and Biotechnology, 161(1–8), 511 – 22. https://doi.org/10.1007/s12010-009-8891-6
Khan, N., Muge, E., Mulaa, F. J., Wamalwa, B., von Bergen, M., Jehmlich, N., & Wick, L. Y. (2023). Mycelial nutrient transfer promotes bacterial co-metabolic organochlorine pesticide degradation in nutrient-deprived environments. Isme Journal, 17(4), 570–578. https://doi.org/10.1038/s41396-023-01371-7
Sardar, H., Ali, M. A., Anjum, M. A., Nawaz, F., Hussain, S., Naz, S., & Karimi, S. M. (2017). Agro-industrial residues influence mineral elements accumulation and nutritional composition of king oyster mushroom (Pleurotus eryngii). Scientia Horticulturae, 225, 327–334. https://doi.org/10.1016/j.scienta.2017.07.010
Acknowledgements
The authors are very thankful to all the associated personnel in any reference that contributed in/for the purpose of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibbini, J., Al-Kofahi, S., Davis, L.C. et al. Investigating the Potential of Fusarium solani and Phanerochaete chrysosporium in the Removal of 2,4,6-TNT. Appl Biochem Biotechnol 196, 2713–2727 (2024). https://doi.org/10.1007/s12010-023-04735-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04735-z