Abstract
Filamentous fungi were isolated from semiarid soil in which the lead (Pb) concentrations were above regulatory limits as determined by the Brazilian standards. Among these fungi, four isolates were tested for their abilities to grow in a culture medium containing lead nitrate. Chaetomium aureum was the species that presented a comparatively better performance including mycelial growth in a lead-containing medium, ability to use pectin as a carbon source, as well as basophilic and thermotolerant properties. The C. aureum inoculum with either activated or inactivated native microbiota was able to reduce the free Pb in soil (61 and 54%, respectively) after 60 days of inoculation. Although the mechanism involved in decreasing water-soluble and exchangeable lead concentrations in soil has not been studied, either the processes of biosorption by organic molecules (called oosporein, produced by this species) or fungal mineral transformation is among its possible explanations. These findings support the use of filamentous fungi as potential tools for the bioremediation of contaminated sites and highlight C. aureum as a promising tool for environmental biotechnology. In addition, the ability of this fungus (collected in a lead-contaminated area) to grow efficiently in the presence of this metal indicates that the bioprospecting strategy of the indigenous microbiota should be encouraged, as they appear to be more likely to succeed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) has been recognized as an extremely toxic metal of special interest among environmental pollutants (Casas and Sordo 2006). Pollution caused by anthropogenic activities mostly originates from mining, smelting, industrial uses, wastes incineration, and burning of coal and leaded gasoline (Cheng and Hu 2010). From toxicological perspective, lead causes extensive damage in different organisms and it is considered a neurotoxic agent (Skerfving and Bergdahl 2014).
Microorganisms in soil are particularly susceptible to the effects of Pb. Unlike many other metals, Pb has no biological role and is potentially toxic to microorganisms (Sobolev and Begonia 2008). Its excessive accumulation in living organisms is always detrimental (Adelekan and Abegunde 2008). Nevertheless, certain microorganisms can tolerate the presence of high concentrations of heavy metals owing to different physiological mechanisms (Da Silva-Júnior and Pereira 2007, 2014; Li et al. 2015; Kang et al. 2016).
The strategies used by microorganisms to survive adverse environments with high concentrations of heavy metals are particularly interesting to the study of remediation in contaminated sites. In many cases, these organisms can decrease the bioavailability of toxic elements in the environment through mechanisms, including the absorption of lead by secreting extracellular polymers, like the secretion of polysaccharides by Pseudomonas sp. (Shiomi 2015). There is still, mechanisms such as adsorption at the cell wall (for example, in Aspergillus flavus) (Ozer and Ozer 2003), intracellular binding by substances like phytochelatins and siderophores (in Schizosaccharomyces sp.) (Shiomi 2015) or intracellular precipitation of lead (marine bacteria Vibrio harveyi and gram negative bacteria Providencia alcalifaciens) (Mire et al. 2004; Naik et al. 2012).
The efficacy of metal removal from the environment may be enhanced by selecting optimum conditions for parameters such as temperature and pH (Biswas et al. 2015). Determination of these optimal conditions may encourage faster microbial growth (Da Silva-Júnior and Pereira 2007) as well as the production and release of metabolites (Battilani et al. 2010). Furthermore, the specific relationship between the contaminant and the microorganism used for the bioremediation process should be considered.
Currently, the search for mechanisms and organisms for bioremediation programs has gained prominence, due to the increasing levels of contaminated sites. In order to look for these competent organisms we have to take into account their potential to grow in adverse conditions, adaptability, and a deeper investigation of the relationship between microorganisms and the environment
The study aimed to isolate filamentous fungi in a contaminated area of lead, as well as to evaluate the behavior of this isolated fungus tolerant to this metal in culture medium and to use a microcosm scale in the soil.
Materials and methods
Sample collection and processing of soil
Soil sampling was performed at the perimeter of a metallurgical plant located in Belo Jardim, Pernambuco state, Brazil. The results of soil analysis are described in Table 1, and the lead concentration was considered to be above regulatory limits as defined by Brazilian standards (CONAMA 2009).
Soil samples were collected from random points at the depth of 0–20 cm, after removing plant residues from the sampling sites. The samples were then stored in polyethylene bags and were transferred to the Soil Microbiology Laboratory of the Instituto Tecnológico de Pernambuco. At the laboratory, the samples were sieved (2 mm) and homogenized for further assays.
Isolation of filamentous fungi
For the isolation of filamentous fungi, 25 g of soil was suspended in 225 mL of sterile distilled water and the resulting suspension serially diluted. Triplicates were obtained from each suspension, placed in plates containing 0.2 mL potato dextrose agar (PDA) medium, and homogenized with a Drigalski spatula. The plates were incubated in the laboratory at room temperature (approximately 28 °C) for up to 6 days, and CFU g−1 counts were then obtained (Warcup 1950).
Identification of the fungi
The identification was performed by macroscopic and microscopic observations of the isolated fungi. The morphological aspects of the cultures such as the aspects of their border, pigmentation, and color were visualized through macroscopic observation of the fungal colonies grown on either PDA or Czapek media. The microscopic characteristics of these isolates were observed through the description of the reproductive structures under an optical microscope using the slide culture technique. The fungal species were identified as described by Fennell et al. (1975), Domsch et al. (1980), and von Arx et al. (1986).
Growth in culture medium with lead nitrate
Tolerance analysis of the isolated species was performed by investigating the growth of the colonies in culture media containing different concentrations of lead nitrate (0, 93.75, 187.5, and 281.25 Pb mg kg−1). A piece of the monosporic cultures of four isolates from the contaminated soil (C. aureum, Thielavia sp. Aspergillus niger and Penicillium sp.) was transferred to PDA media (in triplicate, individually). After 6 days of incubation, the colony diameters were measured with millimeter precision. Among the isolated colonies, the fungus Chaetomium aureum Chivers (1912) was selected considering the results from previous studies, which demonstrated the tolerance of the genus Chaetomium to high lead concentrations.
Growth using different substrates
Evaluation of the growth of colonies using different substrates was performed after transferring a fragment of monosporic cultures (C. aureum) to Petri dishes (also in triplicate) containing PDA medium with different substrates (dextrose, pectin, casein, and amide). The cultures were incubated at room temperature for 4 days, and the colony diameters were then measured with millimeter precision.
Growth at different temperatures
Fragments of C. aureum monosporic cultures were transferred to PDA media (six replicates) and incubated for 3 days at three temperatures (28, 35, and 42 °C). After this period, the colony diameters were measured with millimeter precision.
Growth in different pH values
Fragments of monosporic cultures of C. aureum were transferred to Petri dishes (six replicates) containing PDA media prepared at 4 different pH values (pH 6, 7, 8, and 9) and were incubated for 3 days. After this period, we measured the colony diameters with millimeter precision.
Experiment at the microcosm scale—C. aureum in contaminated soil
C. aureum inoculums preparation
Replicates of the fungal culture stored in the laboratory were used to prepare concentrated suspensions of spores which were placed in Petri dishes containing PDA. These replicates were incubated at a temperature of 28 °C for 10 days. After this period, the mycelial mass was transferred to tubes which containing a sterile saline solution (0.8%) and the final concentration of the inoculum ranged from 2.5 to 3 g L−1.
Soil
Soil samples from the area, where the fungus was isolated, were used for the microcosm assay. Both natural and autoclaved (at 121 °C for 1 h for two consecutive days) soils were used.
Soil inoculation and analyzed variables
Recipients containing 300 g of soil were inoculated (in triplicate) with fungal suspensions for periods of 0, 30, and 60 days. The following parameters were then measured: pH, organic matter, lead content, and colony forming units per gram of soil (CFU g−1).
The experimental design was entirely random in a scheme factorial 2 × 2 × 3 × 3. The treatments were: (1) non-autoclaved soil without C. aureum inoculum, (2) non-autoclaved soil inoculated with C. aureum, (3) autoclaved soil without C. aureum inoculum, and (4) autoclaved soil inoculated with C. aureum.
Water-soluble and exchangeable Pb determination
The soil samples were shaken with 0.1 M CaCl2 for 16 h at room temperature and centrifuged at 3600 rpm. The supernatant then filtered with a Millipore filter (0.45 µm) (Orroño and Lavado 2009). The Pb was quantified using inductively coupled plasma-atomic emission spectrometry (ICP-OES).
Data analysis
The results were expressed as mean ± SD. The data were analyzed by one-way analysis of variance (ANOVA) following the Tukey’s test. The Kruskal–Wallis nonparametric test was employed for the analysis of nonparametric data. Differences at p < 0.05 were considered as statistically significant.
Results and discussion
Microbial growth in the presence of lead nitrate
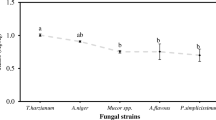
Among the colonies grown in soil from the residue storage patio at the metallurgical plant, four colonies were selected to evaluate growth in the presence of lead: one isolate of C. aureum, one of Thielavia sp., one of Penicillium sp., and one of Aspergillus niger. After 3 days, lead nitrate showed negative effects on the growth of Thielavia sp., A. niger, and Penicillium sp. isolates. However, it caused no significant difference in the growth of the C. aureum isolate (Fig. 1).
After 6 days, the C. aureum isolate showed increased mean growth efficiency in the medium containing lead nitrate, also displaying significantly greater mycelial growth in the two highest concentrations of lead compared to that in the control. In sequence, the Thielavia sp. isolate demonstrated mycelial growth that was superior to the control at a Pb concentration of 187.5 mg kg−1. On the other hand, isolates of the genera Aspergillus and Penicillium showed decreased mycelial growth with increasing lead concentration (Fig. 2).
These findings indicate that the filamentous fungus C. aureum showed tolerance to lead nitrate after 3 days of incubation, and presented a higher mycelial growth depending on the mean lead concentration in the mean after 6 days.
Interactions between fungi and toxic metals are not limited to studies with iron, nor are they restricted to the toxic effects of metals in different species of fungi, these studies also investigated their toxicity, resistance, tolerance, absorption, and excretion (Gola et al. 2016; Kaewdoung et al. 2016; Ruta et al. 2017). Elucidating these processes is an important step in decision-making for microbe bioremediation studies. The relationship between toxic metals and the fungi of the genus Chaetomium was addressed by only a few studies, which have shown both species-specific and metal-specific responses.
The fungi of the Chaetomium genus were isolated from the soils of peri-urban agricultural area irrigated by industrial and mine waste areas, presenting high concentrations of metals such as Fe, Mn, Cu, As, Sr, Mo, Cd, Sb, Ti, Zn, and Pb (Iram et al. 2009). Smith et al. (1978) studied the effects of trace-metals on the growth of urban tree-leaf pathogen fungi and observed that high concentrations of some metals such as Al, Fe, Mg, Pb, and Zn are extremely toxic to Chaetomium sp. However, the growth of this fungus was favored at some intermediate concentrations of Pb and Fe. An example of this high resistance in harsh environments can be seen in a review of the surveys conducted during 1986–2012 in natural ecosystems of the Russian Federation and Ukraine, which were contaminated due to the Chernobyl disaster (Shcheglov et al. 2013), showing a fungal activity of 3.7 × 105–3.7 × 107 Bq/Kg for C. aureum and Paecilomyces lilacinus in medium and high levels of contamination (Zhdanova et al. 1995). Aguileta et al. (2016) also demonstrated a lower prevalence of parasitic fungi at the radiation levels near Chernobyl, but with similar fitness.
In the present study, four investigated isolates of Chaetomium sp. were tolerant to lead, since they were indigenous components of the contaminated soil adjacent to the metallurgical plant. In fact, fungi of this genus have already been used in isolates of contaminated soil from the same study area (Da Silva-Júnior and Pereira 2007) and have proven to be tolerant to the lead-containing mixture of natural soil and scum (Da Silva-Júnior and Pereira 2014). In the current study, C. aureum isolates displayed a better outcome in mycelial growth in relation to other isolates incubated in medium containing lead nitrate. The association between two factors must have contributed to this growth: the tolerance of the fungus to lead and the use of nitrate as a nitrogen source (Feeney and Curran 1992). This explains the necessity of evaluating some culture conditions of this fungus and inoculating it in a lead-contaminated soil in order to monitor some soil parameters at the microcosmic scale.
Analysis of mycelial growth in different substrates and conditions
The C. aureum isolate displayed a stronger ability to grow in the culture medium containing lead nitrate; therefore, it was used for physiological characterization as well as in the microcosm scale experiments. The ability of C. aureum to grow in different substrates was investigated (Fig. 3). This fungus displayed higher mycelial growth in media containing pectin compared to those containing starch and glucose. On the other hand, it was not capable of growing in media containing casein as the substrate.
Abiotic culture parameters, such as temperature, pH, and water activity, may influence in the physiological state of microorganisms (van Long et al. 2017). The C. aureum isolate demonstrated the ability to use pectin, glucose, and starch as substrates, but not casein. Among the three substrates, pectin promoted the highest mycelial growth. The ability to degrade materials of vegetal origin, such as cellulose and pectin, has already been described for the fungi of the genus Chaetomium by Batista and Pontual (1948), besides the detection of the enzymatic activity of pectinase (Reddy and Sreeramulu 2012).
The genus Chaetomium is composed of fungi with an enzymatic surface machinery, which is capable of breaking down complex molecules such as cellulose (Cragg et al. 2015). This strategy has been investigated in studies on the biodegradability of organic polymers (Oprea and Doroftei 2011), nitrocellulose (Auer et al. 2005), hydrocarbons (Aranda 2016), pesticides (Tiedje and Hagedorn 1975; Hu et al. 2015), and natural products (Zheng et al. 2016).
Other growth parameters investigated were the pH of the culture medium and incubation temperature (Figs. 4, 5). The increase in pH values had a positive effect on the growth of C. aureum, and the best temperature for growth was observed at 35 °C. The C. aureum isolate appeared to be both thermo- and alkaline-tolerant. Both properties have already been described for many Chaetomium species (Milner 1977; Ravindran et al. 2011; Ravindran and Thangaiah Naveenan 2011; Srivastava et al. 2017). However, fungi of the genera Chaetomium have already been isolated in soils with pH higher than 10 in the same study area (unpublished data). This specific case, where the pH value of industrial waste is 10.4, demonstrates a basophilic condition that favors the use of this fungus in future bioremediation programs. Furthermore, since this region is located in the Brazilian semiarid region, fungi that can survive and grow in high temperatures may be considered in planning the recuperation of contaminated sites.
Microcosm scale experiment
In order to evaluate the effect of lead-contaminated soil on the fungus, we prepared an experiment at the microcosm scale by adding an inoculum of C. aureum (concentrations between 2.5 and 3 g L−1) to soil samples. Tables 1 and 2 show that the four treatments, with or without C. aureum inoculum in autoclaved and non-autoclaved soil, reduced the concentration of water-soluble and exchangeable lead after 60 days.
Treatment 2 (non-autoclaved soil with the fungus inoculum) showed a reduction of approximately 40% in the levels of exchangeable lead in the first 30 days of inoculation. This reduction is twice as large as that observed in treatment 1 (non-autoclaved soil without the fungus inoculum). Between 30 and 60 days, treatment 4 (autoclaved soil with fungus inoculum) was the most efficient in reducing the concentration of free Pb. The reduction rate after 60 days revealed that treatments with the fungus inoculum were at least in two different settings, non-autoclaved and autoclaved soil, more effective at decreasing the concentrations of exchangeable lead than in the treatments without the inoculum.
The effect on pH and the organic matter content in the soil is illustrated in Table 3. These two parameters only varied after 60 days of inoculation. The pH decreased significantly in treatments 1, 2, and 3, whereas organic matter content increased significantly in treatment 2 after the same period. Microbial density during the microcosm experiments is illustrated in Fig. 6. This parameter displayed similar behavior in treatments with both autoclaved and non-autoclaved soils. In the former experiment, microbial density grew after 30 and 60 days of inoculation, whereas in the latter, treatments 1 and 2 displayed an increase in density after 30 days, followed by a decrease after 60 days of inoculation.
C. aureum thus seemed to be a promising bioremediation element at the microcosm scale. Its presence in soil, associated with the native microbiota, reduced the levels of exchangeable lead by two times more than that in the absence of the fungal inoculum after 60 days of incubation. During the same period, inoculation of C. aureum played an important role in maintaining the pH values of the soil, while inoculation of the fungus in association with the native microbiota increase the organic matter concentration in the soil. On the other hand, the responses in microbial abundance in soil were related to the process of autoclaving.
Even though the number of studies encompassing microbial remediation focused on organic matter decomposition is highly varied, during the last few years, an increased interest in fungal species with the capacity for efficient metal removal has been observed (Sargin et al. 2016; Xin et al. 2016). The most recent studies show the importance of biosorption to reduce the bioavailability of metals in the environment (Gola et al. 2016) and as in the current study, the high tolerance strategy is accompanied by a decrease in the availability of metals in the environment (Dey et al. 2016). Manoliu et al. (1999) suggest that the cellulolytic fungus C. globosum is capable of internalizing iron, thus removing it from the surroundings through molecules called siderophores. In the current study, the mechanism for decreasing exchangeable lead concentration is not clear, but two hypotheses can proposed.
The first hypothesis is that exchangeable lead may be removed through biosorption by molecules released by the fungus, thus reducing its availability in soil. In fact, it is reported that C. aureum produces a metabolite called oosporein, which we believe, may bind to the metal, thus forming a complex. Oosporein is a red-colored pigment that presents the typical properties of a polyhydroxyquinone (Lloyd et al. 1955). Molecules of this nature are capable of binding to metals and actively participate in oxy-reduction reactions (Ragimov et al. 1974; Greenaway and Dabrowiak 1982).
The other hypothesis is the mineral transformation of lead by the fungus. This kind of biologically mediated mineralization has already been described for two fungal species, Metarhizium anisopliae and Paecilomyces javanicus, which transformed lead into pyromorphite (Rhee et al. 2012). Although it has not been investigated in C. aureum, mineralization of lead is a possible bioremediation mechanism, as it reduces metal solubility, and therefore, its bioavailability.
Irrespective of the strategy used by the fungus, our findings are relevant to the study of lead contamination in terrestrial environments. C. aureum, aided by the native microbial community in soil, was capable of influencing both the availability of this metal and the chemical parameters observed in the soil at the microcosm scale. This ability of interfering in edaphic processes is only possible due to the physiological mechanisms of lead tolerance, which have not been enlightened yet for C. aureum. In this context, we believe that the strategy of using indigenous organisms in attempts of bioremediation may be useful to increase their success rates. Recently, the authors also associated the increased efficiency in the removal of metals by fungi when used in consortia (Awasthi et al. 2017). Therefore, microbial processes must be considered in recuperation techniques at lead-contaminated sites.
Conclusions
Lead showed no evidence of toxicity in C. aureum at the concentrations tested in vitro. In terms of substrate use, the C. aureum isolate was considered versatile, temperature-tolerant, and basophilic. In addition, when inoculated in lead-contaminated soil, it was capable of decreasing the exchangeable lead concentration, irrespective of its association with the non-indigenous microbiota.
These findings support the use of filamentous fungi as potential tools for the bioremediation of contaminated sites by metals and highlight C. aureum as a promising tool for environmental biotechnology. The strategy of using indigenous species seems promising to seek candidates for bioremediation of contaminated sites.
References
Adelekan BA, Abegunde KD (2008) Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. Int J Phys Sci 6(5):1045–1058. https://doi.org/10.5897/ijps10.495
Aguileta G, Badouin H, Hood ME, Møller AP, Prieur S, Snirc A, Siguenza S, Mousseau TA, Shykoff JA, Cuomo CA, Giraud T (2016) Lower prevalence but similar fitness in a parasitic fungus at higher radiation levels near Chernobyl. Mol Ecol 25:3370–3383. https://doi.org/10.1111/mec.13675
CONAMA - Conselho Nacional do Meio Ambiente (2009) Resolução no 420, de 28 de dezembro de 2009. “Dispõe sobre critérios e valores orientadores de qualidade do solo quanto à presença de substâncias químicas e estabelece diretrizes para o gerenciamento ambiental de áreas contaminadas por essas substâncias em decorrência de atividades antrópicas.”, Diário Oficial [da República Federativa do Brasil], Brasília, DF, n° 249, de 30/12/2009, pp 81–84
Aranda E (2016) Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr Opin 38:1–8. https://doi.org/10.1016/j.copbio.2015.12.002
Auer N, Hedger JN, Evans CS (2005) Degradation of nitrocellulose by fungi. Biodegradation 16:229–236. https://doi.org/10.1080/07370659508019389
Awasthi AK, Pandey AK, Khan J (2017) Biosorption an innovative tool for bioremediation of metal-contaminated municipal solid waste leachate: optimization and mechanisms exploration. Int J Environ Sci Technol 14(4):729–742. https://doi.org/10.1007/s13762-016-1173-2
Batista AC, Pontual D (1948) Alguns fungos do gênero Chaetomium. Boletim da Secretaria de Agricultura e Comércio 15(1):62–73
Battilani P, Formenti S, Toscani T, Virgili R (2010) Influence of abiotic parameters on ochratoxin A production by a Penicillium nordicum strain in dry-cured meat model systems. Food Control 21(12):1739–1744
Biswas K, Paul D, Sinha SN (2015) Biological agents of bioremediation: a concise review. Front Enviro Microbiol 1:39–43. https://doi.org/10.11648/j.fem.20150103.11
Casas JS, Sordo J (2006) An overview of the historical importance, occurrence, isolation, properties and applications of lead. In: Casas JS, Sordo J (eds) Lead: chemistry, analytical aspects, environmental impact and health effects, 1st edn. Elsevier Science, Amsterdam, p 366
Cheng H, Hu Y (2010) Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: a review. Environ Pollut 158:1134–1146. https://doi.org/10.1016/j.envpol.2009.12.028
Chivers AH (1912) Preliminary diagnoses of new species of Chaetomium. PCPS Am Acad Arts Science 48:83–88
Cragg SM, Beckham GT, Bruce NC, Bugg TDH, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQuenn-Mason SJ, Schnorr K, Walton PH, Watts JEM, Zimmer M (2015) Lignocellulose degradation mechanisms across the Tree of Life. Curr Opin Chem Biol 29:108–119. https://doi.org/10.1016/j.cbpa.2015.10.10.018
Da Silva-Júnior FMR, Pereira SV (2007) Ecologia e fisiologia de fungos filamentosos isolados de solo contaminado por metais pesados. Rev Bras Biocien 5:903–905
Da Silva-Júnior FMR, Pereira SV (2014) Filamentous fungi isolated from Brazilian semiarid tolerant to metallurgical industry wastes: an ex situ evaluation. Braz Arch Biol Technol 57:723–727. https://doi.org/10.1590/S1516-8913201402190
Dey P, Gola D, Mishra A, Malik A, Singh DK, Patel N, von Bergen M, Jehmlich N (2016) Comparative performance evaluation of multi-metal resistant fungal strains for simultaneous removal of multiple hazardous metal. J Hazard Mater 318:679–685. https://doi.org/10.1016/j.jhazmat.2016.07.025
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi, vol II. Academic Press, London, p 85
Feeney N, Curran PMT (1992) Biodeterioration of woods by marine fungi and Chaetomium globosum in response to an external nitrogen source. Int Biodeterior Biodegrad 29:123–133. https://doi.org/10.1016/0964-8305(92)90012-D
Fennell DI, Lillehoj EB, Kwoler WF (1975) Aspergillus flavus and other fungi associated with insect-damaged field corn. Cereal Chem J 52:314–321
Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath N, Ahamnad SZ (2016) Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Biores Technol. https://doi.org/10.1016/j.biortech.2016.06.096
Greenaway FT, Dabrowiak JC (1982) The binding of copper ions to daunomycin and adriamycin. J Inorg Biochem 16:91–107. https://doi.org/10.1016/S0162-0134(00)80218-4
Hu P, Hollister EB, Somenahally AC, Hons FM, Gentry TJ (2015) Soil bacterial and fungal communities respond differently to various isothiocyanates added for biofumigation. Front Microbiol 5:729. https://doi.org/10.3389/fmicb.2014.00729
Iram S, Ahmad I, Stuben D (2009) Analysis of mines and contaminated agricultural soil samples for fungal diversity and tolerance to heavy metals. Pak J Bot 41:885–895
Kaewdoung B, Sutjaritvorakul T, Gadd GM, Whalley AJS, Sihanonth P (2016) Heavy metal tolerance and biotransformation of toxic metal compounds by new isolates of wood-rotting fungi from Thailand. Geomicrobiol J 33(3–4):283–288. https://doi.org/10.1080/01490451.2015.1048394
Kang C-H, Oh SJ, Shin YJ, Han S-H, Nam I-H (2016) Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol Eng 74:402–407. https://doi.org/10.1016/j.ecoleng.2014.10.009
Li X, Peng W, Jia Y, Lu L, Fan W (2015) Bioremediation of lead contaminated soil with Rhodobacter sphaeroides. Chemosphere 156:228–235. https://doi.org/10.1016/j.chemosphere.2016.04.098
Lloyd G, Obertson A, Sankey GB, Whalley WB (1955) The chemistry of fungi. Part XXV.* Oosporein, a metabolite of Chaetomium aureum chivers. J Chem Soc. https://doi.org/10.1039/jr9550002163
Manoliu A, Antohe L, Creanga DE, Cotae C (1999) The influence of petroleum ferrofluids upon the cellulosolytic fungi Chaetomium globosum Kunze: Fr. J Magn Magn Mater 201:446–448. https://doi.org/10.1016/S0304-8853(99)00113-4
Milner PD (1977) Radial growth responses to temperature by 58 Chaetomium species, and some taxonomic relationships. Mycologia 69:492–502. https://doi.org/10.2307/3758552
Mire CE, Tourjee JA, O’Brian WF, Ramanujachary KV, Hecht GB (2004) Lead precipitation by Vibrio harveyi: evidence for novel quorum-sensing interactions. Appl Environ Microbiol 70:855–864. https://doi.org/10.1128/AEM.70.2.855-864.2004
Naik MM, Pandey A, Dubey SK (2012) Pseudomonas aeruginosa strain WI-1 from Mandovi estuary possesses metallothionein to alleviate leal toxicity and promotes plant growth. Ecotoxycol Environ Saf 79:129–133
Oprea S, Doroftei F (2011) Biodegradation of polyurethane acrylate with acrylated epoxidized soybean oil blend elastomers by Chaetomium globosum. Int Biodeterior Biodegrad 65:533–538. https://doi.org/10.1016/j.ibiod.2010.09.011
Orroño DI, Lavado RS (2009) Distribution of extractable heavy metals in different soil fractions. Chem Speciat Bioavailab 21(4):193–198. https://doi.org/10.3184/095422909X12473204137916
Ozer A, Ozer D (2003) Comparative study of the biosorption of Pb(II) and Cr(IV) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 100:219–229
Ragimov AV, Sadykh-Zade SI, Suleimainova SS, Liogon-kii BI (1974) Redox properties of polyhydroxyquinones. J Polym Sci 16:1413–1419. https://doi.org/10.1016/0032-3950(74)90402-X
Ravindran C, Thangaiah Naveenan T (2011) Adaptation of marine derived fungus Chaetomium globosum (NIOCC 36) to alkaline stress using antioxidant properties. Process Biochem 46(4):847–857. https://doi.org/10.1016/j.procbio.2010.12.005
Ravindran C, Varatharajan GR, Karthikeyan A (2011) Role of alkaline-tolerant fungal cellulases in release of total antioxidants from agro-wastes under solid state fermentation. BioResources 6:31–42
Reddy PL, Sreeramulu A (2012) Isolation, identification and screening of pectinolytic fungi from different soil samples of Chittoor district. Int J Life Sci Pharma Rev 3:183–193
Rhee YJ, Hillier S, Gadd GM (2012) Lead transformation to pyromorphite by fungi. Curr Biol 22:237–241. https://doi.org/10.1016/j.cub.2011.12.017
Ruta LL, Kissen R, Nicolau I, Neagoe AD, Petrescu AJ, Bones AM, Farcasanu IC (2017) Heavy metal accumulation by Saccharomyces cerevisiae cells armed with metal binding hexapeptides targeted to the inner face of the plasma membrane. Appl Microbiol Biotechnol 101(14):5749–5763
Sargin I, Arslan G, Kaya M (2016) Microfungal spores (Ustilago maydis and U. digitariae) immobilized chitosan microcapsules for heavy metal removal. Carbohydr Polym 138:201–209. https://doi.org/10.1016/j.carbpol.2015.11.065
Shcheglov AL, Tsvetnova OB, Stolbova VV (2013) Bioindication of radioactive contamination of natural ecosystems. Soil Sci Bull 66:185–191. https://doi.org/10.3103/S0147687413040066
Shiomi N (2015) An assessment of the causes of lead pollution and the efficiency of bioremediation by plants and microorganisms. In: Shiomi N (ed) Advances in bioremediation of wastewater and polluted soil, p 282. https://doi.org/10.5772/59328
Skerfving S, Bergdahl IA (2014) Lead. In: Nordberg GF, Fowler BA, Nordberg M (eds) Handbook on the toxicology of metals, 4th edn, vol II. Academic Press, pp 911–967, ISBN 978-0-12-398293-3
Smith WH, Staskawicza BJ, Harkova RS (1978) Trace-metal pollutants and urban-tree leaf pathogens. Trans Br Mycol Soc 70:29–33. https://doi.org/10.1016/S0007-1536(78)80166-1
Sobolev D, Begonia MFT (2008) Effects of heavy metal contamination upon soil microbes: lead-induced changes in general and denitrifying microbial communities as evidenced by molecular markers. Int J Environ Res Public Health 5(5):450–456
Srivastava N, Srivastava M, Mishra PK, Gupta VK, Molina G, Rodriguez-Couto S, Manikanta A, Ramteke PW (2017) Applications of fungal cellulases in biofuel production: advances and limitations. Renew Sustain Energy Rev 82:2379–2386. https://doi.org/10.1016/jf.rser.2017.08.074
Tiedje JM, Hagedorn ML (1975) Degradation of alachlor by a soil fungus Chaetomium globosum. J Agric Food Chem 23:77–81. https://doi.org/10.1021/jf60197a029
van Long NN, Vasseur V, Coroller L, Dantigny P, Le Pense S, Weill A, Mounier J, Rigalma K (2017) Temperature, water activity and pH during conidia production affect the physiological state and germination time of Penicillium species. Int J Food Microbiol 241:151–160. https://doi.org/10.1016/j.ijfoodmicro.2016.10.022
von Arx JA, Guarro J, Figueras MJ (1986) The Ascomycete genus Chaetomium. Beih Nova Hedwigia 84:1–162
Warcup JH (1950) The Soil plate method for isolations of fungi from soil. Nature (London) 166:117–118
Xin S, Zeng Z, Zhou X, Luo W, Shi X, Wang Q, Deng H, Du Y (2016) Recyclable Saccharomyces cerevisiae loaded nanofibrous mats with sandwich structure constructing via bio-electrospraying for heavy metal removal. J Hazard Mater 324:365–372. https://doi.org/10.1016/j.jhazmat.2016.10.070
Zhdanova NN, Zakharchenko VA, Vasilevskaja AI, Pushkarev AV, Nakonechnaja LT, Artyshkova LV (1995) New approach to the revelation of micromycetes - bioindicators of soil pollution in Ukrainian woodlands. Mikologiya i Fitopatologiya 29:23–29
Zheng Y-K, Qiao X-G, Miao C-P, Liu K, Chen Y-W, Xu L-H, Zhao L-X (2016) Diversity, distribution and biotechnological potential of endophytic fungi. Ann Microbiol 66:529–542. https://doi.org/10.1007/s13213-015-1153-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Da Silva Júnior, F.M.R., Volcão, L.M., Hoscha, L.C. et al. Growth of the fungus Chaetomium aureum in the presence of lead: implications in bioremediation. Environ Earth Sci 77, 275 (2018). https://doi.org/10.1007/s12665-018-7447-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7447-x