Abstract
Corncob is an abundant agricultural residue containing high content of hemicellulose. In this paper, the hemicellulosic hydrolysate was prepared from the hydrolysis of corncob using the solid acid sulfated zirconia as a catalyst. According to response surface analysis experiments, the optimum conditions for preparing hemicellulosic hydrolysate catalyzed by sulfated zirconia were determined as follows: solid (sulfated zirconia)–solid (corncob) ratio was 0.33, solid (corncob)–liquid (water) ratio was 0.09, temperature was 153 °C, and time was 5.3 h. Under the optimized conditions, the soluble sugar concentration was 30.12 g/L with a yield of 033 g/g corncob. Subsequently, xylitol production from the resulting hemicellulosic hydrolysate was demonstrated by Candida tropicalis, and results showed that the yield of xylitol from the hemicellulosic hydrolysate could be significantly improved on a basis of decolorization and detoxification before fermentation. The maximum yield of xylitol from the hemicellulosic hydrolysate fermented by C. tropicalis was 0.76 g/g. This study provides a new attempt for xylitol production from the hemicellulosic hydrolysate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulose is the main component of crop residues which is considered as one of the most promising renewable biomass. For example, hemicellulose accounts for around 40% of corncob, which is an important residue in the corn processing industry [1]. Hemicellulose has been used in the production of pig feed, adhesives, and insulating materials [2, 3]. In particular, hemicellulose is usually hydrolyzed to hemicellulosic hydrolysate containing xylose, which is used as industrial raw materials [4].
At present, the methods for hydrolyzing hemicellulose into hemicellulosic hydrolysate include acid method, alkali method, and enzymatic method. Compared with the other methods, the hydrolysis rate of hemicellulose by acid method is as high as 80–90% and the cost is relatively low. Therefore, the acid method has been usually applied for hemicellulose hydrolysis because of its simple operation [5]. However, the acid method must consume a large amount of alkali to neutralize the hydrolysate.
Solid acid needs no neutralization and is easy to be separated and reused, which is an ideal green substitute for liquid acids. A solid acid carbon catalyst with high active site density generating from biochar was verified by alkali titration and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FT-IR) analysis, and it was used for hydrolysis of xylan. The highest conversion rate was observed to be 85% within 2 h [6]. Another nanoscale catalyst, solid acid SO42−/Fe2O3, has been proved to be capable of selectively hydrolyzing hemicellulose from wheat straw, with the highest conversion rate of 63.5% [7]. Xylitol has been widely used as a functional sweetener [8]. Due to the high production cost and the difficulty of purification, how to produce xylitol at low cost and high efficiency has always been a research focus. Hemicellulosic hydrolysate can be converted into xylitol by xylose reductase in microorganisms. In particular, many previous studies have shown that yeast can effectively convert hemicellulosic hydrolysate into xylitol [9]. Candida guilliermondii has been used to evaluate the production of xylitol from sorghum hemicellulosic hydrolysates, and the maximum xylitol yield was 0.35 g/g [10]. Furthermore, Debaryomyces hansenii which converted rapeseed straw hemicellulosic hydrolysate into xylitol was also investigated to obtain a xylitol yield of 0.45 g/g [11]. Furtherly, a Pichia stipitis YS-30 mutant fermented the stover hydrolysate into xylitol, reaching the maximum xylitol yield of 0.61 g/g [12]. In addition, the application of Candida tropicalis in the production of xylitol from xylose or hemicellulosic hydrolysate has been widely found in previous publications [1, 13,14,15].
In this paper, a process for producing hemicellulosic hydrolysate by hydrolyzing corncob with sulfated zirconia was proposed. The solid acid sulfated zirconia was prepared and characterized using FT-IR, XRD, and acidity determination. Response surface design was adopted to optimize the preparation of the hemicellulosic hydrolysate catalyzed by sulfated zirconia. Afterwards, hemicellulosic hydrolysate fermented by C. tropicalis was proved to be suitable for xylitol production. The influencing factors in the demonstration of xylitol production were investigated.
Materials and Methods
Materials
Corncob (cellulose 39.42%; hemicellulose 35.37%; lignin 13.25%) was purchased from Nantong, Jiangsu, China. ZrOCl2·8H2O was supplied by Aladdin Co. Ltd. All other reagents used were of analytical grade unless otherwise mentioned.
Preparation and Characterization of the Solid Acid Sulfated Zirconia
ZrOCl2·8H2O (100 g/L) solution was adjusted to pH 9.0 with ammonia solution and stirred for 30 min, then the mixture was filtrated and dried at 110 °C overnight. The filter cake was immersed in H2SO4 (0.5 mol/L) in a ratio of 15 mL/g for 1 h, then titrated and dried. Finally, the obtained sulfated zirconia was calcined at 600 °C for 3 h, and then cooled for use.
FT-IR Analysis of the Solid Acid Sulfated Zirconia
FT-IR analysis of solid acid was recorded on a Nicolet iS5 Fourier Transform Infrared Spectrometer (Thermo Fisher Scientific) using the KBr tableting method (solid acid:KBr, 1:100). The resolution is 4 cm−1, and the scanning range is 4000–500 cm−1.
XRD Analysis of the Solid Acid Sulfated Zirconia
XRD was recorded on an X’TRA X-ray Diffraction System (Swiss ARL Ltd.). The light source is a Cu-Kα target with a scan range of 15–90° and a scan rate of 5°/min.
Determination of the Total Acidity of the Solid Acid Sulfated Zirconia
A dried solid acid sulfated zirconia sample (0.2 g) was mixed with 100 mL of NaOH (10 mmol/L). After ultrasonic treatment for 30 min, the mixture was oscillated at a speed of 150 rpm for 24 h and the supernatant was collected by centrifugation. Finally, the supernatant was titrated with HCl (10 mmol/L) using phenolphthalein as indicators.
Optimization of the Preparation of the Hemicellulosic Hydrolysate from Corncob Catalyzed by Sulfated Zirconia via Response Surface Design
Corncob was ground 150-mesh powder firstly. The preparation of the hemicellulosic hydrolysate from corncob powder catalyzed by sulfated zirconia was generally as follows. According to a certain solid (sulfated zirconia)–solid (corncob) ratio and solid (corncob)–liquid (water) ratio, corn cob powder (10 g), and the obtained sulfated zirconia are added into a flask filled with deionized water. The reaction starts with stirring at a certain temperature. After the reaction, the precipitate was removed by filtration, and the amount of the soluble sugar in the hemicellulosic hydrolysate was measured. Based on Box–Behnken design, an experimental design including solid–liquid ratio, solid–solid ratio, temperature, and time factors was selected, as shown in Table 1.
Detoxification of the Hemicellulosic Hydrolysate
The detoxification process of the hemicellulosic hydrolysate with activated charcoal is as follows [16]. Activated charcoal was activated in HCl (0.4 mol/L) for 24 h, and then the activated charcoal was added to the hemicellulosic hydrolysate with a solid–liquid ratio of 5%. The mixture was stirred at 60 °C and 200 rpm for 30 min, and the solution was collected by filtration and stored at 4 °C for use.
The modified sodium sulfite method was also used to detoxify hemicellulosic hydrolysate according to the following steps [17]. CaCO3 was added into the hemicellulosic hydrolysates (pH 10) at 70 °C with agitation for 10 min. Then Na2SO3 was added to the mixture, and the pH was adjusted to pH 7.0 with H3PO4. After filtering the precipitate, the solution should be kept at 4 °C unless it is used.
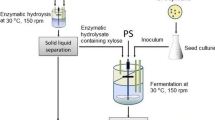
Xylitol Production from Hemicellulosic Hydrolysate by C. tropicalis
The activated C. tropicalis strains were selected to ferment the hemicellulosic hydrolysate into xylitol. The medium was composed of 60 g/L xylose in hemicellulose hydrolysate, 2.25 g/L yeast extract, and 3.75 g/L peptone. The biotransformation was conducted with 10% (by volume) inoculum at the initial pH 6.0 and 30 °C [18]. The rotation speed at 0–24 h was controlled at 210 r/min, and it was reduced to 180 r/min at 24 h to the end. Samples were analyzed every 4 h.
Determination of the Soluble Sugar, Xylose, and Xylitol
The soluble sugar assay was conducted according to anthrone–sulfuric acid method referred to reference [19]. Xylose and xylitol assay was employed as high-performance liquid chromatography (HPLC) method. The HPLC analysis was performed on a HPLC (Agilent1260, MA, USA) equipped with a ZORBAX column (4.6 × 150 mm, 5 μm) and RID detector. A solution of acetonitrile and deionized water with a ratio of 3:1 was used as a mobile phase and the flow rate is 2 mL/min.
Results and Discussion
FT-IR Analysis of Sulfated Zirconia
The FT-IR spectra (400–4000 cm−1) of ZrO2 and sulfated zirconia calcined at 600 °C are presented (Fig. 1). Most bands in the spectrum of sulfated zirconia are the same as those in the spectrum of ZrO2. The peaks, especially at 1070, 1155, and 1117 cm−1, are ascribed to the asymmetric and symmetric stretching vibrations of the bonds between oxygen and sulfur [20], which means the presence of sulfate.
XRD Pattern of Sulfated Zirconia
The XRD patterns of ZrO2 and sulfated zirconia are shown in Fig. 2. The monoclinic phase ZrO2 was obtained by calculating ZrO2 at 600 °C, and the results were consistent with the literature [21]. However, under the same conditions, a stable tetragonal sulfated zirconia was achieved. The resulting tetragonal sulfated zirconia was in agreement with the results that prepared using a similar method [22]. The reason may be that the sulfate additive to ZrO2 stabilizes the metastable phase of the precursor and inhibits the transition to monoclinic phase ZrO2 [23]. In another study, sulfated zirconia was prepared from ZrOCl2·8H2O and (NH4)2SO4, and results also showed the importance of sulfate in hindering the formation of monoclinic phase [24].
The Total Acidity of Sulfated Zirconia
The acidity of solid acid is related to the surface distribution of Brönsted and Lewis acid sites. The total acidity of the resulting sulfated zirconia measured by base titration is 2.72 mmol/g. Similar research has achieved a slightly lower total acidity of 1.83 mmol/g in a solid acid prepared by impregnating Zr(OH)4 with chlorosulfonic acid [25]. In addition, in the presence of acetyl trimethyl ammonium bromide, the total acidity of bifunctional solid catalyst prepared from zirconium ammonium carbonate complex and borax aqueous solution is close to 1.55 mmol/g [26].
Optimization of the Preparation of the Hemicellulosic Hydrolysate via Response Surface Design
Before response surface design, we carried out preliminary single factors experiments to determine the variable ranges of time, temperature, solid (corncob)–liquid (water), and solid (sulfated zirconia)–solid (corncob). Afterwards, the preparation of the hemicellulosic hydrolysate from corncob catalyzed by sulfated zirconia was optimized based on response surface design (Table 2).
The quadratic multi-fitting equation is deduced according to the design expert analysis (Eq. 1):
where A is the time; B is the temperature; C is the solid (corncob)–liquid (water) ratio; and D is the solid (sulfated zirconia)–solid (corncob) ratio.
In general, significant factors were ranked on the basis of the F-test of variance with a 95% confidence level. The larger F-value and P value (P > F) less than 0.05 show more significance of the term. The coefficient of determination (R2) is 0.9515, indicating that 95.15% variability of experimental data can be explained by the regression model (Table 3). The P value less than 0.0001 which means this model is very important. According to statistical analysis, A, B, A2, B2, and C2 are significant terms in this model.
The optimized reaction conditions obtained from response surface analysis were as follows: time 5.3 h, temperature 153 °C, solid (corncob)–liquid (water) ratio 0.09, and solid (sulfated zirconia)–solid (corncob) ratio 0.33. Under the optimized conditions, the soluble sugar concentration reached 30.12 g/L with a yield of 0.33 g/g corncob. Recently, a rice husk-based solid acid (RH-SO3H) has been prepared and applied to saccharified corncob, achieving the maximum reducing sugar yield of around 0.49 g/g at 160 °C for 3 h [27]. Another study showed a carbon-based solid (C-SO3H) acid catalyst can convert corncob into hemicellulosic hydrolysate with the calculated soluble sugar yield of 0.29 g/g at 140 °C for 6 h [28]. However, it should be noted that compared with the dilute acid method, solid acid method requires a higher temperature and longer time. For instance, hemicellulosic hydrolysate from corncob after diluting hydrochloric acid (2%, wt) hydrolysis at 100 °C for 2 h gave a calculated highest soluble sugar yield of approximately 0.35 g/g [29]. Hemicellulosic hydrolysate of kenaf stem has been prepared through dilute nitric-acid hydrolysis, and the maximum yield of xylose could reach around 0.31 g/g with 2% (v/v) HNO3 at 130 °C for 1 h [30].
Xylitol Production from Different Hemicellulosic Hydrolysate by C. tropicalis
Under acidic and heated conditions, xylose can be readily decomposed into furfural and hydroxymethylfurfural, and even further decomposed into formic acid and acetic acid [31]. These chemicals are toxic to microorganisms which can inhibit the production of xylitol. We conducted fermentations by C. tropicalis using the detoxified corncob hemicellulosic hydrolysates as substrates and the untreated hydrolysate as a control. However, a previous study verified the yeast gives the highest xylitol yield of 0.71 g/g from non-detoxified rice straw hydrolysate that had been prepared by the dilute acid pretreatment [32]. However, the detoxification of the corncob hemicellulosic hydrolysate is apparently necessary for the production of xylitol in our study (Fig. 3). Compared with the untreated hydrolysate (Fig. 3(a)), the yield of xylitol from corn cob hemicellulose hydrolysate was increased by 27.5% and 52.5% by activated charcoal detoxification (Fig. 3(b)) and sodium sulfite detoxification (Fig. 3(c)). In particular, for detoxification with activated charcoal and sodium sulfite coupled, the xylitol production from the hemicellulosic hydrolysate can be remarkably raised by 87.5% (Fig. 3(d)). Similar studies have shown that the yield of xylitol can be improved by detoxification of corncob hydrolysate [33] and sugarcane bagasse hemicellulosic hydrolysate [34]. As an example, after the sugarcane bagasse, hemicellulosic hydrolysate was treated by the combination of the over-liming method and activated charcoal adsorption method, the resulting hydrolysate was fermented by C. guilliermondii to achieve the highest xylitol yield of 0.79 g/g [35]. Another investigation showed the maximum xylitol production from activated charcoal detoxified sago trunk hydrolysate fermented by C. tropicalis was 0.78 g/g, which is around 2.5 times more than that of the untreated [36].
Optimization of Xylitol Production Conditions
Generally speaking, the optimum pH value for yeast growth is 4.0–6.0. Previous studies showed that pH value is one of the factors affecting xylitol production by yeast fermentation [37, 38]. Formic acid and acetic acid in the hemicellulosic hydrolysate would lower the pH value, further inhibiting yeast growth and reducing xylitol production (Fig. 4(a)). The possible cause of the toxicity of acetic acid is that acetic acid penetrates the cell membrane and dissociates in the cytoplasm, which leads to toxicity to the cell [39, 40]. As is shown in Fig. 4(a), the optimal initial pH value for xylitol production from the corncob hemicellulosic hydrolysate fermented by C. tropicalis is 6.0.
Inoculant plays an important role in fermentation. Low inoculation amount will prolong the fermentation time and decrease xylitol production. Higher inoculation amount results in too many nutrients for bacterial growth in the early stage of fermentation, which is not conducive to xylitol accumulation in the later stage. In order to determine the optimal inoculation amount, the seeds were added to the fermentation medium in the proportion of 1%, 5%, 10%, 15%, and 20%, respectively. Nevertheless, over-inoculation would lead to accelerated consumption of xylose and decreased xylitol production. The xylitol yield was the highest when the inoculation amount was 10% (Fig. 4(b)). The time course of xylitol production from the detoxified corncob hemicellulosic hydrolysate was investigated at initial pH 6.0 and inoculation amount 10%, and the yield of xylitol increased first and then decreased over time (Fig. 4(c)). The possible reason is that xylitol can be metabolized by cells to grow when sugars are used up. The optimized conditions for xylitol production from corncob hemicellulose hydrolysate are pH 6.0, inoculation amount 15%, and time 28 h. Under these conditions, the highest yield of xylitol was 0.76 g/g. Recently, a newly evolved C. maltosa CHH65 has also been used to ferment corncob hemicellulosic hydrolysate into xylitol, and its xylitol yield was about 0.73 g/g, which is close to our result [41]. Similar research shows hemicellulose hydrolysate of rapeseed straw was fermented into xylitol by Debaryomyces hansenii and C. guilliermondii, and the xylitol yields were 0.55 g/g and 0.45 g/g, respectively, which is significantly lower than that of this study [11]. However, when steam-exploded bagasse hydrolysate is used as a substrate for producing xylitol by C. tropicalis JA2, the yield of xylitol can reach 0.86 g/g [40].
Conclusion
Hemicellulosic hydrolysate was prepared directly from corncob with the self-made solid acid sulfated zirconia. The reaction conditions were optimized by response surface analysis. The optimized reaction conditions were as follows: temperature 153 °C, solid (corncob)–liquid (water) ratio 0.09, solid (sulfated zirconia)–solid (corncob) ratio 0.33, and time 5.3 h. Under these conditions, the soluble sugar concentration in the hemicellulosic hydrolysate was 30.12 g/L with a yield of 033 g/g corncob. Subsequently, the hemicellulosic hydrolysate was used to evaluate the possibility of xylitol production by C. tropicalis. Results indicated that detoxification before fermentation could significantly improve the xylitol production from the hemicellulosic hydrolysate. Finally, a yield of xylitol can reach 0.76 g/g by fermenting hemicellulosic hydrolysate with C. tropicalis. This study provides a new attempt to produce xylitol from hemicellulosic hydrolysate, making the value-added application of corncob possible.
Data Availability
All data are fully available without restriction.
References
Rao, R. S., Jyothi, C. P., Prakasham, R. S., Sarma, P. N., & Rao, L. V. (2006). Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresource Technology, 97(15), 1974–1978.
Yu, J., Li, Z., Ye, Q., Yang, Y., & Chen, S. (2010). Development of succinic acid production from corncob hydrolysate by Actinobacillus succinogenes. Journal of Industrial Microbiology & Biotechnology, 37(10), 1033–1040.
Cheng, K. K., Zhang, J. A., Chavez, E., & Li, J. P. (2010). Integrated production of xylitol and ethanol using corncob. Applied Microbiology and Biotechnology, 87(2), 411–417.
Xu, Y., & Hanna, M. A. (2010). Optimum conditions for dilute acid hydrolysis of hemicellulose in dried distillers grains with solubles. Industrial Crops and Products, 32(3), 511–517.
Yang, B., & Wyman, C. E. (2008). Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels, Bioproducts and Biorefining, 2(1), 26–40.
Ormsby, R., Kastner, J. R., & Miller, J. (2012). Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catalysis Today, 190(1), 89–97.
Zhong, C., Wang, C., Huang, F., Wang, F., Jia, H., Zhou, H., & Wei, P. (2015). Selective hydrolysis of hemicellulose from wheat straw by a nanoscale solid acid catalyst. Carbohydrate Polymers, 131, 384–391.
Weyda, I., Lübeck, M., Ahring, B. K., & Lübeck, P. S. (2014). Point mutation of the xylose reductase (XR) gene reduces xylitol accumulation and increases citric acid production in Aspergillus carbonarius. Journal of Industrial Microbiology & Biotechnology, 41(4), 733–739.
Rao, L. V., Goli, J. K., Gentela, J., & Koti, S. (2016). Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresource Technology, 213, 299–310.
Camargo, D., Sene, L., Variz, D. I. L. S., & Felipe, M. G. A. (2015). Xylitol bioproduction in hemicellulosic hydrolysate obtained from sorghum forage biomass. Applied Biochemistry and Biotechnology, 175(8), 3628–3642.
López-Linares, J. C., Romero, I., Cara, C., Castro, E., & Mussatto, S. I. (2018). Xylitol production by Debaryomyces hansenii and Candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresource Technology, 247, 736–743.
Rodrigues, R. C. L. B., Kenealy, W. R., & Jeffries, T. W. (2011). Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. Journal of Industrial Microbiology & Biotechnology, 38(10), 1649–1655.
Kim, T. B., & Oh, D. K. (2003). Xylitol production by Candida tropicalis in a chemically defined medium. Biotechnology Letters, 25(24), 2085–2088.
Jeon, Y. J., Shin, H. S., & Rogers, P. L. (2011). Xylitol production from a mutant strain of Candida tropicalis. Letters in Applied Microbiology, 53(1), 106–113.
Jia, H., Shao, T., Zhong, C., Li, H., Jiang, M., Zhou, H., & Wei, P. (2016). Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydrate Polymers, 151, 676–683.
Meinita, M. D. N., Hong, Y. K., & Jeong, G. K. (2012). Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess and Biosystems Engineering, 35(1–2), 93–98.
Alriksson, B., Cavka, A., & Jönsson, L. J. (2011). Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents. Bioresource Technology, 102(2), 1254–1263.
Chen, Y., Dong, B., Qin, W., & Xiao, D. (2010). Xylose and cellulose fractionation from corncob with three different strategies and separate fermentation of them to bioethanol. Bioresource Technology, 101(18), 6994–6999.
Zhang, J. K., Gao, R., Dou, D. Q., & Kang, T. G. (2013). The ginsenosides and carbohydrate profiles of ginseng cultivated under mountainous forest. Pharmacognosy Magazine, 9(Suppl 1), S38–S43.
Said, A. E. A. A., El-Wahab, M. M. A., & El-Aal, M. A. (2014). The catalytic performance of sulfated zirconia in the dehydration of methanol to dimethyl ether. Journal of Molecular Catalysis A: Chemical, 394, 40–47.
Liao, Y., Huang, X., Liao, X., & Shi, B. (2011). Preparation of fibrous sulfated zirconia (SO42−/ZrO2) solid acid catalyst using collagen fiber as the template and its application in esterification. Journal of Molecular Catalysis A: Chemical, 347(1-2), 46–51.
Zalewski, D. J., Alerasool, S., & Doolin, P. K. (1999). Characterization of catalytically active sulfated zirconia. Catalysis Today, 53(3), 419–432.
Mekhemer, G. A. H. (2006). Surface characterization of zirconia, holmium oxide/zirconia and sulfated zirconia catalysts. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 274(1-3), 211–218.
Sun, Y. Y., Ma, S. Q., Du, Y. C., Yuan, L., Wang, S. C., Yang, J., Deng, F., & Xiao, F. S. (2005). Solvent-free preparation of nanosized sulfated zirconia with Brønsted acidic sites from a simple calcination. The Journal of Physical Chemistry B, 109(7), 2567–2572.
Yan, H. P., Yang, Y., Tong, D. M., Xiang, X., & Hu, C. W. (2009). Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO42−/ZrO2 and SO42−/ZrO2-Al2O3 solid acid catalysts. Catalysis Communications, 10(11), 1558–1563.
Gupta, P., & Paul, S. (2014). Solid acids: green alternatives for acid catalysis. Catalysis Today, 236, 153–170.
Chen, N., Zhang, G., Zhang, P., Tao, X., Wu, Y., Wang, S., & Nabi, M. (2019). Rice husk-based solid acid for efficient hydrolysis and saccharification of corncob. Bioresource Technology, 292, 121915.
Qi, W., He, C., Wang, Q., Liu, S., Yu, Q., Wang, W., Leksawasdi, N., Wang, C. G., & Yuan, Z. H. (2018). Carbon-based solid acid pretreatment in corncob saccharification: specific xylose production and efficient enzymatic hydrolysis. ACS Sustainable Chemistry & Engineering, 6(3), 3640–3648.
Dominguez, J. M., Cao, N., Gong, C. S., & Tsao, G. T. (1997). Dilute acid hemicellulose hydrolysates from corn cobs for xylitol production by yeast. Bioresource Technology, 61(1), 85–90.
Shah, S. S. M., Luthfi, A. A. I., Low, K. O., Harun, S., Manaf, S. F. A., Illias, R. M., & Jahim, J. M. (2019). Preparation of kenaf stem hemicellulosic hydrolysate and its fermentability in microbial production of xylitol by Escherichia coli BL21. Scientific Reports, 9(1), 4080.
Jönsson, L. J., Alriksson, B., & Nilvebrant, N. O. (2013). Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnology for Biofuels, 6(1), 16.
Huang, C. F., Jiang, Y. F., Guo, G. L., & Hwang, W. S. (2011). Development of a yeast strain for xylitol production without hydrolysate detoxification as part of the integration of co-product generation within the lignocellulosic ethanol process. Bioresource Technology, 102(3), 3322–3329.
Kumar, V., Sandhu, P. P., Ahluwalia, V., Mishra, B. B., & Yadav, S. K. (2019). Improved upstream processing for detoxification and recovery of xylitol produced from corncob. Bioresource Technology, 291, 121931.
De Carvalho, W., Canilha, L., Mussatto, S. I., Dragone, G., Morales, M. L., & Solenzal, A. I. N. (2004). Detoxification of sugarcane bagasse hemicellulosic hydrolysate with ion-exchange resins for xylitol production by calcium alginate-entrapped cells. Journal of Chemical Technology & Biotechnology, 79(8), 863–868.
Alves, L. A., Felipe, M. G. A., Silva, J. B. A. E., Silvio, S. S., & Prata, A. M. R. (1998). Pretreatment of sugarcane bagasse hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Applied Biochemistry and Biotechnology, 72, 89–97.
Kamal, S. M. M., Mohamad, N. L., Abdullah, A. G. L., & Abdullah, N. (2011). Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production. Procedia Food Science, 1, 908–913.
Cao, N. J., Tang, R., Gong, C. S., & Chen, L. F. (1994). The effect of cell density on the production of xylitol from D-xylose by yeast. Applied Biochemistry and Biotechnology, 45–46(1), 515–519.
Sampaio, F. C., de Moraes, C. A., De Faveri, D., Perego, P., Converti, A., & Passos, F. M. L. (2006). Influence of temperature and pH on xylitol production from xylose by Debaryomyces hansenii UFV-170. Process Biochemistry, 41(3), 675–681.
Hahn-Hägerdal, B., Karhumaa, K., Fonseca, C., Spencer-Martins, I., & Gorwa-Grauslund, M. F. (2007). Towards industrial pentose-fermenting yeast strains. Applied Microbiology and Biotechnology, 74(5), 937–953.
Morais Junior, W. G., Pacheco, T. F., Trichez, D., Almeida, J. R. M., & Gonçalves, S. B. (2019). Xylitol production on sugarcane biomass hydrolysate by newly identified Candida tropicalis JA2 strain. Yeast., 36(5), 349–361.
Jiang, X., He, P., Qi, X., Lin, Y., Zhang, Y., & Wang, Q. (2016). High-efficient xylitol production by evolved Candida maltosa adapted to corncob hemicellulosic hydrolysate. Journal of Chemical Technology & Biotechnology, 91(12), 2994–2999.
Funding
The authors received financial support from NSFC (21676142), the National Key Research and Development Program of China (2018YFA0902200), Jiangsu Agricultural Science and Technology Innovation Fund Project (CX(19)2001), Qing Lan Project of Jiangsu Universities, Six Talent Peaks Project in Jiangsu Province, and the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
This study does not contain any individual person’s data.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 334 kb)
Rights and permissions
About this article
Cite this article
Wan, L., Gao, Z., Wu, B. et al. Hydrolysis of Corncob Hemicellulose by Solid Acid Sulfated Zirconia and Its Evaluation in Xylitol Production. Appl Biochem Biotechnol 193, 205–217 (2021). https://doi.org/10.1007/s12010-020-03412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03412-9