Abstract
Corncob as an abundant and low-cost waste resource has received increasing attention to produce value-added chemicals, it is rich in xylan and regarded as the most preferable feedstock for preparing high value added xylooligosaccharides. The use of xylooligosaccharides as core products can cut costs and improve the economic efficiency in biorefinery. In this study, maleic acid, as a non-toxic and edible acidic catalyst, was employed to pretreat corncob and produce xylooligosaccharides. Firstly, the response surface methodology experimental procedure was employed to maximize the yield of the xylooligosaccharides; a yield of 52.9% (w/v) was achieved with 0.5% maleic acid (w/v) at 155 °C for 26 min. In addition, maleic acid pretreatment was also beneficial to enhance the enzymatic hydrolysis efficiency, resulting in an enzymatic glucose yield of 85.4% (w/v) with a total of 10% solids loading. Finally, a total of 160 g of xylooligosaccharides and 275 g glucose could be produced from 1000 g corncob starting from the maleic acid pretreatment. Overall, a cascade processing for converting corncob to xylooligosaccharides and glucose by sequential maleic acid pretreatment and enzymatic hydrolysis was successfully designed for the corncob wastes utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biorefinery technologies provide routes to utilize lignocellulosic biomass, especially in the agricultural and forestry waste processing industries, for producing energy, bio-chemicals, and bio-fuels [1,2,3]. Corncob, as the byproduct of corn processing, is a suitable candidate for biorefinery because it is inexpensive and abundance [4]. In 2020, the world corn production was 885.3 million tons and China holds the second position with 260.7 million tons [5]. That means about 41.5–46.1 million tons of corncob was generated in China from corn processing industry. However, due to lack of policy support and poor management, a large amount of corncob was burned or discarded, resulting in serious environmental pollution and waste of resources. Therefore, it is urgent to develop an efficient approach for the efficient utilization of this renewable feedstock. The mainly components of corncob are cellulose (39–45%), hemicellulose (25–35%), and lignin (17–21%), and its ash and extractives content are very low [6, 7]. The efficient utilization and conversion of polysaccharide components from cellulose and hemicellulose plays a crucial role to develop an economically feasible biorefinery process of lignocellulosic biomass.
Cellulose can be transformed into glucose by pretreatment and enzymatic hydrolysis, and then glucose can be further converted to various fuels and organic acids via fermentation [8,9,10]. Unlike cellulose, hemicellulose is heteropolymer, including xylan, xyloglucan, arabinoxylan, glucomannan, and glucuronoxylan. The hemicellulose of corncob is mainly composed of xylan that can be transformed to xylose, furfural, furfuralcohol, xylitol, and xylooligosaccharides (XOS) [11,12,13]. Among these products, XOS are acting as emerging functional oligosaccharides, which show favorable effects on human intestinal flora by selectively promoting the growth of probiotic bacteria [14, 15]. The worldwide market size for XOS is increasing yearly, and it is predicted to increase to $ 130 million by 2025, which makes XOS become one of the most competitive products all over the world [16, 17]. Overall, the corncob biorefinery focused on XOS production can effectively boost the agricultural waste processing industry economy [18].
The first and crucial step of lignocellulosic biorefinery is pretreatment, including enzymatic, alkaline, and acidic treatment methods [9, 19, 20]. Enzymatic pretreatment is not associated with a pollution problem apart from its high cost, while alkaline is environmentally problematic despite its low cost. Thus, the prospect of industrialization is still some way off for both methods. In contrast, acid pretreatment with dual functions for producing XOS and boosting enzymatic hydrolysis is preferable in adding values to lignocellulose materials [20,21,22]. Acid pretreatment could be either mineral acid or organic acid, which both of them have been reported to be capable of hydrolyzing xylan to produce XOS [23]. However, the use of strong mineral acids always leads to an excessive xylan degradation to undesired by-products such as xylose and furfural, leading to a decrease in the yield of XOS. Comparatively, organic acids tend to be more favorable for XOS generation because of their considerable benefits, such as high yields of oligomers and lower amounts of degradation by-products [24].
Regardless of the type of acids used, their separation and recovery are inevitable steps to the production of XOS. However, using edible organic acids like acetic acid and gluconic acid, which could be co-prepared with XOS as feed additives, could reduce the production cost and water treatment cost [25, 26]. Although edible gluconic acid and acetic acid have been reported to achieve high XOS yields of over 50%, the usage of acid concentration over 10% resulted in a low XOS product purity when acid and XOS were simultaneously co-prepared [27, 28]. Maleic acid (MA) is also a type of edible organic acid that has been mainly used in pharmaceutical, and the market price of food food-grade MA with a purity of 95% is about $15/kg [29]. In addition, MA is a dicarboxylic and tiny molecule acid, which allow a low usage to achieve the same effect compared with mono-carboxylic acids, such as acetic acid and gluconic acid. Therefore, MA was used as an acid catalyst for the corncob pretreatment and XOS preparation in this study. To get the highest yield of XOS, the optimum values of the reaction temperature, MA concentration, and hydrolysis time were evaluated using response surface methodology (RSM) experimental design [30]. The interactive effects of the variables were also studied. Subsequently, the enzymatic hydrolysis of MA pretreated corncob solids was evaluated. We have designed a new and cascade biorefinery process for the co-production of XOS and glucose using MA as an acid catalyst.
Materials and Methods
Chemicals and Materials
Corncob was collected from fully mature maize, which provided by the local farmers in the city of Linyi, China. Harvested in October 2020, it was ground to 60–80 mesh and naturally air-dried to a constant moisture content of less than 10%. The air-dried corncob powder was used as feedstock to conduct the subsequent experiments. The chemical composition of corncob was determined by the National Renewable Energy Laboratory’s standard method, and it mainly contained 33.1% glucan, 31.8% xylan, 19.0% lignin, and 4.2% ash [31]. MA, cellulase (310U/g, Cellic CTec2, Novozymes), and the standards (glucose, xylose, and furfural) were purchased from Sigma-Aldrich (Shanghai, China). The standards of xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) were provided by Tokyo Chemical Industry (Japan) which was supplied by Novozymes A/S (Bagsvaerd, Denmark).
Maleic Acid Pretreatment
The reaction temperature (x1), hydrolysis time (x2), and MA concentration (x3), as the key factors for corncob hydrolysis, were investigated and optimized by the response surface method (RSM). The software Design-Expert (Version 11.0) was used for the experimental design and analysis. As shown in Table 1, a total of 13 experimental runs were performed at the reaction temperature ranging from 130 to 170 °C with 0.25 to 0.75% (w/v) MA concentration and hydrolysis time of 15 to 45 min.
The acidic hydrolysis of corncob was conducted in a 50-mL screw-top pressure-resistant steel reactor. Three grams of corncob powder and 30 mL of MA solution were mixed and stirred uniformly, and then, the reactor tube was sealed with caps and immersed in the oil bath while it reached the preset temperatures, and held varying durations accordingly. Once the reaction was finished, the reactor tube was immediately taken out and immersed into the cold water bath. The solution was centrifuged, the supernatant was collected for XOS determination, and the solid residues were used for the main components of glucan, xylan, and lignin analysis and enzymatic hydrolysis process.
Enzymatic Hydrolysis
The corncob residue pretreated with MA was first washed to neutrality by deionized water and then air-dried to a constant weight. The enzymatic hydrolysis assay was performed using a screw-capped 150-mL flask shaken at 50 °C and 150 rpm. The solids loading was 10% and cellulase loading was 20 FPIU/g glucan. Sodium citrate buffer was used for maintaining the pH at 4.8 (0.1 mol/L). After 72 h of the enzymatic hydrolysis process, the slurry was then centrifuged at 6,000×g for 5 min, and the supernatant was collected.
Response Surface Method Analysis
The yield of XOS was used as the evaluation index of the response surface method (RSM). A quadratic polynomial stepwise regression fitting was performed on the response values of the indexes through a quadratic polynomial model to obtain the functional relationship between the respective variables and the response values of XOS yield.
In the above model, \({\beta }_{0}\) was a constant term, while \({\beta }_{i}\), \({\beta }_{ii}\), and \({\beta }_{ij}\) were the coefficients of the first and quadratic terms, respectively. Y represented the response value of xylooligosaccharides yield. Analysis of variance was employed to test the significance of the RSM experiment.
Analytical Methods
Carbohydrates (xylose, arabinose, glucose, and cellobiose), MA, and furfural were determined with high performance liquid chromatograph (HPLC, Agilent 1260, USA) that was equipped with an Aminex Bio-Rad HPX-87 H column (Bio-Rad Laboratories, USA). Xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) were simultaneously detected with the high performance anion exchange chromatograph (HPAEC, Dionex ICS-5000, USA) coupled with a CarboPac™ PA200 column [32]. The yields of furfural, xylose, XOS, and enzymatic hydrolysis were calculated using the following equations:
Results and Discussion
Hydrolysis of Corncob by Maleic Acid
During the acidic hydrolysis process, hydronium ions (H+) can be released from MA and help to break the glycosidic linkages in the interiors and/or exteriors of xylan, which result in the generation of XOS. A higher temperature and acid concentration can effectively improve the activity of molecules and collisions, increasing the reaction rate [30]. In addition, the reaction time should be controlled for obtaining desired products. Therefore, reaction temperature, acid concentration, and reaction time were investigated in this study, and the effects of these three factors on the degradation of corncob to produce XOS were designed by RSM, using the minimum set of runs. The MA concentrations of 0.25 to 0.75% (w/v), hydrolysis time of 15 to 45 min, and reaction temperatures of 130 to 170 °C were selected for evaluating hydrolysis processes of xylan and XOS generation. The experimental designs with 13 runs and the XOS yield are presented in Table 1.
Figure 1 shows the yields of xylose, X2, X3, X4, X5, X6, and furfural from the RSM designed conditions. As depicted in Fig. 1a, when the reaction was conducted at 130 °C, the XOS yield (the sum of the yields of X2, X3, X4, X5, and X6) increased from 0.8% (0.25% MA, 30 min) to 18.7% (0.75% MA, 30 min) and 1.5% (0.5% MA, 15 min) to 14.1% (0.5% MA, 45 min). Although increased MA concentration and hydrolysis time could slightly increase the reaction rates at the low reaction temperature, the yields of xylose and XOS are still very low. Apparently, increase in the reaction temperature could significantly boost the reaction rates, a XOS yield of 52.4% could be achieved at a temperature of 150 °C with 0.5% MA and 30 min duration, which is shown in Fig. 2b. However, in the case of high reaction temperature, the XOS yield would drop sharply with the increasing MA concentration and hydrolysis time. As shown in Fig. 1c, the XOS yield decreased from 28.1% (0.25% MA, 30 min) to 11.6% (0.75% MA, 30 min) and 42.5% (0.5% MA, 15 min) to 8.7% (0.5% MA, 45 min), while the furfural and xylose yields were drastically increased. On the other hand, lower amounts of X5 and X6 but relatively higher amounts of X2 and X3 were obtained.
The results indicated that reaction temperature, hydrolysis time, and MA concentration significantly influenced the XOS yields. For the key goal of higher XOS, reactions at low temperatures require prolonged hydrolysis time and more acid dosage, which is economically unfavorable, but the excessive temperatures would result in XOS degradation and a large amount of xylose accumulation. Taken above, it suggests that the higher levels of reaction temperature with short reaction duration is favored in the formation of XOS and lower by-roducts.
Fitting Models
Design Expert software was employed to fit all polynomial models based on the experimental results, which were summarized in Table 1. The data obtained were fitted to a second order polynomial equation. The response values (yields of XOS) were calculated according to the following equation:
The coefficient of determination (R2 = 0.9644) and adjusted coefficient of determination (Adj. R2 = 0.8574) indicated the feasibility of the fitted model for experimental results and predicted values. Values of Adj. R2 and Adj. R2 greater than 0.80 show the goodness of fit of the regression equation [33]. In addition, the Adeq Precision value of 10.634 (> 4) also suggested that the obtained model by RSM analysis was reasonable. Overall, all data indicated that the model had a good correlation.
The results of the analysis of variance (ANOVA) of the model were given in Table 2. The F-value of 9.02 and the P-value of 0.0484 (< 0.05) suggested that the model was significant. And it could be seen that the P-values of x1, x1 × 2, x12, x22, and x32 were all less than 0.05, indicating all significantly affected the XOS yield. Smaller P-values represent higher significance; therefore, the order of significance of each factor on XOS yields was MA concentration, hydrolysis time, reaction temperature.
The 3D response surface and contour plots, graphical representations of a regression equation, can reveal the interactive effects of two factors on XOS yields. Figure 2 represents the interaction effect between two independent variables. The results indicated that slight changes in the reaction temperature, hydrolysis time, and MA concentration significantly affected the XOS yield. Furthermore, a higher amount of each independent variable led to accelerated degradation of the corncob. As depicted in Fig. 2a, a maximal contour (XOS yield 50%) could be determined under certain conditions (reaction temperature range from 150 to approximately 160 °C and hydrolysis time range from 21 to approximately 33 min), but further increase in the reaction temperature or hydrolysis time would not improve the XOS yield. Additionally, similar phenomena were observed in Fig. 2b and c, indicating the interactive effects of reaction temperature vs. MA concentration and hydrolysis time vs. MA concentration on XOS yield, respectively.
In the case of lower temperature, higher MA concentration or hydrolysis time was required to improve the XOS yield; however, a consistent increase in the MA concentration or hydrolysis time did not continuously improve the XOS yield. On the other hand, excessive temperature and hydrolysis time led to XOS degradation and increased xylose yield. These results indicated that these parameters should be controlled to achieve the expected XOS yield. Overall, the values of x1, x2, and x3 were in the ranges of 150~160 °C, 21~33 min, and 0.45~0.55%, respectively, where the yields of XOS were achieved at approximately 50%.
The 3D response surface plots with convex shapes can suggest the optimum condition for the highest response. In the fitted model, the optimal condition for obtaining the highest XOS yield was 155 °C reaction temperature, 26 min hydrolysis time, and 0.5% MA concentration. The experiment was conducted in triplicate based on the predicted optimal condition. The mean value of the highest XOS yield was 52.9%, with a total amount of 16.8 g/L, which was in agreement with the predicted value (53.8%). Additionally, the X2, X3, X4, X5, and X6 contents in hydrolysate from xylan hydrolysis at optimized conditions were 5.34, 3.69, 3.26, 2.48, and 2.01 g/L, respectively. The experimental result of XOS yield was consistent with the predicted result from the fitted model, suggesting the accuracy of the established regression equation.
Evaluation of Enzymatic Hydrolysis Efficiency for Pretreated Corncob
According to previous reports, acid pretreatment can effectively improve the efficiency of the enzymatic hydrolysis by increasing cellulose accessibility to cellulases [9, 34]. In the case of the optimum condition (0.5% MA at 155 °C for 26 min) that yielded the maximum XOS, the pretreated residue of corncob was found to contain 56.1% glucan, 13.7% xylan, and 24.8% lignin. MA pre-hydrolysis process for XOS production also simultaneously changed the composition of the corncob that featured an ample quantity of etched and rougher surface. Most of the cellulose and lignin were preserved in the solid residues after MA pretreatment. Generally, solids loading dosage is a crucial factor for enzymatic hydrolysis, and an increase in solids loading can decrease water/energy consumption and the cost of downstream processing [35]. Thus, to study the influence of solids loading on enzymatic hydrolysis, 5, 10, and 15% (w/v) of solids loadings were subjected to enzymatic hydrolysis process.
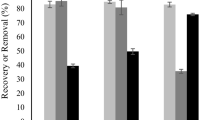
The results of enzymatic hydrolysis with different solids loading indicated that solids loading significantly influenced the efficiency of enzymatic hydrolysis (Fig. 3). Although the final glucose content increased with increasing solids loading, a final content of 73.1 g/L glucose could only be achieved with 15% solids loading and the yield was only 79.1%. Relatively higher enzymatic hydrolysis yields of 87.5% and 85.4% were obtained from that of 5% and 10% solids loadings, respectively. The decrease in enzymatic hydrolysis yield may be caused by the lack of free water in the high solids loading enzymatic system, which also resulted in difficulties in mass transfer [36]. In summary, 10% solids loading is suitable for enzymatic hydrolysis of MA pretreated corncob solids to obtain glucose with a high yield. In addition, it was noted that a small quantity of xylose would be simultaneously released into hydrolysate during the process of enzymatic hydrolysis, and the content ratio of glucose and xylose was 4:1. Thus, as a result starting from 1 kg corncob, approximately 160 g of XOS (mainly X2, X3, and X4) was obtained in pre-hydrolysate by MA pretreatment; and 63 g xylose and 275 g glucose were simultaneously harvested by subsequently enzymatic hydrolysis with 10% solids loading. These results clearly indicated that pretreatment of corncob with MA for co-production of glucose and XOS was a profitable option, which could maximize the economic value of corncob.
Conclusions
A response surface methodology of pretreating corncob using edible MA to co-produce XOS and glucose as high value products was successfully implemented to determine optimal values of MA concentration, temperature, and time. The maximum yield of XOS (52.9%) was achieved with 0.5% MA at 155 °C for 26 min; meanwhile, the enzymatic hydrolysis yield of the retained solids residue was 87.5%. MA pretreatment provides an economical and environmentally friendly method for processing the available lignocellulosic biomass.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Boonchuay, P., Techapun, C., Leksawasdi, N., Seesuriyachan, P., Hanmoungjai, P., Watanabe, M., & Chaiyaso, T. (2018). An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresource Technology, 256, 399–407.
Chen, X., Li, Z., Zhang, L., Wang, H., Qiu, C., Fan, X., & Sun, S. (2021). Preparation of a novel lignin-based film with high solid content and its physicochemical characteristics. Industrial Crops and Products, 164, 113396.
Li, M., Zhang, Q., Luo, B., Chen, C., Wang, S., & Min, D. (2020). Lignin-based carbon solid acid catalyst prepared for selectively converting fructose to 5-hydroxymethylfurfural. Industrial Crops and Products, 145, 111920.
Cai, X., Hu, C. H., Wang, J., Zeng, X. H., & Zheng, Y. G. (2021). Efficient high-solids enzymatic hydrolysis of corncobs by an acidic pretreatment and a fed-batch feeding mode. Bioresource Technol, 326, 124768.
Arumugam, N., Boobalan, T., Pugazhendhi, A., Arun, A., & Kavitha, T. (2021). Particle size influence on the composition of sugars in corncob hemicellulose hydrolysate for xylose fermentation by Meyerozyma caribbica. Bioresource Technol, 340, 125677.
Liao, H., Li, X., Lian, Z., Xu, Y., & Zhang, J. (2021). Two-step acetic acid/sodium acetate and xylanase hydrolysis for xylooligosaccharides production from corncob. Bioresource Technol, 342, 125979.
Ni, J., Di, J., Ma, C., & He, Y. (2021). Valorisation of corncob into furfuryl alcohol and furoic acid via chemoenzymatic cascade catalysis. Bioresources and Bioprocessing, 8, 113.
Ríos-González, L. J., Medina-Morales, M. A., Rodríguez-De La Garza, J. A., Romero-Galarza, A., Medina, D. D., & Morales-Martínez, T. K. (2021). Comparison of dilute acid pretreatment of agave assisted by microwave versus ultrasound to enhance enzymatic hydrolysis. Bioresource Technol, 319, 124099.
Wei, W., Wang, B., Wang, X., Ling, R., & Jin, Y. (2021). Comparison of acid and alkali catalyzed ethylene glycol organosolv pretreatment for sugar production from bagasse. Bioresource Technol, 320, 124293.
Himmel, M. E., Ding, S., Johnson, D. K., Adney, W. S., Nimlos, M. R., Brady, J. W., & Foust, T. D. (2007). Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science, 315(5813), 804–807.
Scopel, E., & Rezende, C. A. (2021). Biorefinery on-demand: Modulating pretreatments to recover lignin, hemicellulose, and extractives as co-products during ethanol production. Industrial Crops and Products, 163, 113336.
Jayapal, N., Samanta, A. K., Kolte, A. P., Senani, S., Sridhar, M., Suresh, K. P., & Sampath, K. T. (2013). Value addition to sugarcane bagasse: Xylan extraction and its process optimization for xylooligosaccharides production. Industrial Crops and Products, 42, 14–24.
Xue, X., Ma, C., Di, J., Huo, X., & He, Y. (2018). One-pot chemo-enzymatic conversion of D-xylose to furfuralcohol by sequential dehydration with oxalic acid plus tin-based solid acid and bioreduction with whole-cells. Bioresource Technol, 268, 292–299.
Lian, Z., Wang, Y., Luo, J., Lai, C., Yong, Q., & Yu, S. (2020). An integrated process to produce prebiotic xylooligosaccharides by autohydrolysis, nanofiltration and endo-xylanase from alkali-extracted xylan. Bioresource Technol, 314, 123685.
Mhetras, N., Mapre, V., & Gokhale, D. (2019). Xylooligosaccharides (XOS) as emerging prebiotics: Its production from lignocellulosic material. Advances in Microbiology, 09(01), 14–20.
Santibáez, L., Henríquez, C., Corro-Tejeda, R., Bernal, S., Armijo, B., & Salazar, O. (2020). Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydrate Polymers, 251, 117118.
Singh, R. D., Nadar, C. G., Muir, J., & Arora, A. (2019). Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. Journal Of Cleaner Production, 241, 118237.
Huang, C., Wang, X., Liang, C., Jiang, X., Yang, G., Xu, J., & Yong, Q. (2019). A sustainable process for procuring biologically active fractions of high-purity xylooligosaccharides and water-soluble lignin from Moso bamboo prehydrolyzate. Biotechnology For Biofuels, 12(1), 189.
Wu, M., Gong, L., Ma, C., & He, Y. (2021). Enhanced enzymatic saccharification of sorghum straw by effective delignification via combined pretreatment with alkali extraction and deep eutectic solvent soaking. Bioresource Technol, 340, 125695.
Ling, Z., Guo, Z., Huang, C., Yao, L., & Xu, F. (2020). Deconstruction of oriented crystalline cellulose by novel levulinic acid based deep eutectic solvents pretreatment for improved enzymatic accessibility. Bioresource Technology, 305, 123025.
Cesaro, A., Conte, A., Carrère, H., Trably, E., Paillet, F., & Belgiorno, V. (2020). Formic acid pretreatment for enhanced production of bioenergy and biochemicals from organic solid waste. Biomass and Bioenergy, 133, 105455.
Yang, Z., Wu, D., Chen, C., Cheong, K., Deng, Y., Chen, L., & Li, S. (2016). Preparation of xylooligosaccharides from xylan by controlled acid hydrolysis and fast protein liquid chromatography coupled with refractive index detection. Separation and Purification Technology, 171, 151–156.
Zhou, X., & Xu, Y. (2019). Eco-friendly consolidated process for co-production of xylooligosaccharides and fermentable sugars using self-providing xylonic acid as key pretreatment catalyst. Biotechnology for Biofuels, 12, 272.
Forsan, C. F., Paz Cedeño, F. R., Masarin, F., & Brienzo, M. (2021). Xylooligosaccharides production by optimized autohydrolysis, sulfuric and acetic acid hydrolysis for minimum sugar degradation production. Bioactive Carbohydrates and Dietary Fibre, 26, 100268.
Moure, A., Gullón, P., Domínguez, H., & Parajó, J. C. (2006). Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochemistry, 41(9), 1913–1923.
Guo, J., Zhao, J., Nawaz, A., Haq, I. U., Chang, W., & Xu, Y. (2021). In situ chemical locking of acetates during xylo-oligosaccharide preparation by lignocellulose acidolysis. Applied Biochemistry and Biotechnology, 193(8), 2602–2615.
Han, J., Cao, R., Zhou, X., & Xu, Y. (2020). An integrated biorefinery process for adding values to corncob in co-production of xylooligosaccharides and glucose starting from pretreatment with gluconic acid. Bioresource Technology, 307, 123200.
Zhang, H., Xu, Y., & Yu, S. (2017). Co-production of functional xylooligosaccharides and fermentable sugars from corncob with effective acetic acid prehydrolysis. Bioresource Technology, 234, 343–349.
Bian, H., Luo, J., Wang, R., Xuelian, Z., & Dai, H. (2019). Recyclable and reusable maleic acid for efficient production of cellulose nanofibrils with stable performance. ACS Sustainable Chemistry & Engineering, 7(24), 20022–20031.
Bian, J., Peng, P., Peng, F., Xiao, X., Xu, F., & Sun, R. C. (2014). Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food Chemistry, 156, 7–13.
Sluiter, J. B., Ruiz, R. O., Scarlata, C. J., Sluiter, A. D., & Templeton, D. W. (2010). Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. Journal of Agricultural and Food Chemistry, 58(16), 9043–9053.
Xu, Y., Fan, L., Wang, X., Yong, Q., & Yu, S. Y. (2013). Simultaneous separation and quantification of linear xylo- and cello-oligosaccharides mixtures in lignocellulosics processing products on high-performance anion-exchange chromatography coupled with pulsed amperometric detection. Bioresources, 8(3), 3247–3259.
Nath, A., & Chattopadhyay, P. K. (2007). Optimization of oven toasting for improving crispness and other quality attributes of ready to eat potato-soy snack using response surface methodology. Journal Of Food Engineering, 80(4), 1282–1292.
Yang, B., & Wyman, C. E. (2010). Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioproducts & Biorefining, 2(1), 26–40.
Martins, L. H. D. S., Rabelo, S. C., & Costa, A. C. D. (2015). Effects of the pretreatment method on high solids enzymatic hydrolysis and ethanol fermentation of the cellulosic fraction of sugarcane bagasse. Bioresource Technology, 191, 312–321.
Cheng, M., Kadhum, H. J., Murthy, G. S., Dien, B. S., & Singh, V. (2020). High solids loading biorefinery for the production of cellulosic sugars from bioenergy sorghum. Bioresource Technology, 318, 124051.
Acknowledgements
The authors acknowledge the support of the Advanced Analysis and Testing Center of Nanjing Forestry University.
Funding
The research was supported by the National Natural Science Foundation of China (32171730).
Author information
Authors and Affiliations
Contributions
Zhina Lian performed the experiments, analyzed the data. Qibo Zhang prepared the draft manuscript. Kankan Jiang and Xin Zhou conceived, designed the study and modified the manuscript. Yong Xu reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
The authors declare that they consent to participate.
Consent for Publication
The authors declare that they consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lian, Z., Zhang, Q., Xu, Y. et al. Biorefinery Cascade Processing for Converting Corncob to Xylooligosaccharides and Glucose by Maleic Acid Pretreatment. Appl Biochem Biotechnol 194, 4946–4958 (2022). https://doi.org/10.1007/s12010-022-03985-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03985-7