Abstract
Xylan is a renewable polysaccharide, readily available in agricultural and forestry residues. It can be hydrolyzed to produce xylooligosaccharides (XOS) with prebiotic activity and xylose, a precursor for several industrial chemicals. Enzymatic hydrolysis of xylan in the lignocellulosic biomass to obtain xylose and XOS requires a pretreatment to facilitate xylanase activity. In this study, organosolv was evaluated for the delignification of corncob while retaining xylan in the pretreated biomass. The treatment at 170 °C for 1 h with 70% ethanol provided 50% lignin removal and 81% xylan recovery. Increasing temperatures and decreasing ethanol fractions decreased the pH and the xylan recovery. Loss of xylan in the organosolv at 190 °C and in the liquid hot water treatment could be prevented by the addition of 100 mM MgO, without compromising lignin removal. Pretreated corncob was suspended in citrate buffer and hydrolyzed by commercial xylanases. Accellerase XY (250 U/ml) at pH 5.5 and 55 °C and Econase XT (0.6 U/ml) at pH 6.0 and 70 °C provided around 65% xylan digestibility and generated xylose (9.8 g/l) and XOS (10.9 g/l), respectively. This approach could decrease xylan loss and degradation in the pretreatment step and yield clear hydrolysates composed of essentially xylose or XOS. Lignocellulosic biorefineries can benefit from the efficient utilization of xylan, increasing sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The biorefinery approach is based on the utilization of biomass to obtain multiple value-added products, such as biofuels, biomaterials, industrial chemicals, food/feed, and other bioproducts, analogous to a petroleum refinery. Lignocellulosic biomass (LCB) contributes to this approach since it is composed mainly of polysaccharides cellulose and hemicellulose and aromatic polymer lignin. LCB includes agricultural residues such as stalk, straw, bagasse, stover, husk, and cob; some food industry wastes such as peel, pomace, and shell; and forestry residues [1]. Compared to first-generation feedstocks like corn and sugar cane/beet, which also have food/feed uses, residual LCB has the potential to develop a more sustainable industry for bio-based products [2]. Various materials, chemicals, and biofuels can be manufactured by the efficient utilization of LCB through thermochemical and biochemical routes [3]. The biochemical approach involves the application of hydrolytic enzymes and microorganisms on carbohydrates derived from LCB to synthesize bioproducts. However, the enzymes generally cannot show activity on the native LCB. The strong network of cellulose, hemicellulose, and lignin in the plant cell wall provides recalcitrance against enzymatic attack. Various physical, chemical, physicochemical, and biological pretreatments are available to overcome the resistance of the lignocellulosic network. These can alter the composition and micro and macro structures of LCB and promote the enzyme attack on the substrates, such as cellulose and hemicellulose [4, 5].
Cellulose constitutes the largest portion of LCB and is very abundant in nature [1]; therefore, most of the LCB-related research has focused on cellulose hydrolysis and utilization. Many bio-based products can be produced by fermentation using glucose derived from cellulose by enzymatic hydrolysis [6]. Lignin and hemicellulose have been considered mostly barriers to cellulolytic enzymes [7]. Hemicellulose is the other carbohydrate polymer (a heteropolymer) commonly found in the LCB. Xylans are the most abundant type of hemicellulose widely found in hardwoods and herbaceous plants [8]. Although xylan structure and components vary in plants, the most common component is the xylose linked with β-1,4 bonds. Xylose chains can carry substitutions, such as arabinose (some are feruloylated), 4-O-methyl glucuronic acid, and acetyl groups. Industrial microorganisms generally cannot assimilate xylose to grow, or the xylose assimilation is repressed by glucose present in the medium [9]. These prevent efficient utilization of xylose in microbial processes. Therefore, xylose and xylan are overlooked in many studies and discarded after pretreatments before cellulose hydrolysis [10]. Still, there have been many attempts to find xylose-assimilating microorganisms or genetically manipulate the organisms to add xylose assimilation capability [10]. The significant chemicals that can be produced from xylose by wild-type or improved microorganisms include ethanol [11], lactic acid [12], succinic acid [13], and others [10]. Xylitol can be produced from xylose microbially [14] or chemically synthesized by hydrogenation of xylose [15]. Dehydration of xylose yields furfural, which is a platform chemical and finds several uses in the chemical industry [16]. Xylan and xylooligosaccharides (XOS) obtained by partial hydrolysis of xylan show prebiotic activity, which is the proliferation of some beneficial bacteria in the colon of the host, improving health [17].
The most common method to extract xylan from LCB is alkaline extraction, which cleaves the ester linkages between hemicellulose and lignin and solubilizes both components to some extent. It involves treatment with a concentrated alkaline solution, neutralization with concentrated acids, and precipitation of xylan with alcohol [18]. Organic solvents, hot water, microwave irradiation, ultrasonication, steam explosion, and supercritical CO2 are among the other methods proposed for the extraction of xylan [16, 18, 19]. In most of the conventional treatments used in LCB biorefineries, xylan is hydrolyzed into xylose, XOS, and its substitutions, such as acetic acid and arabinose. For example, in dilute acid, steam explosion, and hot water treatments, xylan is solubilized and hydrolyzed due to high temperatures and low pH values (because of added or spontaneously formed acids) [10]. Under severe treatment conditions, the monosaccharides formed may be further converted into degradation products, such as furfural, formic acid, acetic acid, and others [20, 21], which may be toxic to the organisms. Therefore, xylan, either in polymeric or smaller forms, is in a mixture composed of LCB-derived compounds. This increases the complexity and cost of the purification steps applied to obtain high-purity xylan and its hydrolysis products or the products derived from those.
An alternative approach is the enzymatic hydrolysis of xylan in the LCB without extracting it from the solid biomass. However, the enzymes are unable to act on the native LCB, thus a pretreatment that does not solubilize the xylan, but decreases the recalcitrance of the lignocellulosic network is required for this approach. Organosolv, which uses organic solvents for the delignification of LCB [22], can serve this purpose. Generally, it is applied to dissolve lignin and hemicellulose simultaneously to decrease the recalcitrance of LCB, thus increasing the digestibility of cellulose in post-enzymatic hydrolysis [23]. Ethanol is the most common solvent in organosolv treatments, and it is generally used in a mixture of water. An acid catalyst may be added to improve the treatment effectiveness [24]. Even without the acids, the xylan is partly solubilized and hydrolyzed during the organosolv treatment because of the hydronium derived from water and acetic acid from xylan, as occurs in other high-temperature treatments, such as dilute acid hydrolysis, liquid hot water treatment, and steam explosion [24]. Enzymatic hydrolysis of xylan in the pretreated LCB was tested in some previous studies. Gong et al. [25] treated corn stover with alkaline organosolv (5% NaOH in methanol) at 80 °C, which retained most of the xylan as well as cellulose and removed lignin. Hydrolysis of the pretreated biomass at a high solid loading with a commercial enzyme complex (Cellic Ctec2 (Novozymes, Denmark)) yielded a mixture of monosaccharides and oligosaccharides. Aqueous ammonia was used to delignify corn stover and cob, which were then hydrolyzed by xylanases into XOS and xylose with a digestibility of around 69% [26]. Li et al. [27] showed that adding 80 mM MgO (a Lewis base catalyst) to aqueous ethanol (50%) in the organosolv treatment could recover 71% of the xylan in the corn stover, in addition to the 89% of the cellulose. The authors hydrolyzed the pretreated biomass with Accellerase 1500 (DuPont, USA), which is an enzyme complex with exo- and endoglucanase, β-glucosidase, and hemicellulase activities, and observed 78% glucose and 41% xylose yields. Li et al. [28] applied an aqueous solution of MgO (100 mM) to corn stover at 190 °C for 40 min and could recover 42% of the xylan, which was twice the amount obtained without MgO addition (liquid hot water (LHW) treatment). This provided a 13% increase in xylose yield without affecting the glucose yield in the enzymatic hydrolysis using Accellerase 1500 enzyme complex. (Note that the last two papers ([27, 28]) were retracted due to some flaws in the experimental method.)
To date, organosolv has typically been applied to decrease the recalcitrance of the lignocellulose to obtain glucose using cellulolytic enzymes, which release some xylose as well due to the xylanolytic enzymes present in the enzyme cocktail. On the other hand, when the xylan in the organosolv-treated biomass is hydrolyzed by xylanases, it may be possible to obtain pure xylose and XOS solutions. In this study, this was explored by application of organosolv to the corncob aiming to increase xylan digestibility and obtain xylose and XOS solutions with no cellulose-derived mono or oligosaccharides. Organosolv conditions were adjusted to maximize xylan recovery and lignin removal, and selected pretreated corncob samples were hydrolyzed by two commercial xylanase enzymes.
2 Materials and methods
2.1 Materials
Ground corncob (particle size < 2 mm) was provided kindly by Dr. Hüseyin Özpınar from Aegean Agricultural Research Center in İzmir, Turkey. The commercial enzymes, namely Accellerase XY and Econase XT, were gifts from Dupont (USA) and AB Enzymes (Germany), respectively. According to the manufacturers, the Accellerase XY hemicellulose complex was designed to aid cellulase for the saccharification of LCB, while Econase XT was an endo-xylanase and used to convert xylan in animal feed into XOS. Both enzymes were produced with genetically modified strains of Trichoderma reesei. The xylan from beechwood and xylooligosaccharide standards (xylobiose, xylotriose, xylotetraose, xylopentaose, and xylohexaose) were purchased from Megazyme (Ireland). All other chemicals were of analytical grade and purchased from Merck (Germany).
2.2 Organosolv treatment
The organosolv treatment was performed in a 500 ml stainless-steel high-pressure reactor (Berghof BR-500, Germany). The treatment conditions were selected based on prior reports on organosolv treatment [29]. Dry corncob powder was mixed with a water–ethanol mixture at a liquid-to-solid ratio of 10 ml/g (250 ml solvent and 25 g biomass). The mixture was heated to set temperatures (150 °C, 170 °C, and 190 °C) with continuous stirring at 300 rpm. After holding the reactor at the set temperature for 1 h, the reactor was cooled to 60 °C in 20–25 min with tap water circulating in the cooling coil. The liquid and solid phases were separated by filtration through cheesecloth under a vacuum. The pH values of the liquids were recorded. The solids were washed with water and dried at 45 °C overnight. The dry weights of the biomass were measured. The cellulose and xylan recoveries and lignin removal were calculated using Eq. 1, Eq. 2, and Eq. 3, respectively.
2.3 Enzymatic hydrolysis
The pretreated corncobs were hydrolyzed with commercial xylanases. Accellerase XY was in liquid form and used as received. Solid Econase XT was mixed with citrate buffer at a concentration of 4% (w/v) to obtain an enzyme solution. The suspended solids were removed by centrifugation at 5000 g for 4 min at 4 °C (Centurion K241R, UK), and the supernatant was used in the hydrolyses.
The xylanase activities of the enzymes were determined by measuring the reducing sugars released upon incubating the enzymes with xylan [30]. Xylan from beechwood was used as the substrate (0.5% (w/v)) in 50 mM citrate buffer at pH 5.5. The diluted enzyme solution (100 μl) was mixed with 900 µl substrate, and the mixture was incubated in a water bath at 50 °C for 5 min. After incubation, 1500 µl DNS solution was added, and the sample was kept in a boiling water bath for 5 min, which developed the color and stopped the enzyme activity. The tubes were cooled in ice-cold water for 1 min before analysis. The amount of reducing sugars released was determined by measuring the absorbance at 540 nm. One unit of xylanase activity was defined as the amount of enzyme required to release 1 μmol of xylose equivalent per min under the assay conditions (pH 5.5 and 50 °C). The calibration curve was drawn using xylose as the standard reducing sugar (Supplementary Fig. S1).
The cellulase activity was determined by measuring the glucose released by the enzyme from Whatman Grade 1 filter paper [31]. Filter-paper strip (1.0 × 6.0 cm) in 1.0 mL 50 mM sodium citrate buffer (pH 5.5) was incubated with 0.5 mL enzyme solution at 55 °C for 60 min in a water bath. The reaction was stopped by the addition of 3.0 mL of DNS reagent, and the absorbance of the solution was read at 540 nm. One filter paper unit (FPU) of enzyme activity was defined as the amount of enzyme that released 1 μmol of reducing sugar in 1 min under the assay condition.
For the hydrolysis, the pretreated corncob was suspended in 50 mM citrate buffer at a liquid-to-solid ratio of 20 ml/g. The pH of the buffer was adjusted to 5.5 for Accellerase XY and 6.0 for Econase XT, according to the recommendations of the manufacturers. The xylose production was carried out using Accellerase XY (250 U/ml) at 55 °C, while XOS production using Econase XT (0.6 U/ml) was at 70 °C in a water bath. The hydrolysis conditions were based on preliminary tests and a parallel study conducted by our group (unpublished results). The samples were taken at regular intervals, and the solids were separated by centrifugation at 25,000 g for 5 min. The Accellerase XY activity in the samples was terminated by keeping them in a water bath at 100 °C for 5 min. The activity of thermostable Econase XT was stopped by the addition of four volumes of cold concentrated acetone, which was then evaporated in a boiling water bath. The supernatants were stored at − 18 °C until HPLC analysis. Xylan digestibility and xylose and XOS yields were calculated using Eq. 4 and Eq. 5, respectively.
2.4 Analyses
Before structural carbohydrate analysis, extractives were removed by treating the biomass sequentially with ultra-pure water and 96% ethanol in a Soxhlet apparatus (Isolab, Germany). The unpretreated and pretreated corncobs were analyzed for structural components according to the NREL/TP-510–42,618 method [32]. Briefly, 3 ml of 72% H2SO4 solution was added to 300 mg dry biomass and incubated for 1 h at room temperature. After the acid concentration was decreased to 4% with the addition of 84 ml of water, the solution was kept at 121 °C for 1 h in an autoclave (ALP, Japan). The mixture was cooled down to room temperature and filtered through porcelain filtering crucibles under a vacuum to collect the remaining solids for lignin and ash analyses. The solids retained were washed with 250 ml water. The crucibles were dried overnight at 105 °C and weighed. Then the ash amount was determined gravimetrically after the crucibles were kept at 525 °C for 3 h. Acid insoluble lignin content was calculated by subtracting ash content from the dry weight of the solid sample remaining after acid hydrolysis. For the determination of the fractions of cellulose and xylan in the samples, the monomers released in acid hydrolysis were analyzed with HPLC after neutralization with CaCO3 [32]. Using the monomer concentrations, the polymers in the solids were calculated by multiplying by the anhydrous factors (0.88 for five-carbon sugars; 0.9 for six-carbon glucose, and 0.72 for acetyl groups).
After enzymatic hydrolysis, hydrolysates were analyzed without further processing with HPLC to determine the amounts of the xylose and the XOS with a degree of polymerization (DP) from two to six. To determine the total amount of XOS (sum of low and high DP XOS), the liquid samples were acid-hydrolyzed to convert all soluble oligomers into the monomer (xylose). Into the 5 ml of liquid sample, 174 µl of 72% (w/w) H2SO4 solution was added, and the mixture was kept at 121 °C for 1 h in the autoclave [33]. The total XOS concentration was calculated by subtracting the xylose concentration in the original hydrolysate from the xylose concentration measured after the acid hydrolysis.
Monosaccharides, xylooligosaccharides, and acetic acid in the samples from pretreatments and enzymatic hydrolyses were measured with HPLC (Perkin Elmer Series 200, USA) equipped with a refractive index (RI) detector. All samples were filtered through 0.45 µm regenerated cellulose syringe filters (Isolab, Germany) to eliminate any impurities. Rezex RPM-monosaccharide column (Phenomenex, USA) was used at 80 °C in the analysis of monosaccharides. Ultra-pure water was used as the eluent at a flow rate of 0.6 ml/min. A deashing guard column (Biorad, USA) was used before the column in the analysis of samples that were hydrolyzed by acid. XOS were detected using the Aminex HPX-42A oligosaccharide column (Bio-Rad, USA) under the same conditions. Acetic acid was measured using Aminex HPX-87H organic acid column (Bio-Rad, USA) at 60 °C. The mobile phase was 5 mM H2SO4 at 0.6 ml/min. Calibration curves were prepared using standard solutions of analytes with known concentrations.
The experiments were carried out at least in duplicates, and average values ± standard deviations were reported. Standard deviations were represented as error bars in the figures.
3 Results and discussion
3.1 Organosolv treatment
The structural analysis of the corncob used in this study showed that it was composed mainly of cellulose and xylan (Table 1). This corncob was treated with ethanol-based organosolv under several different conditions. The treated solids were weighed to determine the solid recovery and analyzed for structural components, such as cellulose, xylan, and lignin (Table 2). The compositions were compared with that of the raw corncob to evaluate the effect of the treatments. The corncob was also treated with LHW for comparison.
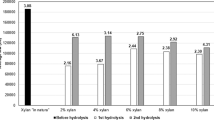
The effect of ethanol concentration was tested at three concentrations (0% (LHW), 50%, and 70% ethanol in water by volume) keeping treatment temperature and time constant at 170 °C and 1 h, respectively. An increase in the ethanol concentration achieved higher xylan and total biomass recovery values (Fig. 1a and Table 2). When the corncob was treated with 70% ethanol, xylan recovery was 81%, whereas it was 55% and 23.5% with 50% and 0% ethanol, respectively. Similarly, more cellulose was recovered with increasing ethanol. Around half of the lignin was removed from the corncob with 50% and 70% ethanol. Omitting ethanol could also remove 41% of lignin from the biomass; however, it did not contribute to the aim of this study due to low xylan recovery. Figure 1a shows that the effect of ethanol was more notable in the xylan recovery in comparison to cellulose recovery and lignin removal.
Effect of organosolv pretreatment conditions on the cellulose (light grey bars) and xylan (dark grey bars) recoveries and lignin removal (black bars) from corncob. a effect of ethanol concentration by volume (T = 170 °C). b effect of temperature (ethanol concentration = 70%). c effect of MgO (100 mM) at different temperatures (0% ethanol in the MgO treatment, 70% ethanol in the others)
In LHW, hydronium from water ionization and the acetic acid released by the removal of acetyl groups from hemicellulose decrease the pH and act as acid catalysts, which facilitates both xylan removal and autohydrolysis [23]. The higher xylan solubilization with 50% ethanol compared to 70% ethanol could also be ascribed to the comparably higher water content in the organosolv medium; thus, ionization and autohydrolysis effects were notable [34, 35]. The pH of liquor obtained after LHW was 3.83, which was considerably lower than that of the organosolv liquors (5.15 and 4.62 with 70% and 50%, respectively). The lower cellulose recovery in LHW can also be explained by the same phenomenon. The positive effect of increasing ethanol concentrations on the recovery of hemicellulose and lignin removal was also documented in previous reports [27, 36,37,38].
It is well-known that, in organosolv, the lignin removal increases with temperature, as well as carbohydrate solubilization and degradation [29]. In this study, the corncob was treated at 150 °C, 170 °C, and 190 °C for 1 h using 70% ethanol as the solvent to quantify the effect of organosolv treatment temperature (Fig. 1b). Higher temperatures solubilized more of the biomass (Table 2). The pH of the liquors after the treatments was slightly different, decreasing with temperature [36]. The composition of the biomass pretreated at 150 °C was comparable to that obtained at 170 °C. The cellulose and xylan recoveries were around 80–85%. At 190 °C, similar cellulose recovery, but much lower xylan recovery (36%) was observed. Almost three-quarters of the lignin could be separated from the corncob at this temperature, so that, biomass with low lignin and xylan and high cellulose was obtained. This could be a convenient feedstock to obtain glucose after post-enzymatic hydrolysis and can be used as a carbon source in bio-productions [22, 39, 40].
The effect of MgO as a catalyst in organosolv treatment was tested at 150 °C, 170 °C, and 190 °C using 70% ethanol as the solvent (Table 2 and Fig. 1c). pH of the treatment liquors decreased with increasing temperature in the presence of MgO like in its absence; however, the values were considerably higher (pH 6.45–7.65) compared to the tests without MgO at the same temperatures (pH 4.81–5.33) (Table 2). At 150 °C, the solubility and the composition of the treated corncob were very close with and without MgO despite the difference in the pH value of about 2.4. At 170 °C, MgO provided slightly higher biomass recovery. A small increase in xylan recovery from about 81% to 87% was observed with the addition of MgO at 170 °C. Cellulose recovery and lignin removal values were comparable with and without MgO. The most drastic effect of MgO was pronounced in the xylan recovery in the organosolv at 190 °C, increasing to 70.1% from 36% (without MgO at 190 °C). The biomass recovery increased, and cellulose recovery did not change with MgO at 190 °C. The lignin removal was high (68.1%), only 10% lower than obtained without MgO. Overall, these results are supporting that lower xylan solubilization (higher recovery) can be explained by the higher organosolv liquor pH values observed with MgO [28]. To extend the understanding of the effect of MgO on pH, MgO was also tested for the treatment of corncob at 170 °C, in the absence of ethanol (MgO treatment). This treatment was similar to LHW, but 100 mM MgO was included. It resulted in a liquor pH of 6.33, which was a 2.5 pH value increase from the treatment without MgO. The catalyst increased the solid recovery to 15.4 g, which was around 40% higher. Under this condition, lignin removal (25.0%) was poorer compared to other treatments. The xylan was recovered to a great extent (69.5%) with a two-fold increase from the recovery without MgO. Separation of xylan from the biomass was hindered by the presence of MgO, which fitted well with the aim of this work; however, the lignin removal was limited. These findings further support the idea that metal oxides can be effective to prevent pH decrease in the thermochemical treatment of LCB [41]. Previously, MgO pretreatment [28] and MgO-catalyzed organosolv treatment [27] were applied on corn stover. In the former, MgO provided higher xylan recovery in the biomass, but no improvement in lignin removal compared to LHW. In organosolv, however, they observed enhanced lignin removal in agreement with this study, with reduced monosaccharide degradation. Ye et al. [42] also treated corncob with MgO to improve lignin removal and reported looser surface area, higher crystallinity, and cellulose content compared to the raw material.
3.2 Xylan digestibility
The enzymatic hydrolysis tests were performed using four pretreated corncob samples selected based primarily on the xylan recovery and the lignin removal values. The biomasses containing 28.7–30.6% xylan obtained after treatments at 150 °C and 170 °C either with or without MgO were used in the tests. They were hydrolyzed by xylanases to check the extent of xylan digestibility. The xylanase activities in Accellerase XY and Econase XT (4% solution) were found to be 28,400 U/ml and 1300 U/ml, respectively. The cellulase (filter paperase) activity of Accellerase XY was 74 FPU/ml, while no cellulase activity was detected in Econase XT.
Accellerase XY was applied at 55 °C for 24 h to hydrolyze the xylan in the pretreated corncob into xylose. The maximum xylan digestibility was 65%, which was obtained with the sample treated at 170 °C in the presence of MgO (Fig. 2). Under this condition, 9.8 g/L xylose was released into the hydrolysis medium. Xylose yield based on the initial xylan in the feedstock was calculated as 57%, corresponding to 15.3% of the raw corncob (dry basis). Without MgO, the digestibility and yield decreased slightly to 58% and 47%, respectively. In the hydrolysis of samples treated at 150 °C with and without MgO, 43% and 33% of the xylan in the pretreated samples were hydrolyzed into xylose, respectively. No glucose was detected in the enzymatic hydrolysate, which was ascribed to the very low cellulolytic activity (0.65 FPU/ml) in the hydrolysis medium.
The compositions of the pretreated corncob samples used in hydrolysis were similar (Table 2); however, their digestibility differed considerably. For example, an increase in organosolv pretreatment temperature from 150 to 170 °C had only a slight effect on the xylan and lignin contents, whereas the digestibility increased more than 1.5 times. Similarly, the inclusion of MgO in the organosolv at 150 °C did not change the breakdown of the compositions but increased digestibility. The effect of MgO in the organosolv at 170 °C was profoundly less on xylan digestibility. It is apparent from these results that the extent of lignin removal, or cellulose and xylan recovery were not the primary determinants in the xylan digestion of organosolv-pretreated corncob. The higher temperature and presence of MgO as a catalyst may have rendered the xylan in the biomass more open to enzymatic hydrolysis. This showed that the effect of the organosolv treatment was not only through delignification but also the disruption of the interaction among the components of the pretreated biomass was effective in the post-enzymatic hydrolysis. This effect was more pronounced at higher temperatures and when MgO was used as a catalyst. Such effects of pretreatments including organosolv have been generally discussed for cellulose digestibility by enzymes, ascribing to the accessible area, cellulose crystallinity, pore volume, particle size, and degree of polymerization as well as the hemicellulose and lignin contents [23, 39].
To understand the potential of organosolv for XOS production, another enzymatic hydrolysis under a different condition was carried out. The corncob pretreated at 170 °C without MgO was selected for this test based on the results of xylose production. It was hydrolyzed at 70 °C using another commercial xylanase, Econase XT, which showed better performance for XOS production than Accellerase XY in the preliminary experiments. The XOS production started rapidly and in 8 h most of the xylan conversion was completed (Fig. 3). Total XOS, which accounted for all the xylan-based oligomers dissolved in the hydrolysis medium, reached 9.6 g/L in 8 h. This corresponded to approximately 65% conversion of the xylan in the pretreated corncob into XOS and 53% XOS yield based on the feedstock xylan. The XOS yield on raw corncob was calculated as 14%. Extending the hydrolysis time to 24 h increased the total XOS concentration slightly to 10.9 g/L. XOS production was accompanied by xylose and in 24 h, 1.9 g/L xylose was released. Xylose formation during XOS-oriented hydrolysis was generally indispensable and reported also in other studies [43,44,45,46].
Xylooligosaccharides (XOS) production from pretreated (170 °C, 70% ethanol, no MgO) corncob by enzymatic hydrolysis using xylanase (Econase XT). Solid square: total XOS; open square: XOS with low degree of polymerization; star: xylose; solid circle: xylobiose; open circle: xylotriose; triange: xylotetraose
The degree of polymerization is a prominent parameter for the prebiotic activity of XOS [47]. Most of the XOS was in the form of a dimer (xylobiose). Unlike total XOS, xylobiose synthesis did not finish in 8 h and increased in 24 h to 6.5 g/L. Between 8 and 24 h, some of the large XOS may have been hydrolyzed into xylobiose. In line with that, there was approximately 1 g/L of other XOS (xylotriose and xylotetraose) after 8 h, but their concentrations decreased in the later stages. Between 24 and 48 h, there was no considerable change in the concentrations. The XOS obtained from the organosolv pretreated corncob to a large extent had low DP, which are known to promote better the growth of Bifidobacterium in the colon [47]. The degree of polymerization of XOS and the fraction of XOS with low DP depend on the characteristics of the xylanase used for the hydrolysis, as well as the type of feedstock and the pretreatment conditions [48]. Application of xylanase for 48 h on the untreated (raw) corncob yielded only 0.96 g/l total XOS, which confirmed the requirement of pretreatment for the enzymatic hydrolysis of LCB [49, 50]. Like in xylose production by Accellerase XY, there was no detectable amount of glucose released during XOS production by Econase XT, owing to the lack of cellulase activity.
When organosolv was applied under specific conditions, it could retain most of the xylan in the corncob biomass, while the biomass was partially delignified simultaneously. Unlike the organosolv treatment at high temperatures or in the presence of an acid catalyst, the xylan was not solubilized or hydrolyzed extensively. In this way, the formation of carbohydrate degradation products can be kept to a minimum. The pretreated biomass can be separated easily from the pretreatment liquor and washed; thus, the enzymatic hydrolysate may have contained no (or a small amount of) compounds that originated in the organosolv and carried along with the solid biomass. This is unlike the organosolv at high severities and the LHW treatment. In those treatments, the xylose and XOS are collected in the treatment liquor, which contains the extractives from the biomass as well as the carbohydrate degradation products [20, 23]. The resulting corncob biomass was amenable to xylanase activity on the xylan, so that it could be hydrolyzed into xylose and XOS. The high selectivity of the commercial xylanase towards xylan hydrolysis provided a hydrolysate composed of primarily xylan hydrolysis products. Proper selection of the enzyme source and the hydrolysis conditions directed the hydrolysis toward either xylose or XOS exclusively.
Organosolv treatment is a costly process because of the high energy demand to provide high temperatures and the requirement of special equipment that should withstand high pressure. The cost of the proposed process may also increase due to the enzyme cost. Besides, the xylose yield in this approach (around 57% based on the initial xylan) was lower than the ones obtained by dilute acid hydrolysis, which can be as high as over 90% [51], whereas XOS yield (53%) was comparable to previous studies [52]. On the other hand, the xylose and XOS purification cost following the process proposed in this study could be low since the hydrolysates do not contain any other carbohydrates, plant components, and sugar degradation products. In addition to that, the cellulose recovered can be hydrolyzed into glucose without any other pretreatment since most of the xylan and lignin are removed. It is also possible to recover the lignin from the organosolv liquor [53]. So, glucose and lignin can be used as feedstock for value-added products.
4 Conclusion
The current study showed that organosolv pretreatment under specific conditions combined with an enzymatic post-hydrolysis can be an alternative approach to obtaining xylose and XOS from xylan in LCB. For improved yields, the dissolution and degradation of xylan in the pretreatments should be prevented. This was achieved in this study by using a high ethanol fraction (70%) in the organosolv pretreatment while adding MgO further improved the xylan recovery. Higher pretreatment temperature had a positive effect on xylose yield in enzymatic hydrolysis, while MgO had a slight effect. Organosolv pretreatment also allowed enzymatic XOS production composed extensively of xylobiose, having a high prebiotic potential. The absence of sugar degradation and cellulose digestion products in the enzymatic hydrolysates, unlike dilute acid and hot water hydrolysates, and not requiring concentrated acid and alkali are the primary advantages of the proposed method. This approach can be applied in lignocellulose biorefineries aiming to add value to xylan in addition to cellulose and lignin, thus increasing the feasibility.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Romaní A, Rocha CMR, Michelin M, Domingues L, Teixeira JA (2020) Valorization of lignocellulosic-based wastes. In: Varjani S, Pandey A, Gnansounou E, Khanal SK, Raveendran S (eds) Current Developments in Biotechnology and Bioengineering. Elsevier, pp 383–410
Hassan SS, Williams GA, Jaiswal AK (2019) Moving towards the second generation of lignocellulosic biorefineries in the EU: drivers, challenges, and opportunities. Renew Sustain Energy Rev 101:590–599. https://doi.org/10.1016/j.rser.2018.11.041
Dutta N, Usman M, Luo G, Zhang S (2022) An insight into valorization of lignocellulosic biomass by optimization with the combination of hydrothermal (HT) and biological techniques: a review. Sustain Chem 3(1):35–55
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Lin H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206. https://doi.org/10.1016/j.fuproc.2016.12.007
Roy R, Rahman MS, Raynie DE (2020) Recent advances of greener pretreatment technologies of lignocellulose. Curr Res Green Sustain Chem 3:100035. https://doi.org/10.1016/j.crgsc.2020.100035
Reshmy R, Paulose TAP, Philip E, Thomas D, Madhavan A, Sirohi R, Binod P, Kumar Awasthi M, Pandey A, Sindhu R (2022) Updates on high value products from cellulosic biorefinery. Fuel 308:122056. https://doi.org/10.1016/j.fuel.2021.122056
Nitsos CK, Lazaridis PA, Mach-Aigner A, Matis KA, Triantafyllidis KS (2019) Enhancing lignocellulosic biomass hydrolysis by hydrothermal pretreatment, extraction of surface lignin, wet milling and production of cellulolytic enzymes. Chemsuschem 12(6):1179–1195. https://doi.org/10.1002/cssc.201802597
Gírio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101(13):4775–4800. https://doi.org/10.1016/j.biortech.2010.01.088
Hua Y, Wang J, Zhu Y, Zhang B, Kong X, Li W, Wang D, Hong J (2019) Release of glucose repression on xylose utilization in Kluyveromyces marxianus to enhance glucose-xylose co-utilization and xylitol production from corncob hydrolysate. Microb Cell Fact 18(1):24. https://doi.org/10.1186/s12934-019-1068-2
Narisetty V, Cox R, Bommareddy R, Agrawal D, Ahmad E, Pant KK, Chandel AK, Bhatia SK, Kumar D, Binod P, Gupta VK, Kumar V (2022) Valorisation of xylose to renewable fuels and chemicals, an essential step in augmenting the commercial viability of lignocellulosic biorefineries. Sustain Energy Fuels 6(1):29–65. https://doi.org/10.1039/D1SE00927C
Harner NK, Wen X, Bajwa PK, Austin GD, Ho C-Y, Habash MB, Trevors JT, Lee H (2015) Genetic improvement of native xylose-fermenting yeasts for ethanol production. J Ind Microbiol Biotechnol 42(1):1–20. https://doi.org/10.1007/s10295-014-1535-z
Qiu Z, Gao Q, Bao J (2018) Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour Technol 249:9–15. https://doi.org/10.1016/j.biortech.2017.09.117
Jiang M, Ma J, Wu M, Liu R, Liang L, Xin F, Zhang W, Jia H, Dong W (2017) Progress of succinic acid production from renewable resources: metabolic and fermentative strategies. Bioresour Technol 245:1710–1717. https://doi.org/10.1016/j.biortech.2017.05.209
Mohamad NL, Mustapa Kamal SM, Mokhtar MN (2015) Xylitol biological production: a review of recent studies. Food Rev Int 31(1):74–89. https://doi.org/10.1080/87559129.2014.961077
Torres-Mayanga PC, Lachos-Perez D, Mudhoo A, Kumar S, Brown AB, Tyufekchiev M, Dragone G, Mussatto SI, Rostagno MA, Timko M, Forster-Carneiro T (2019) Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: a review. Biomass Bioenergy 130:105397. https://doi.org/10.1016/j.biombioe.2019.105397
Naidu DS, Hlangothi SP, John MJ (2018) Bio-based products from xylan: a review. Carbohydr Polym 179:28–41
Zeybek N, Rastall RA, Buyukkileci AO (2020) Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr Polym 236:116076. https://doi.org/10.1016/j.carbpol.2020.116076
Peng F, Peng P, Xu F, Sun R-C (2012) Fractional purification and bioconversion of hemicelluloses. Biotechnol Adv 30(4):879–903. https://doi.org/10.1016/j.biotechadv.2012.01.018
Huang L-Z, Ma M-G, Ji X-X, Choi S-E, Si C (2021) Recent developments and applications of hemicellulose from wheat straw: a review. Front Bioeng Biotechnol 9:440
Surek E, Buyukkileci AO (2017) Production of xylooligosaccharides by autohydrolysis of hazelnut (Corylus avellana L.) shell. Carbohydr Polym 174:565–571. https://doi.org/10.1016/j.carbpol.2017.06.109
Yao K, Wu Q, An R, Meng W, Ding M, Li B, Yuan Y (2018) Hydrothermal pretreatment for deconstruction of plant cell wall: part I. Effect on lignin-carbohydrate complex. AICHE J 64(6):1938–1953. https://doi.org/10.1002/aic.16114
Ferreira JA, Taherzadeh MJ (2020) Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour Technol 299:122695. https://doi.org/10.1016/j.biortech.2019.122695
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82(5):815–827. https://doi.org/10.1007/s00253-009-1883-1
Zhang Z, Harrison MD, Rackemann DW, Doherty WOS, O’Hara IM (2016) Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem 18(2):360–381. https://doi.org/10.1039/C5GC02034D
Gong Z, Wang X, Yuan W, Wang Y, Zhou W, Wang G, Liu Y (2020) Fed-batch enzymatic hydrolysis of alkaline organosolv-pretreated corn stover facilitating high concentrations and yields of fermentable sugars for microbial lipid production. Biotechnol Biofuels 13(1):13. https://doi.org/10.1186/s13068-019-1639-9
Zhu Y, Kim TH, Lee Y, Chen R, Elander RT (2006) Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Appl Biochem Biotechnol 130(1):586–598
Li J, Zhang M, Wang D (2019) Enhancing delignification and subsequent enzymatic hydrolysis of corn stover by magnesium oxide-ethanol pretreatment. Bioresour Technol 279:124–131. https://doi.org/10.1016/j.biortech.2019.01.123
Li J, Li W, Zhang M, Wang D (2018) Boosting the fermentable sugar yield and concentration of corn stover by magnesium oxide pretreatment for ethanol production. Bioresour Technol 269:400–407. https://doi.org/10.1016/j.biortech.2018.08.102
Borand MN, Karaosmanoğlu F (2018) Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: a review. J Renew Sustain Energy 10(3):033104. https://doi.org/10.1063/1.5025876
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23(3):257–270
Adney B, Baker J (2008) Measurement of cellulase activities. https://www.nrel.gov/docs/gen/fy08/42628.pdf. Accessed 10 November 2022
Sluiter JB, Ruiz RO, Scarlata CJ, Sluiter AD, Templeton DW (2010) Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J Agric Food Chem 58(16):9043–9053. https://doi.org/10.1021/jf1008023
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. https://www.nrel.gov/docs/gen/fy08/42623.pdf. Accessed 10 November 2022.
Matsakas L, Raghavendran V, Yakimenko O, Persson G, Olsson E, Rova U, Olsson L, Christakopoulos P (2019) Lignin-first biomass fractionation using a hybrid organosolv – steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour Technol 273:521–528. https://doi.org/10.1016/j.biortech.2018.11.055
Nitsos C, Rova U, Christakopoulos P (2018) Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 11(1):50
Kim TH, Ryu HJ, Oh KK (2019) Improvement of organosolv fractionation performance for rice husk through a low acid-catalyzation. Energies 12(9):1800
Pan X, Gilkes N, Kadla J, Pye K, Saka S, Gregg D, Ehara K, Xie D, Lam D, Saddler J (2006) Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: optimization of process yields. Biotechnol Bioeng 94(5):851–861. https://doi.org/10.1002/bit.20905
Santos TM, Rigual V, Domínguez JC, Alonso MV, Oliet M, Rodriguez F (2022) Fractionation of Pinus radiata by ethanol-based organosolv process. Biomass Convers. Biorefin. https://doi.org/10.1007/s13399-022-02329-z
Zhou Z, Lei F, Li P, Jiang J (2018) Lignocellulosic biomass to biofuels and biochemicals: a comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnol Bioeng 115(11):2683–2702. https://doi.org/10.1002/bit.26788
Malik K, Sharma P, Yang Y, Zhang P, Zhang L, Xing X, Yue J, Song Z, Nan L, Yujun S, El-Dalatony MM, Salama E-S, Li X (2022) Lignocellulosic biomass for bioethanol: insight into the advanced pretreatment and fermentation approaches. Ind. Crop Prod. 188:115569. https://doi.org/10.1016/j.indcrop.2022.115569
Li J, Zhang M, Li J, Wang D (2018) Corn stover pretreatment by metal oxides for improving lignin removal and reducing sugar degradation and water usage. Bioresour Technol 263:232–241. https://doi.org/10.1016/j.biortech.2018.05.006
Ye K, Tang Y, Fu D, Chen T, Li M (2021) Effect of magnesium oxide pretreatment on the delignification and enzymatic hydrolysis of corncob. Ind Crop Prod 161:113170. https://doi.org/10.1016/j.indcrop.2020.113170
Jayapal N, Samanta AK, Kolte AP, Senani S, Sridhar M, Suresh KP, Sampath KT (2013) Value addition to sugarcane bagasse: xylan extraction and its process optimization for xylooligosaccharides production. Ind Crop Prod 42:14–24. https://doi.org/10.1016/j.indcrop.2012.05.019
Samanta A, Jayapal N, Jayaram C, Roy S, Kolte A, Senani S, Sridhar M (2015) Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact Carbohydr Dietary Fibre 5(1):62–71
Surek E, Buyukkileci AO, Yegin S (2021) Processing of hazelnut (Corylus avellana L.) shell autohydrolysis liquor for production of low molecular weight xylooligosaccharides by Aureobasidium pullulans NRRL Y–2311–1 xylanase. Ind Crop Prod 161:113212. https://doi.org/10.1016/j.indcrop.2020.113212
Uçkun Kiran E, Akpinar O, Bakir U (2013) Improvement of enzymatic xylooligosaccharides production by the co-utilization of xylans from different origins. Food Bioprod Process 91(4):565–574. https://doi.org/10.1016/j.fbp.2012.12.002
Ho AL, Kosik O, Lovegrove A, Charalampopoulos D, Rastall RA (2018) In vitro fermentability of xylo-oligosaccharide and xylo-polysaccharide fractions with different molecular weights by human faecal bacteria. Carbohydr Polym 179:50–58. https://doi.org/10.1016/j.carbpol.2017.08.077
Yegin S (2022) Microbial xylanases in xylooligosaccharide production from lignocellulosic feedstocks. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03190-w
Feng X, Yao Y, Xu N, Jia H, Li X, Zhao J, Chen S, Qu Y (2021) Pretreatment affects profits from xylanase during enzymatic saccharification of corn stover through changing the interaction between lignin and xylanase protein. Front Microbiol 12:754593. https://doi.org/10.3389/fmicb.2021.754593
Huang C, Zhao C, Li H, Xiong L, Chen X, Luo M, Chen X (2018) Comparison of different pretreatments on the synergistic effect of cellulase and xylanase during the enzymatic hydrolysis of sugarcane bagasse. RSC Adv 8(54):30725–30731. https://doi.org/10.1039/C8RA05047C
Harahap BM (2020) Degradation techniques of hemicellulose fraction from biomass feedstock for optimum Xylose production: a review. Jurnal Keteknikan Pertanian Tropis dan Biosistem 8(2):107–124. https://doi.org/10.21776/ub.jkptb.2020.008.02.01
Chen Y, Xie Y, Ajuwon KM, Zhong R, Li T, Chen L, Zhang H, Beckers Y, Everaert N (2021) Xylo-oligosaccharides, preparation and application to human and animal health: a review. Front Nutr 8:731930. https://doi.org/10.3389/fnut.2021.731930
Pongchaiphol S, Suriyachai N, Hararak B, Raita M, Laosiripojana N, Champreda V (2022) Physicochemical characteristics of organosolv lignins from different lignocellulosic agricultural wastes. Int J Biol Macromol 216:710–727. https://doi.org/10.1016/j.ijbiomac.2022.07.007
Funding
This work was supported by The Scientific and Technological Research Council of Türkiye (grant number 218M252).
Author information
Authors and Affiliations
Contributions
Ali Oguz Buyukkileci: conceptualization, methodology, funding acquisition, resources, supervision, and writing—reviewing and editing; Nuran Temelli: investigation, methodology, and writing—original draft preparation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buyukkileci, A.O., Temelli, N. Organosolv pretreatment of corncob for enzymatic hydrolysis of Xylan. Biomass Conv. Bioref. 13, 6385–6394 (2023). https://doi.org/10.1007/s13399-023-03786-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03786-w