Abstract
Endophytes either be bacteria, fungi, or actinomycetes colonize inside the tissue of host plants without showing any immediate negative effects on them. Among numerous natural alternative sources, fungal endophytes produce a wide range of structurally diverse bioactive metabolites including anticancer compounds. Considering the production of bioactive compounds in low quantity, genetic and physicochemical modification of the fungal endophytes is performed for the enhanced production of bioactive compounds. Presently, for the treatment of cancer, chemotherapy is majorly used, but the side effects of chemotherapy are of prime concern in clinical practices. Also, the drug-resistant properties of carcinoma cells, lack of cancer cells-specific medicine, and the side effects of drugs are the biggest obstacles in cancer treatment. The interminable requirement of potential drugs has encouraged researchers to seek alternatives to find novel bioactive compounds, and fungal endophytes seem to be a probable target for the discovery of anticancer drugs. The present review focuses a comprehensive literature on the major fungal endophyte-derived bioactive compounds which are presently been used for the management of cancer, biotic factors influencing the production of bioactive compounds and about the challenges in the field of fungal endophyte research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytes show a mutualistic relationship with the plants and are a rich source of bioactive compounds, which are used in medicine, agriculture, and pharmaceutical industry. Endophytes can be bacteria, fungi, or actinomycetes, which colonize underneath the epidermal layer of plant tissues without showing any adverse effect or symptoms on the respective host plant [94]. The distribution of fungal endophytes has been examined from various plant tissues such as stem, stem bark, root, leaf, seeds, fruits, and flowers. It colonizes different range of host plants including phanerogams [44] as well as cryptogams [35]. Geographically, endophytes have been isolated from plants distributed over a wide range from Arctic to Antarctic and Tropics to Temperate zone [128]. Various advantages of endophytes such as their presence in wide range of ecological niches, in almost every plant, and in vitro growing ability make an outstanding candidate to explore the fungal endophyte-derived bioactive metabolite. At the metabolic level, the mutualistic or antagonistic symbiotic relationship of host and endophyte leads to the production of bioactive metabolites with various biological activities including antifungal, antibacterial [11], antioxidant, antidiabetic [89], antiparasitic, and anticancer activities [50]. Besides, fungal endophytes have also given significant contribution in the field of medicine and agriculture. Several applications have been shown through reports suggesting plant growth-promoting activity of fungal endophytes [20, 39], and activity in crop production and sustainable agriculture [45, 69]. Phomopsichalasin, first cytochalasin-type compound, was isolated from a fungal endophyte of Phomopsis sp. with antibacterial activity. Phomopsichalasin showed significant antibacterial activity against Bacillus subtilis and Staphylococcus aureus with 12 mm and 8 mm of zone of inhibition, respectively [41]. Fungal endophytes are reported to produce various industrial enzymes including extracellular enzymes amylases, cellulases, lipases, chitinases, and proteases. Cylindrocephalum sp., a fungal endophyte associated with Alpinia calcarata has been reported as a proficient producer of lipase and amylase enzyme [111]. Beside several biological activities, fungal endophytes are also known to produce bioactive metabolites which are used in the management of various types of cancer [86, 120]. Current review presents a comprehensive idea about anticancer mode of action of fungal endophyte-derived bioactive compounds. This also provides an insight about biotic factors such as epigenetic modifiers which are being used to activate biosynthetic gene clusters and produce cryptic bioactive compounds.
Cancer develops as a result of accumulation of genetic and epigenetic variations, and when alterations accumulate at higher level than in normal cells, the disease become life-threatening. Due to the variation, there is an uncontrolled growth of cells, which leads to formation of tumor. The abnormal cells sometimes also invade other parts of the body, forming secondary tumors, eventually spreading the cancer cells. According to GLOBOCAN 2020 estimation, 19.3 million new cancer cases have been recorded worldwide (excluding non-melanoma skin cancer), and approximately 10 million deaths due to cancer (excluding non-melanoma skin cancer) [109]. The World Health Organization (WHO), through the estimates of year 2019, has declared cancer as first or second foremost cause of death. In India, cancer is responsible for 0.3 million deaths every year [73]. Plants have been served through many years in cancer treatment through production of metabolites of various classes like alkaloids, flavonoids, polyphenols, and terpenoids [87]. Moreover, several microbes have also been reported to exhibit anticancer compounds, including endophytic bacteria [95, 98, 112] and fungi [5, 57, 81, 119]. The fungal endophyte provides effortless culture-dependent isolation, less requirement of complex media and labour, easy scale up for the production of metabolites and being non-toxic to mammalian cells therefore, grasp more attention to be employed for bioactive procurement. The fungal endophyte interaction with the host plant has been established in the long evolutionary process and can be explained through the concept of co-evolution. The mutualistic interaction between fungal endophyte and host can possibly be a consequence of evolution of strategies during inhabiting the host plant and also due to the evolution of counter strategies [110]. This process has driven the adaptation of both host and fungal endophytes towards each other that triggered the sharing of common biosynthetic pathway of secondary metabolites by both partners [3]. Another concept to explain the production of similar bioactive compounds has been given through the process of horizontal gene transfer (HGT) occurred between both partners [100, 101]. The HGT events occurred between host plant and fungal endophyte has been accounted in several reports [33, 107].
For more than 30 years, the extracts of fungal endophytes associated with selected medicinal plants are used against different diseases [85]. However, the production of metabolites by fungal endophytes is genus or species specific and might be variable with respect to abiotic conditions and biotic factors like pathogens. There are many synthetic drugs for the treatment of cancer. Still, the side effects of synthetic drugs, drug-resistant property of carcinoma cells, lack of cancer cell-specific medicine, and the curb of chemotherapeutic drugs create the biggest obstacles for the treatment of cancer [19]. Considering the aforementioned limitations, researchers have intended towards fungal endophytes to obtain anticancer compounds showing minimum side effects. The surge in demand has increased the exploitation of medicinal plants with many of them becoming endangered. Using plant-associated microorganisms such as fungal endophytes, we not only isolate the potential anticancer metabolites but also maintain the biodiversity of medicinal plants. In the present review, we attempted to present an update on selected categories of bioactive compounds derived from fungal endophytes of medicinal plants as reliable sources of anticancer compounds.
Bioactive Compounds with Anticancer Activity
Plant-associated fungal endophytes are essential and untapped resource of natural bioactive metabolites. With accelerating advancement in the field of endophytic research, various fungal endophytes have been identified as prolific source of anticancer compounds (Fig. 1). According to a report, approximately 57% of compounds are derived from plants (bioactive compound and their derivatives), which are used in the clinical trials for cancer therapy [26]. The plants reported with anticancer activity are selected for the isolation of fungal endophytes in contemplation to obtain similar anticancer compounds from fungal endophytes as they mimic their host metabolites. The prime source of Taxol (anticancer drug) which is the Taxus brevifolia has been studied for their fungal endophyte, and Taxomyces andreanae, a fungal endophyte, revealed the presence of Taxol that gained much attention [103]. After the identification and characterization of anticancer compound Taxol from fungal endophyte, researchers have focused on manipulating and optimizing the growth conditions which ultimately leads to the discovery of various compounds and novel semi-synthetic analogues with remarkable biochemistry [105]. Besides, several bioactive compounds derived from fungal endophytes have been moved for patent and industrial production (Table 1). Previous findings have also showed that a medicinal plant Crescentia cujete L. with anticancer property was selected for screening the anticancer activity of fungal endophytes. The results revealed that the fungal extracts possess significant cytotoxic potential against hepatocellular carcinoma cell lines (HepG2) [78]. A rare medicinal plant of China, Dysosma versipellis with anticancer property was investigated for the fungal endophytes with antimicrobial activities and for the production of podophyllotoxin (PTOX). The fungal strains Fusarium sp. WB5122 and WB5121 were reported to produce PTOX and therefore could be explored for its anticancer activities [113]. Another report showed fungal endophytes, Phialocephala fortinii, Juniperus communis L. Horstmann, and Trametes hirsuta, as a potential source of podophyllotoxin isolated from plants with anticancer potential, Podophyllum peltatum and Juniperus recurve [6]. Since fungal endophyte-derived bioactive compounds are of low abundance. Therefore, several strategies including tissue culture, optimization of the fermentation process, biotransformation, and synthetic/semisynthetic strategies, are being used to overcome such limitations [75]. Here, we discuss some of the major fungal endophyte-derived bioactive compounds that have a potential for the management of cancer (Fig. 2).
Pictorial representation of the anticancer activity of the fungal endophyte derived bioactive compounds. Plant tissues are used to culture onto solid media for the isolation of associated fungal endophytes. After the appearance of fungal mycelia, individual fungal strains are sub-cultured to get specific fungal strains. Furthermore, the strains are subjected to liquid fermentation for the mass production of bioactive compounds. The compounds present in the crude fungal extract are purified and characterized using HPLC, UPLC–QTOF MS, FT-IR, NMR, IR, LC-MS/MS, GC-MS, and ESI-Q-TOF. Isolated and characterized bioactive compound may show potential anticancer activity by mediating cell cycle arrest, prevention of the tubulin-microtubule assembly, and induction of apoptosis
Structure of fungal endophyte derived bioactive compounds with potential anticancer property. (1). Paclitaxel; (2). Podophyllotoxin; (3). Camptothecin; (4). Vinblastine; (5). Vincristine; (6). Dicatenarin; (7). Cladosporol A; (8). Cladosporol G; (9). Cytochalasin A; (10). Diaporthichalasin H; (11). Jammosporin A; (12). Multirostratin A; (13). 20-oxo-deoxaphomin; (14). Ergocytochalasin A; (15). 18-deoxycytochalasin H; (16). Cytochalasin H; (17). Penochalasin I; (18). 5-methylmellein; (19). 6-hydroxymellein; (20). 4-hydroxymellein; (21). 5-hydroxymellein; (22). cis-4-hydroxymellein; (23). Mellein; (24). Botryoisocoumarin A; (25). Fumitremorgin C; (26). Fumitremorgin D; (27). 12,13-dihydroxyfumitremorgin C
Paclitaxel (1) (generic name “Taxol”) belongs to the taxane family, having anticancer activity with a unique mode of action. The Food and Drug Administration (FDA) has approved to use Paclitaxel™ either alone or in combination with other anticancer therapy for the treatment of breast cancer, non-small cell lung cancer (NSCLC), ovarian cancer, non-HIV-associated Kaposi sarcoma, and AIDS-related Kaposi sarcoma [55]. Moreover, paclitaxel can also be used in the management of several other cancers, including bladder, cervical, head and neck, esophagus, and endometrial cancer. Initially, the crude extract of medicinal plants was used to harvest paclitaxel for cancer treatment. The anticancer activity of paclitaxel and its analogs have been determined by carrying out several cytotoxic assays. However, the low yield of paclitaxel was quite challenging. Therefore, the efforts were made to find a safe and potent approach for the enhanced production of paclitaxel to overcome such limitations. Discovery of paclitaxel-producing fungal endophyte Taxomyces andreanae has given a significant breakthrough which was isolated from the inner bark of plant Taxus brevifolia for the first time [104]. In one of the report, a group of researchers have performed genome sequence analysis of fungal endophyte Penicillium aurantiogriseum NRRL 62,431 and demonstrated the independent production of paclitaxel at a larger scale [127]. Moreover, an increased concentration of paclitaxel has been derived from fungal endophyte with the aid of optimized culture parameters and culture fermentation supplemented with various substances such as precursors, inducers, metabolic bypass inhibitors, nitrogen sources, and carbon sources [134].
Paclitaxel-producing fungal endophytes and associated plants are found in large number such as Seimatoantlerium nepalense from Taxus wallichiana [12], Seimatoantlerium tepuiense from Venezuelan and Guyana [106], Metarhizium anisopliae, Pestalotiopsis terminaliae, and Tubercularia sp. fungal strain TF5 in batch culture [34]. Furthermore, several other fungal endophyte including Alternaria, Cladosporium, Fusarium, Aspergillus, Pestalotia, Botryodiplodia, Pestalotiopsis, Metarhizium, Periconia, Taxomyces, Botrytis, Tubercularia, Mucor, and Pithomyces are reported to produce paclitaxel and its derivatives such as baccatin III and 10-deacetylbaccatin III [21]. Paclitaxel stabilizes the assembly of microtubules during cell division by binding to the N-terminal end of β-tubulin subunit. The unusual stabilization of the microtubules network impedes the construction of the mitotic spindle and chromosome segregation and leads to the cell cycle arrest at the G2/M phase, therefore leading to apoptosis of cancer cells [123]. Beside cell cycle arrest, paclitaxel also shows anticancer activity by targeting the mitochondria, apoptotic inhibitor protein such as B-cell Leukemia 2 (Bcl-2) and immune cells [32]. Previous study shows that paclitaxel (Taxol) enhances the pro-apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in metastatic cervical cancer (HeLa) cells by decreasing the expression level of Bcl-2 and increasing the expression levels of DR5 and cleaved caspase‑3 [108]. Therefore, potential mechanism of paclitaxel on TRAIL-induced apoptosis suggests that paclitaxel could be a promising candidate in the treatment of cervical cancer.
Another compound is podophyllotoxin (2) which is a popular aryl tetralin lignan, produced from both angiosperm as well as gymnosperm plants, with anticancer properties [71]. Podophyllotoxin and its analogs are of great pharmacological importance because of their cytotoxic potential [6]. Beside anticancer activity, podophyllotoxin is also effective in viral diseases, oxidative stress, microbial infections, and immunological disorders. Podophyllotoxin show toxic side effects in cancer treatment, so instead semi-synthetic derivatives like Teniposide™, Etoposide™, and Etopophos™ are being used as cytotoxic drugs [8]. These chemical compounds are used in the management of various types of cancer including leukemia, testicle cancer, and other solid tumors. In one of the report, it was shown that fungal strain JRE1 of Fusarium oxysporum from Juniperus recurva produces podophyllotoxin at a larger scale, 28.8 µg/g of the dry mass of mycelia [54]. Another study has shown that the Trametes hirsuta associated with the rhizome of the Podophyllum hexandrum plant could produce podophyllotoxin-related compounds, demethoxypodophyllotoxin and podophyllotoxin glycoside. The production of podophyllotoxin from fungal endophyte Trametes hirsuta was initiated at 72 h while rapidly declined after 96 h [80]. Another report discovered a podophyllotoxin-producing endophyte Alternaria tenuissima, associated with the root of Sinopodophyllum emodi [63]). Another report shows that Chaetomium globosum strain MF564 and Pseudallescheria sp. T55 of Ascomycota division produce podophyllotoxin [122]. A study reported isolation of podophyllotoxin with the highest yield of 277 µg/g of dry weight mycelia from Fusarium strain WB5121 associated with Dysosma versipellis [113].
Podophyllotoxin also prevents the assembly of tubulin-microtubule which leads to a cell-cycle arrest and ultimately causes apoptosis. The carbamate derivative 4β-(1,2,3-triazol-1-yl) podophyllotoxin shows promising cytotoxic activities against cancer cell lines, human lung adenocarcinoma cells (A549), human colon carcinoma cells (HCT-8), human promyelocytic leukemia cells (HL-60), and HeLa cells. Fluorescence-activated cell sorting (FACS) analysis revealed that compound 4′-O-demethyl-4β-[(4-hydroxymethyl)-1,2,3-triazol-1-yl]-4-desoxypodophyllotoxin cyclopentyl carbamate, a carbamate derivative of podophyllotoxin, induces apoptosis and cell cycle arrest at G2/M phase, inhibits microtubules formation, and inhibits DNA topoisomerase II (TOP-II) [67].
Camptothecin (3) is a five-ring quinoline alkaloid, sold under commercial names irinotecan and topotecan. Camptothecin-producing plant sources other than Camptotheca acuminata belongs to seven families such as Meliaceae (Dysoxylum binectariferum), Betulaceae (Alnus nepalensis), Piperaceae (Piper betel), Apocynaceae (Ervatamia heyneana and Chonemorpha fragrans), Gelsemiaceae (Mostueabrunonis), Violaceae (Rinorea anguifera), and Rubiaceae (Ophiorrhiza alata, Ixora coccinea) [79]. The FDA-approved drugs topotecan and irinotecan are being used to treat various cancers, including small-cell lung cancer, colorectal cancer, and ovarian cancer, and were manufactured by GlaxoSmithKline [7] and Pharmacia [91], respectively. Camptothecin derivatives such as 10,11-methylenedioxy camptothecin, rubitecan (9-nitrocamptothecin), and IDEC-132 (9-aminocamptothecin) are an excellent anticancer agent [102]. Besides these, other derivatives have also been investigated and are under clinical trial for their probable anticancer activity.
Chloroform extract of endophyte Entrophospora infrequens isolated from the inner bark tissue of Nothapodytes foetida yields camptothecin of 18 µg/mg of dry weight mycelia. Camptothecin isolated from fungal endophyte Entrophospora infrequens showed cytotoxic activity against human ovarian cancer cells (OVCAR-5), liver cancer cell (HEp2), and lung cancer cells (A549) [81]. Further, study revealed that E. infrequens produces a detectable quantity of camptothecin of approximately 4.96 mg/100 g of dry weight mycelia under bioreactor in 48 h [5]. Two fungal strains of Fusarium solani viz. MTCC9668 and MTCC9667 were isolated from Apodytes dimidiata found in the Western Ghats of India, reported to yield 53 µg/100g and 37 µg/100g of camptothecin, respectively from dry weight of mycelia [99]. A group has optimized the fungal culture parameters and isolated the highest yield of camptothecin (146 mg/l) with mixed fungal cultures (F1 + F2), while the monoculture of Colletotrichum fructicola (F1) and Corynespora cassiicola (F2) yields 33 mg/l and 69 mg/l of camptothecin, respectively. Furthermore, the monoculture of Fusarium oxysporum isolated from the same plant Nothapodytes nimmoniana yielded 90 mg/l of camptothecin [13]. Another study revealed that a total of 94 fungal strains were isolated from Camptotheca acuminata and only one fungal strain Fusarium solani S-019 has produced camptothecin (40 ± 5 µg/g) [88]. A study showed that camptothecin (0.175 mg/l) was isolated from Aspergillus niger associated with the Indian Piper betel, and the compound showed anticancer activity against colon cancer cell line (HCT-15) with an IC50 value of 31.25 µg/ml [9].
Camptothecin is water-insoluble, but the water-soluble derivatives of camptothecin such as Hycamtin [72] and Camptosar [16] showed anticancer activity against ovarian cancers and colorectal carcinomas, respectively, and have been approved as potential anticancer agent. Initially, DNA and RNA synthesis were believed to be the target site of camptothecin by which it induces cytotoxicity in cancer cells. But in the 1980s, the actual cellular target of camptothecin was identified as topoisomerase I [42]. The DNA supercoiling relaxing enzyme topoisomerase-I forms a cleavage complex that is stabilized by camptothecin and its analogs [2]. This stabilization leads to the initiation of apoptotic events, which ultimately results in cell death [132]. The combinational therapeutic approach has been used for the enhanced cytotoxic activity of camptothecin and its analogs. Camptothecin and its analogs, such as irinotecan in combination with mTOR inhibitors, may provide a promising approach in the treatment of colorectal carcinomas [90].
Coupling of two indole alkaloids, catharanthine and vindoline, generates vinblastine (4) and vincristine (5) that are promising candidates to be employed as an anticancer agent. Vinblastine was reported for the first time in 1998 derived from Alternaria sp., which was isolated from the phloem tissue of Catharanthus roseus [15]. After that, vincristine and vinblastine were successfully characterized by using electron spray ionization-mass spectroscopy (ESI-MS), MS/MS, 1 H NMR, and UV-Vis spectroscopy and yielded about 67 and 76 µg, respectively, from 1 L of culture filtrate of Fusarium oxysporum isolated from Catharanthus roseus [56]. The kinetin and alpha-naphthalene acetic acid are two phytohormones that are known to play a crucial role in vincristine production [49]. In a study, out of 22 fungal endophytes isolated from Catharanthus roseus, only Talaromyces radicus-CrP20 showed expression of tryptophan decarboxylase (TDC) gene (involved in alkaloids biosynthetic pathway) and produces vinblastine and vincristine. Cytotoxicity assay showed that HeLa cells are highly susceptible to vincristine with IC50 value of 4.2 µg/ml [77].
In vitro analysis of vinblastine and vincristine isolated from C. roseus showed anticancer activity by mediating cell cycle arrest at M-phase, irreversible binding with spindle protein and microtubule, depolarization of mitochondrial membrane potential, and induction of apoptosis [77]. Vinblastine and vincristine are also used in the prevention of progression of Hodgkin lymphoma and cervical cancer [76]. Vincristine is known to interfere with microtubule assembly, disrupts the intracellular transport and mitotic spindle dynamics, and decreases blood flow in cancer tissues leading to anti- angiogenesis. However, the cellular pharmacology of vinblastine, vindesine, and vincristine used in chemotherapeutics of cancer still needs to be established.
Dicatenarin (6) is an anthraquinone derivative isolated from the fungal endophyte of the Allium schoenoprasum plant [52]. Dicatenarin belongs to the anthraquinone class of the bioactive metabolites. The metabolites from this class have been recognized as cytotoxic/proapoptotic factor. The presence of phenolic hydroxyl group in dicatenarin plays a significant part in the bioactivity and oxidation process. The phenolic hydroxyl group at carbon-4 increase the generation of reactive oxygen species, showing cytotoxic activity against the pancreatic cancer cells (MIA PaCa-2). Furthermore, the compound elevates the release of cytochrome C from the transition pore of mitochondria, which in turn enhances the expression of caspase-3 protein for programmed cell death. It also alters the peptides that are targeted by mitochondria by generating ROS leading to apoptosis [53].
Cladosporol A (7) is tetralone derivatives isolated from Cladosporium cladosporioides from Datura innoxia plant. It has been reported as an inhibitor of β-1,3-glucan biosynthesis [58]. A chiral isomer of cladosporol A, Alterfungin, shows potential antifungal activity [23]. Cladosporol A has also been reported to exhibit antitumor activity in nude mice with gastric cancer xenografts [24]. It induces apoptosis in human breast cancer cells (MCF-7). Cladosporol A increases the release of cytochrome C, depolymerizes microtubules, induces loss of mitochondrial membrane potential, and condenses chromatin and its fragments. Moreover, cladosporol A also downregulates Bcl-2, upregulates Bax (Bcl-2-associated X protein), and converts microtubule-associated protein light chain 3, LC3-I to LC3-II by increasing monodansylcadaverine [53]. Cladosporol A is also known to exhibit antiproliferative activity against human colorectal cancer cell lines [137]. Cladosporol shows antiproliferative activity by modulating the expression of cell cycle genes like cyclin D1 and p21 (waf1/cip1). This activity is mediated by PPAR-γ (peroxisome proliferator-activated receptor-γ) in colorectal cancer cells [137]. Another cladosporol derivative cladosporol G (8) compound was extracted from C. cladosporioides fungi from deep Indian Ocean Sea. This compound showed cytotoxic activity against the HeLa cell line [133].
Cytochalasans are polyketide-amino acid compounds having perhydroisoindolone moiety in the core structure. It is a promising drug for chemotherapy and has various derivatives. The first compound to be characterized in this group was cytochalasin A (9). These compounds are known to disrupt the actin cytoskeleton, further altering cellular motility, morphology, and adhesive nature of transformed cancer cells. It also weakens the ion channels of cancer cells [118]. One of its forms diaporthichalasin H (10) was isolated from the Diaporthe sp. SC-J0138 associated with Cyclosorus parasiticus leaves. This compound showed cytotoxic activity against human cancer cells like A549, HeLa, HepG2, and MCF–7 with IC50 values of 13.90, 20.00, 9.90, and 32.10 µM, respectively [126]. Jammosporin A (11) is another cytochalasan isolated from Rosellinia sanctae-cruciana associated with A. lebbeck plant. It exhibited cytotoxic activity against human leukaemia cancer cell line [96]. Some of the other derivatives of cytochalasans extracted from fungal endophytes having anticancer activities are multirostratin A (12), 20-oxo-deoxaphomin (13), ergocytochalasin A (14), 18-deoxycytochalasin H (15), cytochalasin H (16), and penochalasin I (17) [25, 43]. The detail about the compounds along with their cytotoxic activity is mentioned in Table 2.
Melleins are the secondary metabolites that belong to polyketide family, isocoumarins. Melleins are mostly isolated from fungi. Derivatives of melleins are mainly known for their antimicrobial property [46], but increasing body of evidences has also suggested their anticancer potential. Three mellein derivatives 5-methylmellein (18), 6-hydroxymellein (19), and 4-hydroxymellein (20) were isolated from the Penicillium sp. from Senecio flavus. These compounds showed cytotoxicity against MCF-7 cancer cell line with IC50 values of > 10 mg/mL, 6.1 mg/mL, and 8.3 mg/mL, respectively [30]. The metabolites, 5-hydroxymellein (21), cis-4-hydroxymellein (22), mellein (23), and botryoisocoumarin A (24), which were isolated from endophyte Aspergillus flocculus associated with plant Markhamia platycalyx, showed cytotoxic activity against K562 (myelogenous leukemia) cell line [116].
Fumitremorgin (FTMs) belongs to the alkaloid family and is also known as tremorgenic metabolites due to its ability to cause tremor. They are produced by Aspergillus and Penicillium species. FTMs have been extensively studied due to their versatile biological activity and synthetic derivatives. Many reports have shown their potential in activities like neurotoxicity, cytotoxicity, and cell-cycle inhibition. Fumitremorgin C (25) shows a potent chemo-sensitizing property and reverses the overexpression of the gene which codes for breast cancer resistance protein (BCRP). This protein was found to be overexpressed in the human colon cell (S1-M1-3.2) line [84]. The derivatives of fumitremorgin were extracted and characterized from the marine-derived fungal strain Aspergillus sp. (BRF 030). Compounds fumitremorgin C and 12,13-dihydroxy fumitremorgin C (27) showed cytotoxic activity against HCT-116 tumor cell line having IC50 values of 15.17 µM and 4.53 µM, respectively [93]. Compounds like fumitremorgin D (26) and 12,13-dihydroxyfumitremorgin C were isolated from the liquid culture of fungus Aspergillus fumigatus which showed cytotoxic activity against HepG2 (Human hepatocellular carcinoma) cell line [64].
Biotic Factors in Improving the Production of Fungal Bioactive Compounds
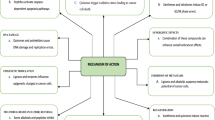
The production of bioactive compounds by fungal endophytes has greatly impacted the human life. However, the production of bioactive compounds is substantially affected by the variation in the biosynthetic pathway and culture conditions [121]. The limited production of these bioactive compounds posed a challenge to meet the market demand and that is due to some of the unexpressed genes that limit the production of bioactive compounds. The production of bioactive compounds is extensively triggered by biosynthetic gene clusters (BGCs). These BGCs are responsible for the insufficiency or lack of bioactive compound production as they are present in heterochromatin form that remain unexpressed under laboratory condition. Some of the epigenetic modifiers and threat-inducing techniques cause activation of these BGCs that leads to the euchromatin state and confers the production of complex bioactive compounds [10]. The strategies involved in the activation of gene clusters are co-culture techniques and employment of epigenetic modifiers (inducers). These inducers play significant role in enhancing the bioactive compound production either through overexpressing activator or repressor or may lead to deletion [14]. These chemical inducers modify the activity of crucial enzymes like histone deacetylase (HDACs) enzymes and DNA methyltransferase (DNMT) [36]. These modifications are highly responsible for the manipulation of pathway-specific regulator and invoke the production of novel bioactive compounds of significant interest [74]. Modern analytical tools and techniques along with the advent of genomic era have brought a dramatical change in the hunt for novel bioactive compounds. Furthermore, the elicitors, epigenetic modifiers, and co-culture technique have been studied for their active involvement in inducing the expression of silent genes in previously identified fungal endophytes that provide new avenues for the enhancement of bioactive compound production from same fungal endophytes studied so far and to explore cryptic bioactive compound [27]. A schematic diagram represented by Fig. 3 shows the action of epigenetic modifiers on silent biosynthetic gene clusters (BGCs) leading to their activation. The challenge of instability of bioactive compound produced by endophyte Acremonium sp. KM 677,335 isolated from Taxus baccata has been overcome through addition of 0.0001% crushed bark of T. baccata serving as an unusual elicitor. The production of paclitaxel by Acremonium sp. KM 677,335 has shown to be enhanced by four-fold and also the instability of paclitaxel during in vitro production has been sorted by employing crushed bark of T. baccata as a novel elicitor [28]. The induced production of taxol by fungal endophyte Periconia sp. associated with Torreya grandifolia has been demonstrated in a report through the application of benzoic acid that caused an increase of 8-fold in taxol production. The benzoic acid acts as an activator of fungal metabolism [59]. In another study, valproic acid has been reported to induce the silent secondary metabolites in the endophytic fungus Nigrospora sphaerica [68]. The secondary metabolite profile was altered through the application of valproic acid that modulates the pathway of fumiquinazoline C and enhanced its production [70]. Fumiquinazoline C isolated from endophyte Aspergillus fumigatus of Liverwort Heteroscyphus tener (Steph.) Schiffn. has been reported to exhibit anticancer activity against the human prostate cancers PC3, human lung cancer cell line (NCI-H460), multiple drug resistance PC3D cells, and the human lung adenocarcinoma epithelial cell line (A549) [125]. An endophytic fungus Diaporthe sp. isolated from Datura inoxia has been reported for their enhanced bioactivity in the presence of valproic acid (histone deacetylases inhibitor). The modulation of secondary metabolite profile by valproic acid resulted in the discovery of 3 unknown novel compounds xylarolide A, diportharine A, and xylarolide B and one known compound xylarolide. The significant growth inhibition has been observed in MIAPaCa-2 by both xylarolide A and xylarolide [97]. In a previous report, epigenetic modifiers, nicotinamide, and sodium butyrate belonging to class histone deacetylases (HDACs) inhibitors have been shown to induce the production of cryptic bioactive compounds from a marine-derived fungus Penicillium brevicompactum. The bioactive compounds syringic acid, sinapic acid, and acetosyringone exhibited potent antiproliferative activity against HepG2 cancer cell line [29]. Enhanced production of camptothecin has been obtained from endophytic fungus Entrophospora infrequens isolated from a medicinal plant Nothapodytes foetida through supplementation of precursors including tryptophan, leucine, tryptamine, mevalonic acid, geraniol, and citral. These precursors were used either alone or in combination like tryptophan and leucine, tryptophan and geraniol, tryptophan and mevalonic acid and tryptophan and citral, and tryptophan and leucine which significantly induced the production of camptothecin by 2.5 fold and the compound was tested on HL-60 cells where the morphology of the cells was observed to be more like apoptotic cells [4]. An epigenetic modifier quercetin has been shown in a report to significantly induce the production of vinblastine in the endophytic fungi Penicillium concavoradulozum VE89L and Aspergillus amstelodami VR177L derived from Vinca plants [37]. Vinblastine has been shown in previous studies to exhibit cytotoxic activity against cancer [117]. The co-culture strategy has also shown to be effective in terms of discovery of novel bioactive compounds. Previous report of co-culturing of an endophytic fungus Aspergillus versicolor KU258497 with the bacterium Bacillus subtilis 168 trpC2 shows procurement of two new 3,4-dihydronaphthalen-(2 H)-1-one (1-tetralone) derivatives, aspvanicin A and its epimer aspvanicin B with some other known compounds. The compound aspvanicin B was reported to exhibit moderate cytotoxicity against the mouse lymphoma cell line L5178Y [1]. In a report, co-culture of two marine-derived mangrove endophytic fungi has been shown to produce two bioactive compounds, namely, marinamide and its methyl ester. Both of the compounds exhibited significant cytotoxic activity against HepG2, 95-D, MGC832, and HeLa cell lines [136].
Two different pathways involved in the activation of silent or attenuated biosynthetic gene clusters through epigenetic modifiers. (a) The methylated state of chromatin remains transcriptionally inactive that is regulated by epigenetic enzyme DNMT (DNA methyltransferase). These pathway-specific regulator, DNMT, upon inhibition through epigenetic modifier results in the activation of silent biosynthetic pathways and clusters of cryptic bioactive compounds are produced. (b) The deacetylated form of chromatin remains in heterochromatin state and many of the genes remain unexpressed. These genes are induced through inhibitory action of chemical epigenetic modifiers on key regulators HDAC (histone deacetylase) that causes deacetylation of chromatin. Acetylation of chromatin activates the repressed biosynthetic gene clusters eventually leading to the production of novel bioactive compounds
These studies suggest that under laboratory conditions, a large number of pathways remain dormant and are activated by epigenetic modifiers. So, the requirement of specific environmental conditions by the organisms should be considered. The presence of neighboring microbes can enhance the silent biosynthetic pathways for bioactive compound production in fungal endophyte or epigenetic modifiers can activate these pathways through modifying the activity of key role-playing enzymes. The epigenetic modifier-mediated activation of silent biosynthetic pathways in endophytic fungi marks these chemicals to imitate the chromosomal conditions in the chemical cross-talk between the host plant and fungi, which may be needed for the production of bioactive compounds. Therefore, identification and activation of silent BGCs through epigenetic modifiers and co-culturing microbes could enhance the production and applicability of these bioactive compounds.
Future Challenges in the Field of Fungal Endophytes Research
Recently, on account of the biochemical diversity of the fungal endophytes, a great curiosity has been developed among researchers for bioprospecting the fungal endophytes for natural bioactive metabolite with pharmacological importance. The first step towards fungal endophyte research is the selection of medicinal plant or a host plant; it is always good to select a plant with great biological diversity and vibrant ecological niches. Besides this, in certain cases, endangered or endemic plants, or the plants having an unusual strategy to cope with the environmental stress, or the plant having medicinal importance can also be selected. But it is always unsure whether the selected plants will have potent endophytes, which will produce bioactive compounds with potential anticancer activity. The research is still limited to many of the concepts regarding endophytes. Many of the mechanisms of physiological and biochemical roles played by endophytes are still undiscovered. During stress condition, endophytes have been found to be involved in triggering the immunity in plants. They generate a cascade of chemicals, within the host plant to mitigate the stress condition. The current findings about the modulation of defense system in plants by endophytes are still like a black box puzzle. Also, multiple facets of plant-fungal interaction and their secretome analysis are needed to be explored. Using the current experimental technique, it is challenging to determine the differences in concentration of metabolite produced by fungal endophyte with and without colonizing the host plant. Therefore, the hypothesis requires further detailed investigations and proper experimental design that could explicit endophyte-plant relationship. In the long evolutionary process, the co-evolution between plants and endophytes has resulted in the production of compounds by endophytes, similar to that of their host plant. The presence of a diverse array of fungal endophytes in a plant often impedes the isolation and bioprospection process due to the production of metabolites dissimilar to their host. Therefore, a well propound research is required for the identification of these fungal endophytes, their interaction with the host, and the biochemical roles associated with them.
The instability of the biosynthetic pathway of fungal endophytes producing anticancer bioactive compounds on successive culturing is another challenge while studying about it. The endophyte-endophyte relationship is another unexplored area that needs to be elucidated at a molecular level. The fungal endophyte, which has a collaborative association with another endophyte either bacterial or fungal, might be the reason for the decreased yield of the compounds on successive culturing. A widely proposed theory for the colonization of fungal endophytes in the host plant is the genetic recombination. Few examples have been seen in which the endophytes produce anticancer compounds (for example L-asparaginase) with the help of plant enzymes thereby completing their biosynthetic pathways. Therefore, a detailed understanding of the fungal-plant interactions, diversity of the plants, and the associated endophytes, ecology, cross-talks, and evolution is needed to be well studied.
Conclusions
Endophytes are a treasure house and a sustainable source of bioactive compounds with potential anticancer activity. With the advent of modern technologies and tools, purification and characterization of fungal endophyte-derived bioactive compounds are now relatively easy. Several biological assays such as MTT cell-proliferation assay, Annexin V FITC/PI flow cytometry, cell cycle analysis, loss of mitochondrial membrane potential, and DNA fragmentation patterns have been used to examine the potential anticancer activities of these compounds. Moreover, with the application of modern ‘omics’ technology and advanced molecular biology approaches, one can better understand and increase the production of fungal endophyte-derived bioactive compounds. Approaches like genetic engineering, amplification of gene cluster, mutagenesis, refinement of fungal culture, and use of elicitors and epigenetic modifiers have been introduced for increased output of fungal endophyte-derived bioactive compounds. As cancer is one of the deadliest diseases in the world, there is an urgent need for a more stable and advanced form of drugs which specifically target the cancer cells. In spite of several research done to explore fungal endophytes, the biosynthetic pathway of many metabolites is still unexplored due to difficulty in experimental verification of biosynthetic gene clusters (BGCs). There is a need to develop advance technology to study uncultivable endophytes. The endophytes with known chemical structure and biological properties have been discussed repeatedly; by using them as a reference, focus is needed to be shift towards ‘hidden gems’. With the deep understanding of endophytes’ interaction with their host and the biosynthetic pathway for metabolite production, a new way will be paved for refactoring of these pathways to produce novel molecules.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Abdelwahab, M. F., Kurtán, T., Mándi, A., Müller, W. E., Fouad, M. A., Kamel, M. S. … Proksch, P. (2018). Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC approaches. Tetrahedron Letters, 59, 2647–2652
Adams, D. J., Wahl, M. L., Flowers, J. L., Sen, B., Colvin, M., Dewhirst, M. W. … Wani, M. C. (2006). Camptothecin analogs with enhanced activity against human breast cancer cells. II. Impact of the tumor pH gradient. Cancer chemotherapy and pharmacology, 57, 145–154
Alam, B., Lǐ, J., Gě, Q., Khan, M. A., Gōng, J., Mehmood, S., Yuán, Y., & Gǒng, W. (2021). Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Frontiers in Plant Science, 12, 791033
Amna, T., Amina, M., Sharma, P., Puri, S., Al-Youssef, H. M., Al-Taweel, A. M., & Qazi, G. (2012). Effect of precursors feeding and media manipulation on production of novel anticancer pro-drug camptothecin from endophytic fungus. Brazilian Journal of Microbiology, 43, 1476–1489
Amna, T., Puri, S. C., Verma, V., Sharma, J. P., Khajuria, R. K., Musarrat, J. … Qazi, G. (2006). Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Canadian Journal of Microbiology, 52, 189–196
Ardalani, H., Avan, A., & Ghayour-Mobarhan, M. (2017). Podophyllotoxin: A novel potential natural anticancer agent. Avicenna Journal of Phytomedicine, 7, 285
Ardizzoni, A., Hansen, H., Dombernowsky, P., Gamucci, T., Kaplan, S., Postmus, P., & Verweij, J. (1997). Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: A phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. Journal of Clinical Oncology, 15, 2090–2096. https://doi.org/10.1200/jco.1997.15.5.2090
Arroo, R., Alfermann, A., Medarde, M., Petersen, M., Pras, N., & Woolley, J. (2002). Plant cell factories as a source for anti-cancer lignans. Phytochemistry Reviews, 1, 27–35
Aswini, A., & Soundhari, C. (2018). Production of camptothecin from endophytic fungi and characterization by high-performance liquid chromatography and anticancer activity against colon cancer cell line. Asian Journal of Pharmaceutical Clinical Research, 11, 166–170
Atanasova-Penichon, V., Legoahec, L., Bernillon, S., Deborde, C., Maucourt, M., Verdal-Bonnin, M. N. … Richard-Forget, F. (2018). Mycotoxin biosynthesis and central metabolism are two interlinked pathways in Fusarium graminearum, as demonstrated by the extensive metabolic changes induced by caffeic acid exposure. Applied Environmental Microbiology, 84, e01705-01717
Atri, N., Rai, N., Singh, A. K., Verma, M., Barik, S., Gautam, V., & Singh, S. K. (2020). Screening for endophytic fungi with antibacterial efficiency from Moringa oleifera and Withania somnifera. Journal of Scientific Research, 64(1), 127–133
Bashyal, B. (1999). Seimatoantlerium nepalense, an endophytic taxol producing coelomycete from Himalayan yew (Taxus wallachiana). Mycotaxon, 72, 33–42
Bhalkar, B. N., Patil, S. M., & Govindwar, S. P. (2016). Camptothecine production by mixed fermentation of two endophytic fungi from Nothapodytes nimmoniana. Fungal Biology, 120, 873–883
Bharatiya, P., Rathod, P., Hiray, A., & Kate, A. S. (2021). Multifarious Elicitors: Invoking biosynthesis of various bioactive secondary metabolite in fungi. Applied Biochemistry and Biotechnology, 193, 668–686
Bo, G., Haiyan, L., & Lingqi, Z. (1998). Isolation of an fungus producting Vinbrastine. Journal of Yunnan University, 20, 214–215
Bodurka, D. C., Levenback, C., Wolf, J. K., Gano, J., Wharton, J. T., Kavanagh, J. J., & Gershenson, D. M. (2003). Phase II trial of irinotecan in patients with metastatic epithelial ovarian cancer or peritoneal cancer. Journal of Clinical Oncology, 21, 291–297
Cao, J., Tu, Y., & Jin, W. J. C. P. (2017a). Paclitaxel-producing Aspergillus flavus Bp6t2 and application thereof 106967622
Cao, J., & Jin, W., Y. T (2017b). Penicillium Sp. BP6T3 producing paclitaxel and application Thereof. 107129936 A. CN Patent
Chakraborty, S., & Rahman, T. (2012). The difficulties in cancer treatment. E-cancer Medical Science, 6, ed16
Chand, K., Shah, S., Sharma, J., Paudel, M. R., & Pant, B. (2020). Isolation, characterization, and plant growth-promoting activities of endophytic fungi from a wild orchid Vanda cristata. Plant Signaling Behavior, 15, 1744294
Chandra, S. (2012). Endophytic fungi: Novel sources of anticancer lead molecules. Applied Microbiology and Biotechnology, 95, 47–59
Chen, H. & Wang, Y. (2012). Screening of New Camptothecin-producing fungus for manufacture of Camptothecin. 102417883 A. CN Patent
Chen, J., Duan, L., Chen, H., Lin, H., Li, W., & Luo, J. (2009a). Studies on the antifungal activities of alterfungin and its derivatives. Zhongguo Kangshengsu Zazhi, 34, 60
Chen, J., Qiu, X., Wang, R., Duan, L., Chen, S., Luo, J., & Kong, L. (2009b). Inhibition of human gastric carcinoma cell growth in vitro and in vivo by cladosporol isolated from the paclitaxel-producing strain Alternaria alternata var. monosporus. Biological Pharmaceutical Bulletin, 32, 2072–2074
Chen, Z., Chen, H. P., Li, Y., Feng, T., & Liu, J. K. (2015). Cytochalasins from cultures of endophytic fungus Phoma multirostrata EA-12. The Journal of Antibiotics, 68, 23–26
Choudhari, A. S., Mandave, P. C., Deshpande, M., Ranjekar, P., & Prakash, O. (2020). Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Frontiers in Pharmacology, 10, 1614
Cichewicz, R. H. (2010). Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Natural Product Reports, 27, 11–22
El-Bialy, H. A., & El-Bastawisy, H. S. (2020). Elicitors stimulate paclitaxel production by endophytic fungi isolated from ecologically altered Taxus baccata. Journal of Radiation Research Applied Sciences, 13, 79–87
El-Hawary, S. S., Sayed, A. M., Mohammed, R., Hassan, H. M., Zaki, M. A., Rateb, M. E. … Abdelmohsen, U. R. (2018). Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicompactum. Marine Drugs, 16, 253
Elkhayat, E. S., & Goda, A. M. (2017). Antifungal and cytotoxic constituents from the endophytic fungus Penicillium sp. Bulletin of Faculty of Pharmacy, Cairo University, 55, 85–89
Eyberger, J. P. (2004). Production of Podophyllotoxin by endophytic fungi. U.S.A. Patent US20040248265A1
Ferlini, C., Raspaglio, G., Mozzetti, S., Distefano, M., Filippetti, F., Martinelli, E. … Scambia, G. (2003). Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Molecular Pharmacology, 64, 51–58
Frisvad, J. C., Andersen, B., & Thrane, U. J. M. (2008). The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycological Research, 112, 231–240
Gangadevi, V., & Muthumary, J. (2009). Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun tree). Biotechnology Applied Biochemistry, 52, 9–15
Gao, Y. H., Bai, W. X., Sun, W. H., Zhou, W. N., Wu, G. L., Zhu, Z. Q. … Li, H. Y. (2019). Diversity of culturable endophytic fungi associated with Bryophytes, Pteridophytes and Spermatophytes from Dawei Mountain. Nature Reserve, China Chiang Mai Journal Of Science, 46, 626–638
González-Menéndez, V., Pérez-Bonilla, M., Pérez-Victoria, I., Martín, J., Muñoz, F., Reyes, F. … Genilloud, O. (2016). Multicomponent analysis of the differential induction of secondary metabolite profiles in fungal endophytes. Molecules, 21, 234
Gulyamova, T., Abdulmyanova, L., Ruzieva, D., Rasulova, G., Yusupov, U., & Sattarova, R. (2019). Effect of epigenetic modifiers on fermentation parameters of endophytic fungi from plants growing in Uzbekistan. International Journal of Current Microbiology and Applied Sciences, 8, 2019
Haibo, C. (2016). Method for improving Paclitaxel yield in endophytic fungi fermented product. China Patent CN105400842A
Hassan, S. E. D. (2017). Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. Journal of Advanced Research, 8, 687–695
Hoffman, A. (2003). Method for isolating Taxane producing endophytic fungi from Angiosperms. US, 6,638,742 B1
Horn, W., Simmonds, M., Schwartz, R., & Blaney, W. (1995). Phomopsichalasin, a novel antimicrobial agent from an endophytic Phomopsis sp. Tetrahedron, 51, 3969–3978
Hsiang, Y. H., & Liu, L. F. (1988). Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Research, 48, 1722–1726
Huang, S., Chen, H., Li, W., Zhu, X., Ding, W., & Li, C. (2016). Bioactive chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Marine Drugs, 14, 172
Huang, Y. L., Zimmerman, N. B., & Arnold, A. E. (2018). Observations on the early establishment of foliar endophytic fungi in leaf discs and living leaves of a model woody angiosperm, Populus trichocarpa (Salicaceae). Journal of Fungi, 4, 58
Hubbard, M., Germida, J., & Vujanovic, V. (2014). Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. Journal of Applied Microbiology, 116, 109–122
Hussain, H., Jabeen, F., Krohn, K., Al-Harrasi, A., Ahmad, M., Mabood, F. … Green, I. R. (2015). Antimicrobial activity of two mellein derivatives isolated from an endophytic fungus. Medicinal Chemistry Research, 24, 2111–2114
Ibrahim, S. R., Mohamed, G. A., Al Haidari, R. A., Zayed, M. F., El-Kholy, A. A., Elkhayat, E. S., & Ross, S. A. (2018). Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorganic & Medicinal Chemistry, 26, 786–790
Jayeeta, J. C. (2013).Cost-effective process for commercial production of Paclitaxel by Fusarium Solani. WO Patent 2013164834 A1, 7 November 2013.
Kalidass, C., Mohan, V. R., & Daniel, A. (2009). Effect of auxin and cytokinin on vincristine production by callus cultures of Catharanthus roseus L.(apocynaceae). Tropical and Subtropical Agroecosystems, 12, 283–288
Keshri, P. K., Rai, N., Verma, A., Kamble, S. C., Barik, S., Mishra, P. … Gautam, V. (2021). Biological potential of bioactive metabolites derived from fungal endophytes associated with medicinal plants. Mycological Progress, 20(5), 577–594
Kornsakulkarn, J., Choowong, W., Rachtawee, P., Boonyuen, N., Kongthong, S., Isaka, M., & Thongpanchang, C. (2018). Bioactive hydroanthraquinones from endophytic fungus Nigrospora sp. BCC 47789. Phytochemistry Letters, 24, 46–50
Koul, M., Meena, S., Kumar, A., Sharma, P. R., Singamaneni, V., Riyaz-Ul-Hassan, S. … Gupta, P. (2016). Secondary metabolites from endophytic fungus Penicillium pinophilum induce ROS-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Medica, 82, 344–355
Koul, M., & Singh, S. (2017). Penicillium spp.: prolific producer for harnessing cytotoxic secondary metabolites. Anticancer Drugs, 28, 11–30
Kour, A., Shawl, A. S., Rehman, S., Sultan, P., Qazi, P. H., Suden, P. … Verma, V. (2008). Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World Journal of Microbiology and Biotechnology, 24, 1115–1121
Krown, S. E., Moser, C. B., MacPhail, P., Matining, R. M., Godfrey, C., Caruso, S. R., & Gottshall, B. (2020). Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: A three-arm, open-label, randomised, non-inferiority trial. The Lancet, 395, 1195–1207
Kumar, A., Patil, D., Rajamohanan, P. R., & Ahmad, A. (2013). Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One, 8, e71805
Kusari, S., Lamshöft, M., & Spiteller, M. (2009). Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. Journal of Applied Microbiology, 107, 1019–1030
Li, H. L., Li, X. M., Mándi, A., Antus, S., Li, X., Zhang, P. … Wang, B. G. (2017). Characterization of Cladosporols from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399 and configurational revision of the previously reported Cladosporol derivatives. The Journal of Organic Chemistry, 82, 9946–9954
Li, J. Y., Sidhu, R. S., Ford, E., Long, D., Hess, W., & Strobel, G. (1998). The induction of taxol production in the endophytic fungus—Periconia sp. from Torreya grandifolia. Journal of Industrial Microbiology Biotechnology, 20, 259–264
Li, Q., Chen, C., Cheng, L., Wei, M., Dai, C., He, Y. … Liu, J. (2019a). Emeridones A–F, a series of 3, 5-demethylorsellinic acid-based meroterpenoids with rearranged skeletons from an endophytic fungus Emericella sp. TJ29. The Journal of Organic Chemistry, 84, 1534–1541
Li, X. H., Han, X. H., Qin, L. L., He, J. L., Cao, Z. X., Gu, Y. C. … Deng, Y. (2019b). Isochromanes from Aspergillus fumigatus, an endophytic fungus from Cordyceps sinensis. Natural Product Research, 33, 1870–1875
Li, S., Chen, J. F., Qin, L. L., Li, X. H., Cao, Z. X., Gu, Y. C. … Deng, Y. (2020). Two new sesquiterpenes produced by the endophytic fungus Aspergillus fumigatus from Ligusticum wallichii. Journal of Asian Natural Products Research, 22, 138–143
Liang, Z., Zhang, J., Zhang, X., Li, J., Zhang, X., & Zhao, C. (2016). Endophytic fungus from Sinopodophyllum emodi (Wall.) Ying that produces Podophyllotoxin. Journal of Chromatographic Science, 54, 175–178
Liang, Z., Zhang, T., Zhang, X., Zhang, J., & Zhao, C. (2015). An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules, 20, 1424–1433
Lin, P. C., Wu, Y. Z., Bao, T. W., Wang, Y. N., Shang, X. Y., & Lin, S. (2018). A new cytotoxic 12-membered macrolactone from the endophytic fungus Exserohilum rostratum LPC-001. Journal of Asian Natural Products Research, 20, 1093–1100
Liu, H. X., Tan, H. B., Chen, Y. C., Li, S. N., Li, H. H., & Zhang, W. M. (2020). Cytotoxic triquinane-type sesquiterpenoids from the endophytic fungus Cerrena sp. A593. Natural Product Research, 34, 2430–2436
Liu, J. F., Sang, C. Y., Xu, X. H., Zhang, L. L., Yang, X., Hui, L. … Chen, S. W. (2013). Synthesis and cytotoxic activity on human cancer cells of carbamate derivatives of 4β-(1, 2, 3-triazol-1-yl) podophyllotoxin. European Journal of Medicinal Chemistry, 64, 621–628
Lopes, A., da Silva, D., Lopes, N., & Pupo, M. (2012). Epigenetic modulation changed the secondary metabolite profile in the endophyte Nigrospora sphaerica SS67. Planta Medica, 78, PL38
Lugtenberg, B. J., Caradus, J. R., Johnson, L. J. (2016). Fungal endophytes for sustainable crop production. FEMS Microbiology Ecology, 92, 01–17
Magotra, A., Kumar, M., Kushwaha, M., Awasthi, P., Raina, C., Gupta, A. P., & Chaubey, A. (2017). Epigenetic modifier induced enhancement of fumiquinazoline C production in Aspergillus fumigatus (GA-L7): An endophytic fungus from Grewia asiatica L. AMB Express, 7, 1–10
Majumder, A., & Jha, S. (2009). Characterization of podophyllotoxin yielding cell Lines of Podophyllum hexandrum. Caryologia, 62, 220–235
Manci, N., Marchetti, C., Di Tucci, C., Giorgini, M., Esposito, F., Palaia, I. … Panici, P. B. (2011). A prospective phase II study of topotecan (Hycamtin®) and cisplatin as neoadjuvant chemotherapy in locally advanced cervical cancer. Gynecologic Oncology, 122, 285–290
Mann, K. (2020). An analysis of cancer incidences and mortality in India. Asian Journal of Multidimensional Research, 9, 79–88
Mathur, S., & Hoskins, C. (2017). Drug development: Lessons from nature. Biomedical Reports, 6, 612–614
Morales-Sánchez, V., Fe Andrés, M., Díaz, C. E., & González-Coloma, A. (2020). Factors affecting the metabolite productions in endophytes: Biotechnological approaches for production of metabolites. Current Medicinal Chemistry, 27, 1855–1873
Ozdemir, N., Dogan, M., Sendur, M. A. N., Yazici, O., Abali, H., Yazilitas, D., & Zengin, N. (2014). Efficacy and safety of first line vincristine with doxorubicin, bleomycin and dacarbazine (ABOD) for Hodgkin’s lymphoma: A single institute experience. Asian Pacific Journal of Cancer Prevention, 15, 8715–8718
Palem, P. P., Kuriakose, G. C., & Jayabaskaran, C. (2015). An endophytic fungus, Talaromyces radicus, isolated from Catharanthus roseus, produces vincristine and vinblastine, which induce apoptotic cell death. PLoS One, 10, e0144476
Prabukumar, S., Rajkuberan, C., Ravindran, K., & Sivaramakrishnan, S. (2015). Isolation and characterization of endophytic fungi from medicinal plant Crescentia cujete L. and their antibacterial, antioxidant and anticancer properties. International Journal of Pharmacy and Pharmaceutical Sciences, 7, 316–321
Pu, X., Zhang, C. R., Zhu, L., Li, Q. L., Huang, Q. M., Zhang, L., & Luo, Y. G. (2019). Possible clues for camptothecin biosynthesis from the metabolites in camptothecin-producing plants. Fitoterapia, 134, 113–128
Puri, S. C., Nazir, A., Chawla, R., Arora, R., Riyaz-ul-Hasan, S., Amna, T. … Sagar, R. (2006). The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. Journal of Biotechnology, 122, 494–510
Puri, S. C., Verma, V., Amna, T., Qazi, G. N., & Spiteller, M. (2005). An endophytic fungus from Nothapodytes foetida that produces Camptothecin. Journal of Natural Products, 68, 1717–1719
Qiao, Y., Tu, K., Feng, W., Liu, J., Xu, Q., Tao, L. … Xue, Y. (2018). Polyketide and prenylxanthone derivatives from the endophytic fungus Aspergillus sp. TJ23. Chemistry Biodiversity, 15, e1800395
Qin, D., Shen, W., Wang, J., Han, M., Chai, F., Duan, X. … Zuo, S. (2019). Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4. 1850. Phytochemistry, 158, 56–66
Rabindran, S. K., Ross, D. D., Doyle, L. A., Yang, W., & Greenberger, L. M. (2000). Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Research, 60, 47–50
Rai, N., Kumari Keshri, P., Verma, A., Kamble, S. C., Mishra, P., Barik, S. … Gautam, V. (2021). Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology, 12(3), 139–159
Rai, N., Keshri, P. K., Gupta, P., Verma, A., Kamble, S. C., Singh, S. K., & Gautam, V. (2022). Bioprospecting of fungal endophytes from Oroxylum indicum (L.) Kurz with antioxidant and cytotoxic activity. PLOS ONE, 17(3), e0264673. https://doi.org/10.1371/journal.pone.0264673
Ramakrishna, W., Kumari, A., Rahman, N., & Mandave, P. (2021). Anticancer activities of plant secondary metabolites: Rice Callus Suspension culture as a new paradigm. Rice Science, 28, 13–30
Ran, X., Zhang, G., Li, S., & Wang, J. (2017). Characterization and antitumor activity of camptothecin from endophytic fungus Fusarium solani isolated from Camptotheca acuminate. African Health Sciences, 17, 566–574
Ranjan, A., Singh, R. K., Khare, S., Tripathi, R., Pandey, R. K., Singh, A. K. … Singh, S. K. (2019). Characterization and evaluation of mycosterol secreted from endophytic strain of Gymnema sylvestre for inhibition of α-glucosidase activity. Scientific Reports, 9, 1–13
Reita, D., Bour, C., Benbrika, R., Groh, A., Pencreach, E., Guérin, E., & Guenot, D. (2019). Synergistic anti-tumor effect of mTOR inhibitors with irinotecan on colon cancer cells. Cancers, 11, 1581
Rougier, P., Van Cutsem, E., Bajetta, E., Niederle, N., Possinger, K., Labianca, R. … Wils, J. (1998). Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. The Lancet, 352, 1407–1412
Ruan, B. H., Yu, Z. F., Yang, X. Q., Yang, Y. B., Hu, M., Zhang, Z. X. … Ding, Z. T. (2018). New bioactive compounds from aquatic endophyte Chaetomium globosum. Natural Product Research, 32, 1050–1055
Saraiva, N. N., Rodrigues, B. S., Jimenez, P. C., Guimarães, L. A., Torres, M. C., Rodrigues-Filho, E. … de Mattos, M. C. (2015). Cytotoxic compounds from the marine-derived fungus Aspergillus sp. recovered from the sediments of the Brazilian coast. Natural Product Research, 29, 1545–1550
Schulz, B., & Boyle, C. (2005). The endophytic continuum. Mycological Research, 109, 661–686
Sebola, T. E., Uche-Okereafor, N. C., Mekuto, L., Makatini, M. M., Green, E., & Mavumengwana, V. (2020). Antibacterial and anticancer activity and untargeted secondary metabolite profiling of crude bacterial endophyte extracts from Crinum macowanii Baker leaves. International Journal of Microbiology, 2020, 8839490
Sharma, N., Kushwaha, M., Arora, D., Jain, S., Singamaneni, V., Sharma, S. … Jaglan, S. (2018a). New cytochalasin from Rosellinia sanctae-cruciana, an endophytic fungus of Albizia lebbeck. Journal of Applied Microbiology, 125, 111–120
Sharma, V., Singamaneni, V., Sharma, N., Kumar, A., Arora, D., Kushwaha, M. … Gupta, P. (2018b). Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorganic Medicinal Chemistry Letters, 28, 2217–2221
Shweta, S., Bindu, J. H., Raghu, J., Suma, H., Manjunatha, B., Kumara, P. M. … Shaanker, R. U. (2013). Isolation of endophytic bacteria producing the anti-cancer alkaloid camptothecine from Miquelia dentata Bedd.(Icacinaceae). Phytomedicine, 20, 913–917
Shweta, S., Zuehlke, S., Ramesha, B., Priti, V., Kumar, P. M., Ravikanth, G. … Shaanker, R. U. (2010). Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry, 71, 117–122
Slot, J. C., & Rokas, A. (2011). Horizontal transfer of a large and highly toxic secondary metabolic gene cluster between fungi. Current Biology, 21, 134–139
Soanes, D., & Richards, T. A. J. A. R. P. (2014). Horizontal gene transfer in eukaryotic plant pathogens. Annual Review of Phytopathology, 52, 583–614
Sriram, D., Yogeeswari, P., Thirumurugan, R., & Ratan Bal, T. (2005). Camptothecin and its analogues: A review on their chemotherapeutic potential. Natural Product Research, 19, 393–412
Stierle, A., Strobel, G., & Stierle, D. (1993). Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science, 260, 214–216
Stierle, A., Strobel, G., Stierle, D., Grothaus, P., & Bignami, G. (1995). The search for a taxol-producing microorganism among the endophytic fungi of the Pacific yew, Taxus brevifolia. Journal of Natural Products, 58, 1315–1324
Strobel, G., Daisy, B., Castillo, U., & Harper, J. (2004). Natural products from endophytic microorganisms. Journal of Natural Products, 67, 257–268
Strobel, G. A., Ford, E., Li, J., Sears, J., Sidhu, R. S., & Hess, W. (1999). Seimatoantlerium tepuiense gen. nov., a unique epiphytic fungus producing taxol from the Venezuelan guyana. Systematic Applied Microbiology, 22, 426–433
Sudhakar, T., Dash, S., Rao, R., Srinivasan, R., Zacharia, S., Atmanand, M. … Nayak, S. (2013). Do endophytic fungi possess pathway genes for plant secondary metabolites. Current Science, 104, 178
Sun, X., Cui, M., Wang, D., Guo, B., & Zhang, L. (2018). Tumor necrosis factor-related apoptosis inducing ligand overexpression and Taxol treatment suppresses the growth of cervical cancer cells in vitro and in vivo. Oncology Letters, 15, 5744–5750
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249
Suryanarayanan, T., Thirunavukkarasu, N., Govindarajulu, M., Sasse, F., Jansen, R., & Murali, T. (2009). Fungal endophytes and bioprospecting. Fungal Biology Reviews, 23, 9–19
Suryanarayanan, T. S., Thirunavukkarasu, N., Govindarajulu, M. B., & Gopalan, V. (2012). Fungal endophytes: An untapped source of biocatalysts. Fungal Diversity, 54, 19–30
Taechowisan, T., Chaisaeng, S., & Phutdhawong, W. S. (2017). Antibacterial, antioxidant and anticancer activities of biphenyls from Streptomyces sp. BO-07: An endophyte in Boesenbergia rotunda (L.) Mansf. A Food Agricultural Immunology, 28, 1330–1346
Tan, X., Zhou, Y., Zhou, X., Xia, X., Wei, Y., He, L., & Yu, L. (2018). Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; A rare medicinal plant endemic to China. Scientific Reports, 8, 1–9
Tang, X., Wu, X., Liu, X., & Ma, Y., X F (2016). Method for preparing Cytochalasin H from mangrove endophytic fungi. 105925646 A. CN Patent
Tantapakul, C., Promgool, T., Kanokmedhakul, K., Soytong, K., Song, J., Hadsadee, S. … Kanokmedhakul, S. (2020). Bioactive xanthoquinodins and epipolythiodioxopiperazines from Chaetomium globosum 7s-1, an endophytic fungus isolated from Rhapis cochinchinensis (Lour.) Mart. Natural Product Research, 34, 494–502
Tawfike, A. F., Romli, M., Clements, C., Abbott, G., Young, L., Schumacher, M. … Edrada-Ebel, R. (2019). Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. Journal of Chromatography B, 1106, 71–83
Thirumaran, R., Prendergast, G. C., & Gilman, P. B. (2007). Cytotoxic chemotherapy in clinical treatment of cancer. Cancer Immunotherapy (pp. 101–116). Elsevier
Van Goietsenoven, G., Mathieu, V., Andolfi, A., Cimmino, A., Lefranc, F., Kiss, R., & Evidente, A. (2011). In vitro growth inhibitory effects of cytochalasins and derivatives in cancer cells. Planta Medica, 77, 711–717
Verekar, S. A., Mishra, P. D., Sreekumar, E. S., Deshmukh, S. K., Fiebig, H. H., Kelter, G., & Maier, A. (2014). Anticancer activity of new depsipeptide compound isolated from an endophytic fungus. The Journal of Antibiotics, 67, 697–701
Verma, A., Gupta, P., Rai, N., Tiwari, R. K., Kumar, A., Salvi, P., Kamble, S. C., Singh, S. K., & Gautam, V. (2022). Assessment of biological activities of fungal endophytes derived bioactive compounds Isolated from Amoora rohituka. Journal of Fungi, 8(3), 285. https://doi.org/10.3390/jof8030285
Von Bubnoff, A. (2006). Seeking new antibiotics in nature’s backyard. Cell, 127, 867–869
Wang, T., Ma, Y., Ye, Y., Zheng, H., Zhang, B., & Zhang, E. (2017). Screening and identification of endophytic fungi producing podophyllotoxin compounds in Sinopodophyllum hexandrum stems. Chinese J Exp Trad Med Formul, 18, 493–532.
Weaver, B. A. (2014). How Taxol/paclitaxel kills cancer cells. Molecular Biology of the Cell, 25, 2677–2681
Xiao, J., Lin, L. B., Hu, J. Y., Duan, D. Z., Shi, W., Zhang, Q. … Wang, X. L. (2018). Pestalustaines A and B, unprecedented sesquiterpene and coumarin derivatives from endophytic fungus Pestalotiopsis adusta. Tetrahedron Letters, 59, 1772–1775
Xie, F., Li, X. B., Zhou, J. C., Xu, Q. Q., Wang, X. N., Yuan, H. Q., & Lou, H. X. (2015). Secondary metabolites from Aspergillus fumigatus, an endophytic fungus from the liverwort Heteroscyphus tener (Steph.) Schiffn. Chemistry Biodiversity, 12, 1313–1321
Yang, X., Wu, P., Xue, J., Li, H., & Wei, X. (2020). Cytochalasans from endophytic fungus Diaporthe sp SC-J0138. Fitoterapia, 145, 104611
Yang, Y., Zhao, H., Barrero, R. A., Zhang, B., Sun, G., Wilson, I. W. … Bruce, R. (2014). Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genomics, 15, 1–14
Yu, N. H., Kim, J. A., Jeong, M. H., Cheong, Y. H., Hong, S. G., Jung, J. S. … Hur, J. S. (2014). Diversity of endophytic fungi associated with bryophyte in the maritime Antarctic (King George Island). Polar Biology, 37, 27–36
Zhang, Y. (2011a). Induction of Nothapodytes nimmoniana endophyte to produce sugar derivative of Camptothecin. 102080110 A. CN Patent
Zhang, Y. (2011b). Method for inducing Nothapodytes nimmoniana endophyte to produce 10-HydroxyCamptothecin. 102080111 A. CN Patent
Zhang, Y. (2011c). Induction of Nothapodytes nimmoniana endophyte to manufacture 9-Methoxycamptothecin. 102080112 A. CN Patent
Zhang, J., Zhang, S., Song, J., Sun, K., Zong, C., Zhao, Q. … Wei, L. (2014). Autophagy inhibition switches low-dose camptothecin-induced premature senescence to apoptosis in human colorectal cancer cells. Biochemical Pharmacology, 90, 265–275
Zhang, Z., He, X., Liu, C., Che, Q., Zhu, T., Gu, Q., & Li, D. (2016). Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342. RSC Advances, 6, 76498–76504
Zhao, K., Yu, L., Jin, Y., Ma, X., Liu, D., & Wang, X. (2016). Advances and prospects of taxol biosynthesis by endophytic fungi. Chinese Journal of Biotechnology, 32, 1038–1051
Zhencheng, L. (2014). High Paclitaxel-producing endophytic fungi Botryosphaeria dothidea for manufacture of Paclitaxel. China Patent CN103911293A
Zhu, F., Chen, G., Wu, J., & Pan, J. (2013). Structure revision and cytotoxic activity of marinamide and its methyl ester, novel alkaloids produced by co-cultures of two marine-derived mangrove endophytic fungi. Natural Product Research, 27, 1960–1964
Zurlo, D., Assante, G., Moricca, S., Colantuoni, V., & Lupo, A. (2014). Cladosporol A, a new peroxisome proliferator-activated receptor γ (PPARγ) ligand, inhibits colorectal cancer cells proliferation through β-catenin/TCF pathway inactivation. Biochimica et Biophysica Acta -General Subjects, 1840, 2361–2372
Zhao, C., Zhu, Y., Liang, Z., Zhang, J., & Qian, Z. (2012). One endophytic fungi from Sinopodophyllum emodi and the application thereof. CN Patent 102559517 A, 11 July 2012
Acknowledgements
NR would like to thank the University Grants Commission, New Delhi, India, for the Junior Research Fellowship. PG and PKK would like to thank the Science and Engineering Research Board (SERB) India for Junior Research Fellowship under Empowerment and Equity Opportunities for Excellence in Science (EMEQ) scheme (EEQ/2019/000025). AV would like to thank the Council of Scientific and Industrial Research, New Delhi, India, for the Junior Research Fellowship.
Funding
This work is funded by a Start-up grant from University Grants Commission, New Delhi, India to Dr. Vibhav Gautam. The VG laboratory is also supported by the SERB-EMEQ project (EEQ/2019/000025) and Banaras Hindu University, Varanasi, India Institution of Eminence Seed Grant.
Author information
Authors and Affiliations
Contributions
NR, PG and PKK wrote and compiled the manuscript. AV, PM, DK, AK and SKS revised the manuscript. Study and entire writing of the manuscript was supervised by VG (from the compilation of the first draft to the final draft).
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Research Involving Human Participants and/or Animals
The article does not include any human and/or animal-based study.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rai, N., Gupta, P., Keshri, P.K. et al. Fungal Endophytes: an Accessible Source of Bioactive Compounds with Potential Anticancer Activity. Appl Biochem Biotechnol 194, 3296–3319 (2022). https://doi.org/10.1007/s12010-022-03872-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03872-1