Abstract
Bryophytes comprise one of the richest microfungal microhabitats in the Antarctic environment. The maritime Antarctic is very vulnerable to rapid environmental change caused by global warming. The aim of this study was to investigate the importance of bryophytes as a microhabitat for fungal species in the maritime Antarctic by surveying endophytic fungal diversity from several bryophytes including Andreaea sp., Barbilophozia hatcheri, Chorisodontium aciphyllum, Polytrichum alpinum, Polytrichum strictum, Sanionia uncinata, and Warnstorfia sarmentosa. We collected 13 bryophyte samples at four localities on Barton Peninsula, King George Island. In total, 31 endophytic fungi morphotypes were isolated from bryophyte tissues by a thorough surface sterilization method. Using internal transcribed spacer sequence analysis, 16 endophytic fungal strains belonging to Ascomycota (12), Basidiomycota (1), Oomycota (1), and Zygomycota (2) phyla were obtained. Our results suggest the presence of a diverse range of fungal species even in a very limited area, and those bryophytes play an important role in conserving fungal diversity in this harsh environment. Growth rate measurements at a wide range of temperatures confirmed that most of the fungal strains were both mesophilic and psychrotolerant. This is the first report of endophytic fungi in Antarctic moss tissue by fluorescence in situ hybridization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctica, considered an extreme environment, is characterized by high-stress and high-disturbance conditions (Pugh 1980) that are amplified by low temperatures, high aridity, high incidence of ultraviolet radiation, low and sporadic availability of nutrients, and strong thermal excursions, particularly at the microhabitat level (Vishniac 1993). King George Island is the largest of the South Shetland Islands belonging to the maritime Antarctic zone (Lee et al. 2008), where the ameliorating effect of the ocean produces a milder climate (Kanda and Komárková 1997). King George Island is dominated by a diversity of bryophyte and lichen species with only two vascular plant species (Øvstedal and Lewis-Smith 2002). Microbial diversity and activity are also affected by extreme conditions, and it is generally accepted that microbial species richness is more restricted in such environments than in temperate regions (Wynn-Williams 1996).
The Southern Ocean represents a major barrier to the colonization and distribution of new species to Antarctica. Terrestrial organism propagules arrive in the Antarctic almost exclusively via wind dispersal, animals, or humans (Ellis-Evans and Walton 1990). This geographical isolation and the relatively low anthropogenic impact offer ideal conditions within which to study the fungal population composition in different substrates because airborne spores that could generate fungal flora in isolation experiments are relatively rare and the expected diversity is low when compared with temperate or tropical climates (Mőller and Dreyfuss 1996).
In the past, mycological studies conducted in Antarctic regions have been generally restricted to soil (Tubaki and Asano 1965; Boyd et al. 1966; Heal et al. 1967; Bailey and Wynn-Williams 1982) or freshwater habitats (Ellis-Evans 1985; Montemartini-Corte 1991), and only plants, mosses, and other organic substrates have been used as sources for the isolation of Antarctic microfungi (Pugh and Allsopp 1982; Fletcher et al. 1985; Gamundi and Spinedi 1988; Caretta and Frate 1990; Baublis et al. 1991; Onofri et al. 1994; Hoshino et al. 1999; Bradner et al. 2000). The study of fungi in Antarctica is related to host distribution such as birds, invertebrates, and vegetation, with the latter consisting of bryophyte and lichen communities (Tosi et al. 2002) and focused on species that live in nonvascular plants. These include bryophytes (i.e., mosses, liverworts, and hornworts), which are functionally important producers (Upson et al. 2007; Hoshino et al. 2009; U’ren et al. 2010; Siciʼnski et al. 2011; Zhang et al. 2013). Samples collected in mid-Victoria Land during 1988/1998 indicate that microfungi occur most frequently in mosses and the soil underneath them (Caretta and Frate 1990; Caretta et al. 1994). Mosses are a suitable substrate for the growth of some nematode-trapping fungi of the genus Arthrobotrys (Caretta et al. 1994). Within the Antarctic environment, mosses are one of the richest microfungal microhabitats, particularly in regard to indigenous psychrophilic species (Tosi et al. 2002).
Associations between mosses and fungi were long thought to be uncommon and rare (Grasso and Scheirer 1981). Although interactions between bryophilous ascomycetes and bryophytes have been investigated by Dőbbeler (1997), endophyte and bryophyte interactions have remained largely unstudied (Davey and Currah 2006).
In this study, we isolated fungal endophytes from Antarctic bryophytes and conducted a phylogenetic study. Moreover, we tested the growth rate of the endophytic fungi isolated at a range of temperatures. Finally, we attempted to detect fungal endophyte hyphae in Polytrichum strictum using selective staining and in-tube fluorescence in situ hybridization (FISH) coupled with confocal laser scanning microscopy (CLSM).
Materials and methods
Sample collection
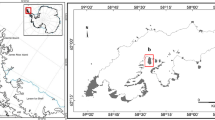
We analyzed 13 bryophytes belonging to seven species collected from different locations in the maritime Antarctic, King George Island (Fig. 1; Table 1). Bryophyte samples devoid of apparent fungal infection were collected under aseptic conditions into sterile polyethylene tubes and preserved at 4 °C.
Fungal endophytic isolation
Isolation of the internal fungus portion was performed as previously described by Li et al. (2007). Briefly, moss fragments were washed for 3 h in streaming water and immersed in 75 % ethanol for 1 min, in 2 % sodium hypochlorite for 3 min, and then in 75 % ethanol for 30 s. Each fragment was then gently rinsed with sterilized distilled water, which was subsequently analyzed by polymerase chain reaction to check for fungal contamination of the thallus surface. Sterilized samples were then dried with sterile paper towels, after which the rinsed fragments were dried with sterile filter papers and then plated on potato dextrose agar with 0.01 % streptomycin and incubated at 15 °C. Fungi growing from each fragment were isolated from pure cultures, purified on 2 % MY solid medium (Malt Extract Broth; BD Difco, Sparks, MD, USA), and then deposited in the Korea Lichen and Allied Bioresources Center (KOLABIC), Korea Lichen Research Institute (KoLRI), Sunchon National University.
DNA extraction, amplification, sequencing, and phylogeny analysis
All isolates were grouped into different morphospecies based on the following morphological characteristics: colony color, texture, and growth rate on MY solid medium. All isolates were subjected to molecular identification. Fungal DNA extraction was performed using a DNeasy® Plant Mini Kit according to the manufacturer’s protocols (Qiagen, Hilden, Germany). The internal transcribed spacer (ITS) region of the rDNA gene was amplified with the universal primers ITS4 (5′-TCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) (White et al. 1990). The amplified DNA was then concentrated and purified using a PCR quick-spin™ PCR Product Purification Kit (INTRON Biotechnology, Seongnam, Korea). For the species-level identification by rDNA gene sequencing, the consensus sequence was aligned with all related species sequences retrieved from the GenBank database. The consensus sequences were then deposited into GenBank under the accession numbers HQ335293–HQ335308. Phylogenetic analysis was performed using the ITS region sequences of the endophytic fungi retrieved from the GenBank database (Supplementary Table 1) and the 16 endophytes isolated in this study. Sequence alignment was conducted in BioEdit, and a phylogenetic tree was generated by the neighbor-joining (NJ), minimum evolution (ME), and maximum likelihood (ML) analysis methods performed in MEGA 5.0 (Tamura et al. 2011) with the number of bootstrap trials set to 1,000.
Temperature preference
Temperature preference was tested using 15 representative fungal isolates. Samples were inoculated on MY medium plates (90 × 15 mm diameter) and incubated at 4 ± 1, 10 ± 1, 15 ± 1, 25 ± 1, and 30 ± 1 °C. Fungal growth was recorded daily for 1 month, and the mean growth rate was expressed in millimeter per day. All analyses were conducted in triplicate.
Selective fungal staining
Sterilized P. strictum (sample no. 4) were directly embedded in Tissue-Tek OCT compound (Sakura Fintek, Torrance, CA, USA), which prevents tissue damage. The samples were then rapidly frozen at −22 °C and cut into 8 μm-thick sections on a cryomicrotome (Microm HM 520, Labcon, Germany). The cryosections were then directly placed into a 1.5 ml microcentrifuge tube, after which they were cleared with 10 % KOH for 10 min at 90 °C and washed three times in deionized water. The sections were then treated with H2O2 for 3 min and 2 % HCl for 4 min, after which they were stained with 0.5 % trypan blue for 12 h at 50 °C. Finally, the sections were placed on a regular microscopic glass slide and immediately mounted with 50 % glycerol and cover slipped.
Fixation
The endophytic fungal strain EFOMIA 03 cultured in MY liquid medium and sterilized P. strictum thalli were fixed with freshly prepared 3.7 % paraformaldehyde solution in PBS for 12 h at 4 °C and then washed three times in ice-cold phosphate-buffered saline (PBS) before storage at −20 °C in 1:1 ice-cold PBS/96 % ethanol.
Probe design and evaluation
All 28S rDNA fungal endophyte sequences were aligned with the 28S rDNA-specific probe sequences (Baschien et al. 2008), and an NME-2 of 13-mer probe was designed to detect fungal endophytes (5′-GTTCAGCGGGTAT-3′). The NME-2 probes were labeled with fluorescein maleimide (Vector Laboratories, Burlingame, VT, USA) using a FastTag R Basic labeling kit (Vector Laboratories), and fluorescein labeling efficiency was confirmed using dot blot (Vector Laboratories). The fluorescein-labeled NME-2 probe was stored in the dark at −20 °C.
In-tube FISH
Small fragments (10–15 mm length) of fixed P. strictum and EFOMIA 03 were embedded in Tissue-Tek® O.C.T. Compound (Sakura Fintek), rapidly frozen at −22 °C, and cut into 8 μm-thick sections on a cryotome. The cryosections were then placed directly in a 1.5 ml microcentrifuge tube. To decrease the inherent autofluorescence signals, the cryosections were treated with 100 mM Tris–HCl buffer (pH 8) with 50 mM sodium borohydride (NaBH4) for 30 min. The samples were subsequently embedded in permeabilization buffer [1 × PBS, 1 % sodium dodecyl sulfate (SDS), pH 5.5] and lysing enzymes from Trichoderma harzianum (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 1 mg of lysing enzymes per milliliter of buffer. The samples were then incubated at 30 °C for 1 h, after which they were rinsed twice with ice-cold PBS. An ethanol series (50–70–85–96 % ethanol solutions, 3 min each) was carried out in the same tube. The samples were then quickly rinsed and washed for 3 min at room temperature. All hybridizations were performed at 42 °C for 12 h in a buffer containing 0.9 M NaCl, 20 mM Tris–HCl (pH 8), 0.01 % SDS, and 40 % formamide, and the fluorescent probes were gently mixed to give a final oligonucleotide concentration of 5 ng/μl. The hybridization buffer was immediately removed, and the samples were rinsed twice with prewarmed washing buffer [20 mM Tris–HCl (pH 8) and 31.4 mM NaCl] for 2–3 h at 42 °C. The washing buffer was then removed, and the samples were rinsed with ice-cold water. The section embedded with 1 × ISH blocking solution was subsequently warmed for 30 min at 37 °C and incubated with 10 μg of biotinylated anti-fluorescein of 1 × blocking solution ml for 30 min at room temperature, after which it was washed twice for 3 min each in 1 × blocking solution to remove the unconjugated biotinylated anti-fluorescein. Next, the section was incubated with fluorescein-avidin DCS solution (10 μg/ml in 1 × blocking solution) for 30 min at room temperature and washed twice for 5 min in 4 × SSC + 0.1 % Tween 20. The sections were finally placed on a regular glass microscope slide and quickly dried with compressed air. The sections were immediately mounted with Vectashield® HardSet™ Mounting Medium (Vector Laboratories), incubated for 12 h at 4 °C as recommended by the manufacturer, and observed by CLSM.
Results
Phylotype identification and phylogeny
In total, 31 morphologically distinct fungal endophytes were obtained from 13 different Antarctic bryophytes and grouped into 16 different fungal endophyte strains by ITS sequence analysis (Table 1). Of these endophytes, three strains (EFOMIA 02, 04, and 15) were 100 % homologous with fungi retrieved from the GenBank database. EFOMIA 02 was 100 % homologous with Lecythophora hoffmannii (GenBank: AB231012). EFOMIA 04 showed 100 % similarity to a fungal sp. (GenBank: HM123665), and EFOMIA 15 was 100 % homologous with Antarctomyces psychrotophicus (GenBank: AM489755) isolated from the larvae of the introduced chironomid Eretmoptera murphyi on Signy Island.
Nine strains exhibited 98–99 % similarity to fungi retrieved from the GenBank database, most of which were homologous with uncultured fungi. The remaining strains shared less than 97 % similarity to fungi retrieved from the GenBank database. Almost all of the endophytes were previously unidentified.
All of the endophytic isolates were grouped into 16 different strains and identified by molecular analysis. Phylogenetic analysis revealed that 12 strains belonged to Ascomycota, two strains to Zygomycota, and one strain each to Basidiomycota and Oomycota (Fig. 2).
Phylogenetic tree of fungal taxa recovered from Antarctic bryophytes and their closest relatives. The tree was constructed based on the rDNA gene sequence (ITS1-5.8S-ITS2) by the NJ, ME, and ML analysis methods in MEGA 5.0 with 1,000 bootstrap trials. The bootstrap values are presented near the corresponding branch (NJ/ME/ML), and those above 95 % are indicated by bold lines
Temperature preference
The temperature preferences of 15 fungal endophytes are presented in Table 2. The strain EFOMIA 07 isolated from P. strictum (sample no. 4) was not included because of its very slow growth. Most of the fungal strains tested grew at the relatively low temperature of 4 °C, but EFOMIA 05 could only grow between 10 and 30 °C, indicating that it is not psychrotolerant. EFOMIA 03, 06, and 10 grew at temperatures in the range of 4–15 °C, while optimum EFOMIA 10 growth occurred at 15 °C. EFOMIA 08, 09, 11, and 15 grew at temperatures in the range of 4–25 °C, and EFOMIA 11 displayed different morphotypes under different temperatures. Specifically, its color changed from white to orange, and the texture of its hyphae appeared cottonless from 4 to 10 °C. However, the hyphae produced a dendritic pattern from 15 to 25 °C (data not shown). Optimum EFOMIA 09 and 10 growth occurred at 15 °C and they also grew well at 4 °C, indicating that they are likely psychrophilic. EFOMIA 01, 04, 13, and 14 grew at 4–30 °C, with EFOMIA 04 and 14 exhibiting very good growth at 25–30 °C, indicating that they are mesophilic. EFOMIA 01, 12, and 16 grew relatively well at 30 °C, and these three strains were relatively more thermotolerant than the other strains investigated.
Selective staining and FISH of fungal hyphae on Antarctic moss
Selective staining of vertical P. strictum (sample no. 4) sections clearly highlighted EFOMIA 03 hyphae (blue) within moss tissues (Fig. 3a). The endophytic fungus hyphae extended across the cell wall of the moss, but were not observed in the cell-wall interspaces. Prior to in-tube FISH application, the NME-2-labeled probe concentrations of 200 ng/μl were confirmed by dot blot analysis (data not shown), indicating that complete labeling was accomplished. To check the applicability of the in-tube FISH method and ability of the NME-2 probe to detect EFOMIA 03, we conducted the in-tube FISH experiment on EFOMIA 03 directly. The EFOMIA 03 hyphae were detected based on the NME-2 probe FISH signal upon fluorescence microscopy (data not shown). Negative controls without FISH probes consistently exhibited no signals.
Colonization of EFOMIA 03 hyphae within P. strictum (4) visualized by selective fungal hyphae staining and in-tube FISH. a The arrow indicates the EFOMIA 03 hyphae pattern. The hyphae (stained blue) extended across the moss cell wall but was not observed in the cell-wall spaces or the sectioned moss tissue. b In-tube FISH with P. strictum (4) using NME-2 probes (CLSM)
The moss cryosections were treated with sodium borohydride to lower the mosses’ inherent autofluorescence. The optimal concentration of formamide for the NME-2 probe was 40 % and a hybridization temperature 42 °C, under which endophytic fungal hyphae could be detected based on FISH signals (Fig. 3b).
Discussion
According to Ruisi et al. (2007), geographical isolation combined with environmental stress makes Antarctica a good place to search for new endemic fungal species. Studies of endophytic fungi in relatively extreme environments and from phylogenetically distinct plant lineages are promising sources of novel species and will contribute to our understanding of fungal diversity, the cryptic ecology of microfungal symbionts, and the evolution of plant–fungal symbioses (Higgins et al. 2007).
In 2000, Penicillium sp., Trichoderma sp., and Embellisia sp. isolated from the Antarctic moss Bryum argenteum were collected from Southern Victoria Land (Bradner et al. 2000). Furthermore, in 2002, 19 Antarctic mosses comprising eight species were collected from 17 sites in Victoria Land; the moss samples primarily consisted of Bryum pseudotriquetrum, Ceratodon purpureus, and Syntrichia princeps. Overall, 120 fungi were isolated from the mosses comprising 18 genera and 28 Ascomycota, Basidiomycota, and Zygomycota species (Tosi et al. 2002). In 1996, 10 unidentified mosses were collected at Arctowski and Jubany on King George Island, and 31 endophytic fungi were identified from them. Overall, nine of the endophytic fungi were previously unidentified and assigned to the Ascomycota, while one belonged to the Zygomycota (Mőller and Dreyfuss 1996).
In this study, 16 endophytic fungi were isolated from 13 bryophytes belonging to seven species collected at the Korea Antarctic Research Station on Barton Peninsula, King George Island. When compared with previous reports by Mőller and Dreyfuss (1996), we identified a smaller number of endophytic fungal isolates, but several fungal species from one bryophyte species. Among them, two endophytes of the Geomyces and Mortiella genera were also found in our study. Moreover, the two genera of our endophytes exhibited similar temperature preferences to those of the same genus isolated in 1996. The isolated endophytic fungi fell into four phyla, Ascomycota, Zygomycota, Basidiomycota, and Oomycota, which suggests that the endophytic fungi isolated in this study are highly diverse on Barton Peninsula, with the moss P. strictum (sample no. 4) exhibiting the richest fungal diversity. Five endophytic fungi from the phyla Ascomycota and Zygomycota were harbored in the bryophyte. The harsh surface sterilization treatment used in this study can affect moss cells in thin thalli and subsequently damage fungal endophytes living in and/or between the cells. Thus, it is highly likely that the majority of isolates came from P. strictum (sample no. 4) because this species has thick tissues compared with those of the other bryophytes examined in this study.
Phylogenetic analysis revealed that EFOMIA 01, EFOMIA 07, and EFOMIA 11 belong to the phylum Ascomycota (Fig. 2). EFOMIA 01 was closely related to Clathrosphaerina zalewskii (GenBank: EF029222). EFOMIA 07 and EFOMIA 11 were closely related to Xenopolyscytalum sp. (GenBank: JX852343) and Helotiales sp. (GenBank: JX852359), respectively, both of which were isolated from the Antarctic region. These findings suggest that the fungal lineage might be adapted to polar regions. Specifically, EFOMIA 11 exhibited different phenotypes depending on growth temperature.
EFOMIA 03, found in several Antarctic mosses, also belonged to the phylum Ascomycota (Fig. 2) and was closely related to Gyoerffyella sp. (GenBank: EF093185) and Varicosporium elodeae (GenBank: GQ152148). Therefore, EFOMIA 03 has a wide range of moss hosts and was the most common endophyte found in the study area. Moreover, EFOMIA 03 grew from 4 to 15 °C and was very sensitive to high temperatures. These findings suggest that the endophyte has adapted to Antarctic environmental conditions over a long time and may be sensitive to future temperature increases in the region.
EFOMIA 09, 10, and 15 were isolated from P. strictum (sample nos. 4 and 12). Phylogenetic analysis indicated that EFOMIA 09 and 10 belonged to the genus Mortierella, Zygomycota. EFOMIA 09 and 10 are psychrophilic fungi because they grew well at 4 °C. EFOMIA 15 was assigned to the Ascomycota (Fig. 2) and is similar to a fungus (GenBank: AM489755) previously isolated from an insect (E. murphyi) found on Signy Island, Antarctica (Fig. 2). Of the fungi isolated from the Antarctic mosses, many live in association with other organisms, nematodes, insects, and other fungi (Tosi et al. 2002).
Phylogenetic analysis indicated that EFOMIA 02, 04, 12, and 16 were closely related (Fig. 2) and belong to the class Sordariomycetidae. EFOMIA 02, 12, and 16 grew at 4–30 °C, and their optimum growth occurred at 30 °C. Their temperature preferences imply that they are tolerant to a wide range of temperatures and that they might have originated from a temperate zone and adapted to cold regions.
EFOMIA 08 closely matched Geomyces pannorum (GenBank: DQ189229) isolated from the mosses B. pseudotriquetrum, C. purpureus, S. princeps, B. argenteum, Schistidium antarctici, and Campylopus pyriformis in Antarctic Victoria Land and one unidentified moss from King George Island (Mőller and Dreyfuss 1996; Tosi et al. 2002). These results indicate that EFOMIA 08 is an endophytic fungus of Antarctic bryophytes. Furthermore, the results of the EFOMIA 08 growth–temperature relationships were the same as those reported for G. pannorum var. pannurm (Tosi et al. 2002).
EFOMIA 05 and 06 were isolated from C. aciphyllum (sample no. 6) and belong to the phylum Ascomycota (Fig. 2). EFOMIA 05 is similar to Massarina sp. (GenBank: AJ972794) in the order Pleosporales (Table 1), which was isolated from boreal forest bryophytes (Kauserud et al. 2008). EFOMIA 05 did not grow at 4 °C, but did so at 10–30 °C, which suggests that EFOMIA 05 might have adapted to the maritime Antarctic as an endophyte.
EFOMIA 14 is the only endophyte included in the phylum Basidiomycota and is similar to the plant pathogen Daedaleopsis confragosa (GenBank: FJ810177) (Fig. 2).
EFOMIA 13 was highly homologous with Pythium sp. (GenBank: AB299389), which was isolated from moss in the Arctic and Antarctic regions. Pythium (Oomycota) has been widely observed in Antarctica (Bridge and Denton 2007), and Pythium tenue has been reported from the Dry Valleys of Victoria Land in continental Antarctica (Knox and Paterson 1973).
Our investigation into the growth–temperature relationships revealed that most fungal isolates had a maximum growth rate within the range of 15–30 °C (Table 2) and that all endophytes, except for EFOMIA 05, grew at 4 °C. These results are similar to previously reported data from numerous studies on Antarctic fungi. Endophytic fungi isolated from Antarctic bryophytes are mesophilic and psychrotolerant (Bailey and Wynn-Williams 1982; Caretta and Frate 1990; Mőller and Dreyfuss 1996).
Like tracheophytes, bryophytes harbor endophytic fungi that have no apparent detrimental effects on their hosts (Jakucs et al. 2003). Endophytic fungi in the roots of vascular plants, e.g., Heteroconium chaetospira, may imbue their host plants with resistance to pathogens such as Verticillium dahliae and Plasmodiophora brassicae (Narisawa et al. 1998; Narisawa et al. 2002). The presence of fungi isolated from Brachythecium rutabulum allows this plant to grow over a much wider pH range than when the fungi are absent (During and Van Tooren 1990). Endophytic fungi may provide bryophyte hosts with greater tolerance to extreme pH or promote vegetative growth (Davey and Currah 2006). It is also possible that the presence of endophytic fungi in Antarctic bryophytes is one of the evolutionary strategies that enabled adaptation to the extreme environment of the region.
In this study, EFOMIA 01, 03, 04, 06, 07, 10, 11, 14, and 16 exhibited high levels of sequence similarity to uncultured fungi sequences (Table 1), which suggests that these endophytic fungi isolated from Antarctic bryophytes are novel species. We found that diverse endophytic fungi are associated with Antarctic bryophytes with a wide range of temperature preferences (Table 2). We have also developed a novel FISH method to visualize the presence of endophytic fungi in Antarctic moss tissue. This method can be applied to monitor cold-adapted endophyte communities, which are highly vulnerable to increasing temperatures caused by global warming, in response to changes in the terrestrial ecosystem in the maritime Antarctic.
References
Bailey AD, Wynn-Williams DD (1982) Soil microbiological studies at Signy Island, South Orkney Island. Br Antarct Surv Bull 51:167–191
Baschien C, Manz W, Neu TR, Marvanova L, Szewzyk U (2008) In situ detection of freshwater fungi in an Alpine stream by new taxon-specific fluorescence in situ hybridization probes. Appl Environ Microbiol 74(20):6427–6436. doi:10.1128/AEM.00815-08
Baublis JA, Wharton RA, Volz PA (1991) Diversity of micro-fungi in an Antarctic Dry Valley. J Basic Microbiol 31(1):3–12
Boyd WL, Staley JT, Boyd JW (1966) Ecology of soil microorganisms of Antarctica. Antarct Res 8:125–159
Bradner JR, Sidhu RK, Yee B, Skotnicki ML, Selkirk PM, Nevalainen KMH (2000) A new microfungal isolate, Embellisia sp., associated with the Antarctic moss Bryum argenteum. Polar Biol 23:730–732
Bridge PD, Denton GJ (2007) Isolation of diverse viable fungi from the larvae of the introduced chironomid Eretmoptera murphyi on Signy Island. Polar Biol 30:935–937
Caretta G, Frate GD (1990) Fungi isolated from Antarctic material. Polar Biol 11:1–7
Caretta G, Frate GD, Mangiarotti AM (1994) A record of Arthrobotrys tortor Jarowaja and Engyodontium album (Limber) de Hoog from Antarctica. Bol micol 9:9–12
Davey ML, Currah RS (2006) Interactions between mosses (Bryophyta) and fungi. Can J Bot 84:1509–1519
Dőbbeler P (1997) Biodiversity of bryophilous ascomycetes. Biodivers Conserv 6:721–738
During HJ, Van Tooren BF (1990) Bryophyte interactions with other plants. Bot J Linn Soc 104(1–3):79–98. doi:10.1111/j.1095-8339.1990.tb02212.x
Ellis-Evans JC (1985) Fungi from maritime Antarctic freshwater environments. Br Antarct Surv Bull 68:37–45
Ellis-Evans JC, Walton D (1990) The process of colonization in Antarctic terrestrial and freshwater ecosystems. Polar Biol 3:151–163
Fletcher LD, Kerry EJ, Weste GM (1985) Microfungi of Mac. Robertson and Enderby Lands, Antarctica. Polar Biol 4:81–88
Gamundi IJ, Spinedi HA (1988) Ascomycotina from Antarctica: new species and interesting collections from Danco Coast, Antarctic Peninsula. Mycotaxon 33:467–482
Grasso SJM, Scheirer DC (1981) Scanning electron microscopic observations of a moss-fungus association. Bryologist 84(3):348–350
Heal OW, Bailey AD, Latter PM (1967) Bacteria, fungi and protozoa in Signy Island soils compared with those from a temperate moorland. Phil Trans Roy Soc Lond B Biol Sci 252:191–197
Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F (2007) Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phylogenet Evol 42(2):543–555. doi:10.1016/j.ympev.2006.07.012
Hoshino T, Tojo M, Okada G, Kanda H, Ohgita S, Ishizaki K (1999) A filamentous fungus, Pythium ultimum Trow var. ultimum, isolated from moribund moss colonies from Svalbard, northern islands of Norway. Polar Biosci 12:68–75
Hoshino T, Xiao N, Tkachenko OB (2009) Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 50:26–38
Jakucs E, Naar Z, Szedlay G, Orban S (2003) Glomalean and septate endophytic fungi in Hypopterygium mosses (Bryopsida). Cryptogam Mycol 24(1):27–37
Kanda H, Komárková V (1997) Antarctic terrestrial ecosystems. In: Wielgolaski FE (ed) Ecosystems of the World 3 Polar and Alpine Tundra. Elsevier, Amsterdam, pp 721–761
Kauserud H, Mathiesen C, Ohlson M (2008) High diversity of fungi associated with living parts of boreal forest. Can J Bot 86:1326–1333
Knox JS, Paterson RA (1973) The occurrence and distribution of some aquatic phycomycetes on Ross Island and The Dry Valleys of Victoria Land, Antarctica. Mycologia 65(2):373–387
Lee JS, Lee HK, Hur JS, Andreev M, Hong SG (2008) Diversity of the lichenized fungi in King George Island, Antarctica, revealed by phylogenetic analysis of partial large subunit rDNA sequences. J Microbiol Biotechnol 18(6):1016–1023
Li WC, Zhou J, Guo SY, Guo LD (2007) Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers 25:69–80
Mőller C, Dreyfuss MM (1996) Microfungi from Antarctic lichens, mosses and vascular plants. Micologia 88(6):922–933
Montemartini-Corte A (1991) Funghi di ambienti acquatici. In: Proceedings of the 1st meeting on biology in Antarctica, pp 67–79
Narisawa K, Tokumasu K, Hashiba T (1998) Suppression of clubroot formation in Chinese cabbage by the root endophytic fungus, Heteroconium chaetospira. Plant Pathol 47(2):206–210
Narisawa K, Kawamata H, Currah RS, Hashiba T (2002) Suppression of Verticillium wilt in eggplant by some fungal root endophytes. Eur J Plant Pathol 108:103–109
Onofri S, Tosi S, Persiani AM, Maggi O, Riess S, Zucconi L (1994) Mycological researches in Victoria Land terrestrial ecosystems. In: Battaglia B, Bisol PM, Varotto V (eds) Proceedings of the 2nd meeting on biology in Antarctica. Padova, Italy, pp 19–32
Øvstedal DO, Lewis-Smith RI (2002) Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Arctic 55(2):109–213
Pugh GJF (1980) Strategies in fungal ecology. Trans Br Mycol Soc 75(1):1–14
Pugh GJF, Allsopp D (1982) Microfungi on Signy Island, South Orkney Islands. Br Antarct Surv Bull 21:79–94
Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007) Fungi in Antarctica. Rev Environ Sci Biotechnol 6:127–141. doi:10.1007/s11157-006-9107-y
Siciʼnski J, Jażdżewski K, Broyer CD, Presler P, Ligowski R, Nonato EF, Corbisier TN, Petti MAV, Brito TAS, Lavrado HP, Błażewicz-Paszkowycz M, Pabis K, Jażdżewska A, Campos LS (2011) Admiralty bay benthos diversity—a census of a complex polar ecosystem. Deep Sea Res Pt II 58:30–48
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tosi S, Casado B, Gerdol R, Caretta G (2002) Fungi isolated from Antarctic mosses. Polar Biol 25:262–268. doi:10.1007/s00300-001-0337-8
Tubaki K, Asano I (1965) Additional species of fungi isolated from Antarctic materials. JARE. 1956–1962 Sci Rep Ser E 27:1–12
Upson R, Read DJ, Newsham KK (2007) Widespread association between the ericoid mycorrhizal fungus Rhizoscyphus ericae and a leafy liverwort in the maritime and sub-Antarctic. New Phytol 176(2):460–471. doi:10.1111/j.1469-8137.2007.02178.x
U’ren JM, Lutzoni F, Miadlikowska J, Arnold AE (2010) Community analysis reveals close affinities between endophytic and endolichenic fungi in mosses and lichens. Microb Ecol 60:340–353. doi:10.1007/s00248-010-9698-2
Vishniac HS (1993) The microbiology of Antarctic soils. In: Friedmann EI (ed) Antarctic microbiology. Wiley, New York, pp 297–341
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application, pp 315–322
Wynn-Williams DD (1996) Antarctic microbial diversity: the basis of polar ecosystem processes. Biodivers Conserv 5:1271–1293
Zhang T, Zhang YQ, Liu HY, Wei YZ, Li HL, Su J, Zhao LX, Yu LY (2013) Diversity and cold adaptation of culturable endophytic fungi from bryophytes in the Fildes Region, King George Island, maritime Antarctica. FEMS Microbiol Lett 341:52–61
Acknowledgments
This work was supported by grants from the Korea National Research Resource Center Program and the Korean Polar Research Institute (KOPRI, PE13030).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, N.H., Kim, J., Jeong, MH. et al. Diversity of endophytic fungi associated with bryophyte in the maritime Antarctic (King George Island). Polar Biol 37, 27–36 (2014). https://doi.org/10.1007/s00300-013-1406-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1406-5