Abstract
Red seaweed, Kappaphycus alvarezii, holds great promise for use in biofuel production due to its high carbohydrate content. In this study, we investigated the effect of fermentation inhibitors to the K. alvarezii hydrolysate on cell growth and ethanol fermentation. In addition, detoxification of fermentation inhibitors was performed to decrease the fermentation inhibitory effect. 5-Hydroxymethylfurfural and levulinic acid, which are liberated from acidic hydrolysis, was also observed in the hydrolysate of K. alvarezii. These compounds inhibited ethanol fermentation. In order to remove these inhibitors, activated charcoal and calcium hydroxide were introduced. The efficiency of activated charcoals was examined and over-liming was used to remove the inhibitors. Activated charcoal was found to be more effective than calcium hydroxide to remove the inhibitors. Detoxification by activated charcoal strongly improved the fermentability of dilute acid hydrolysate in the production of bioethanol from K. alvarezii with Saccharomyces cerevisiae. The optimal detoxifying conditions were found to be below an activated charcoal concentration of 5%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kappaphycus alvarezii (cottonii) is red seaweed, which has been categorized as carrageenophyte [1]. Currently, this species is commercially cultivated in the Philippines, Indonesia, Malaysia, Tanzania, and Micronesia [2]. Carrageenan is a polysaccharide contained in K. alvarezii and can be readily hydrolyzed to monosaccharides using acidic and enzymatic hydrolysis. Acidic hydrolysis is economically more feasible than enzymatic hydrolysis. However, the main drawback of the acid hydrolysis process, when compared to the enzymatic hydrolysis process, is the formation of by-product compounds that severely inhibit cell growth and ethanol production from the hydrolysate. Fermentation inhibitors include furan derivatives such as furfural and 5-hydroxymethylfurfural (5-HMF), aliphatic acids such as formic acid, acetic acid and levulinic acid, and phenolic compounds. Some fermentation inhibitors might be present in the sample before hydrolysis, but most of the inhibitors are formed during the hydrolysis process [3–7].
This study focused on two by-products formed in the hydrolysate of K. alvarezii, i.e. hydroxymethylfurfural and levulinic acid. Hydroxymethylfurfural, a toxic compound originating from the degradation of hexose, is the most important intermediate product in the acidic hydrolysis process. Its inhibitory effect is similar to that of furfural, causing a longer lag phase during growth. However, hydroxymethylfurfural is considered less toxic than furfural. Levulinic acid is formed by the degradation of hydroxymethylfurfural. The toxicity of levulinic acid is mainly due to its undissociated form; thus, the medium pH is important [7, 8]. It will be necessary either to avoid the formation of inhibitors under suitable conditions or to include a detoxification process [5, 6, 9]. Some detoxification processes have been developed to achieve high fermentability, i.e. physical, chemical and biological process. Generally, detoxification processes include precipitation, ionization, adsorption with activated carbon, ion exchange resin, and over-liming in wood hydrolysates. Activated charcoal has been utilized to remove pollutants in water and air due to its high adsorption capacity. Treatment of the hydrolysate with Ca(OH)2 prior to fermentation, referred to as over-liming, is one of the most efficient detoxification methods and has been commonly used in studies reported previously [7, 10, 11]. However, one drawback with over-liming is the formation of a calcium sulfate precipitate. Another limitation is a considerable degradation of fermentable sugars if it is done under too harsh conditions (high pH and high temperature). In addition, a very harsh over-liming condition might cause quantitative degradation of some inhibitors. Thus, detoxifying treatment must be systematically evaluated to determine the optimum conditions where a high improvement in fermentability is achieved with the lowest sugar degradation [11].
Saccharomyces cerevisiae is the most widely used microorganism in ethanol fermentation and has several advantages including (1) its efficiency to produce ethanol from hexoses without oxygen, (2) low pH and (3) high tolerance to ethanol and inhibitors [12]. The development of genetically engineered organisms that are able to ferment all sugars in hydrolysates to ethanol remains a challenge for molecular biologists and engineers [13].
The objectives of this study are: (1) to investigate the inhibitory effect of hydroxymethylfurfural and levulinic acid on ethanol fermentation, (2) to compare microorganism for ethanol fermentation, (3) to remove the fermentation inhibitors in the hydrolysate of K. alvarezii.
Materials and methods
Seaweed sample
Specimens of K. alvarezii were collected from Karimunjawa Island, Indonesia. The seaweed samples were rinsed in distilled freshwater to eliminate salt and debris. The samples were dried in dry oven at 60 °C until it reached a constant weight. The dried seaweeds were finely ground to create a powder using a mortar for 5 min.
Hydrolysate of K. alvarezii
The hydrolysate of K. alvarezii was obtained by autoclaving at 130 °C for 15 min by adding H2SO4.
Detoxification of hydrolysate
Two detoxification methods were used, i.e. activated charcoal and over-liming. For the activated charcoals method, various amounts of activated charcoal were added to the hydrolysates at weight ratios of 0.005, 0.01, 0.03, 0.05, 0.1 and 0.15, then the samples were rotated at 130 rpm in a shaking incubator at 30 °C. For the over-liming method, the hydrolysates were mixed with the different concentrations of Ca(OH)2 (0, 0.2, 0.5, 0.7 and 1% w/v), then stirred for different times at room temperature. Subsequently, both activated charcoals and Ca(OH)2 were separated from the hydrolysates by filtration. The sugar content and by-product formation in the filtrated samples were then analyzes.
Ethanol fermentation
For ethanol fermentation, S. cerevisiae (JENICO, Korea) and Pichia angophorae were used. The strains were maintained in yeast medium containing 10 g yeast extract, 6.4 g urea and 20 g glucose per liter. Fermentation was carried out in 3 and 100 mL volume. In the first experiment, the ability of the two different yeast strains to ferment the pure sugars and hydrolysate of K. alvarezii were compared. In the second experiment, the ethanol fermentability of detoxified hydrolysate was investigated.
Analysis of sugars, by-products and ethanol content
In order to measure the sugar content (glucose and galactose) and by-product formation (HMF and levulinic acid), all samples were analyzed by HPLC, which was equipped with a RI detector and Alltech IOA 1000 organic acid column (300 × 7.8 mm) maintained at 60 °C. The mobile phase used was 0.005 N H2SO4 at a flow rate of 0.3 mL/min. The ethanol content was measured using Gas Chromatography (Agilent model 6890 N, USA) with a 2B-WAX column (Agilent, USA) and FID detector. The injection volume was 2 μL with the inlet split ratio 30:1. The initial and maximum oven temperature was 35 and 250 °C, respectively.
Results and discussion
Fermentation of two yeast strains
To identify a microorganism able to utilize the sugars while withstanding the inhibitory substances contained in hydrolysates, the hydrolysate was fermented using two strains, S. cerevisiae and P. angophorae. Figure 1 shows the cell growth, ethanol production, and ethanol yield from two strains. The cell growth of S. cerevisiae was higher than P. angophorae. 6.0 g/L cell mass was obtained within 24 h of cultivation. However, 3.15 g/L of P. angophorae was obtained within 24 h. In regards to ethanol production, S. cerevisiae and P. angophorae produced 6.8 and 3.0 g/L of ethanol within 24 h, respectively. In regards to ethanol yield, S. cerevisiae showed a higher ethanol yield (0.369 g/g at 24 h) than P. angophorae (0.169 g/g at 24 h). Overall, these results demonstrated that S. cerevisiae performed better than P. angophorae in ethanol fermentation of the hydrolysates.
Inhibitory effect of HMF and levulinic acid
The hydrolysate of K. alvarezii contained 0.89 ± 0.13 g glucose, 23.90 ± 2.83 g galactose, 0.96 ± 0.50 g 5-hydroxymethylfurfural, and 4.23 ± 1.50 g levulinic acid per liter (Table 1). The presence of by-product compounds in hydrolysates is a major problem in bioethanol fermentation. The experiments to examine the inhibitory effect of by-produced were conducted using pure galactose, levulinic acid and HMF, which were fermented by S. cerevisiae. As shown in Fig. 2, HMF started to inhibit ethanol production at a concentration of 0.05 g/L and strongly inhibit ethanol production at concentrations greater than 0.5 g/L. In contrast, levulinic acid started to inhibit ethanol production at a concentration of 0.5 g/L, strongly inhibited ethanol production at a concentration of 2.5 g/L and completely inhibited production at a concentration of 5 g/L.
Figure 3 shows the inhibiting effect of HMF and levulinic acid on cell growth. In the case of HMF, 0.25 g/L HMF inhibited the cell growth by 15% relative to the control (without inhibitors). When the HMF concentration was greater than 0.5 g/L, cell growth was decreased by 27.2% relative to the control. At 5 g/L HMF, cell growth was decreased by about 33.5% compared to the control. In the case of levulinic acid, 0.25 g/L levulinic acid inhibited cell growth by 9%, where 5 g/L of levulinic acid inhibited growth by 63.6% relative to the control. Overall, levulinic acid had a greater inhibitory effect than HMF. In several studies, inhibition of cell growth was observed by both HMF and levulinic acid. Delgenes et al. [14] reported that 1.5 g/L HMF completely inhibited cell growth of P. stipitis. Alves et al. [3] also reported inhibition of cell growth of S. cerevisiae at an HMF concentration of 1 g/L. According to Martinez et al. [15] and Taherzadeh et al. [16], the addition of HMF decreased ethanol production and cell growth in E. coli cultivation.
Detoxification of hydrolysate
In order to improve ethanol fermentation, the hydrolysates were detoxified with activated charcoal and over-liming. In the activated charcoal method, different activated charcoal concentrations and reaction times were used (Fig. 4). HMF and levulinic acid concentrations were reduced significantly at an activated charcoal concentration of 15% (w/v). The removal of HMF and levulinic acid dramatically increased at 1% (w/v) activated charcoal. When the activated charcoal concentration was greater than 1%, the galactose content significantly decreased. The optimum concentration of activated charcoal was 1% (w/v), where the removal of glucose, galactose, levulinic acid and HMF were 14.5, 20.3, 38.8 and 70.37%, respectively.
Figure 5 shows the reaction time on the removal of fermentation inhibitors with activated charcoal. The concentration of galactose decreased linearly with reaction time. On the other hand, the glucose concentration decreased more gradually in removal rate than galactose. The removal yield of galactose was 36% at 60 min, whereas, the 18.7% of glucose was removed. The HMF removal yield increased dramatically at 15 min. However, the removal rate of levulinic acid only gradually increased. The HMF removal yields were 11.1 and 70.4% at 15 min and 30 min, respectively. Overall, the inhibitor (HMF and levulinic acid) concentrations were found to decrease abruptly within 30 min, then remained constantly. This result shows that the activated charcoal can remove the HMF and levulinic acid compounds within a short period. In the study by Miyafuji et al. [17], the wood charcoals were used to remove inhibitors of fermentation in the wood hydrolysate. By removing the inhibitors, the fermentability was enhanced. Hydrolysates treated with wood charcoal prepared at various temperatures were fermented for ethanol production, and the effects of the wood charcoal on the fermentability of the hydrolysate were evaluated.
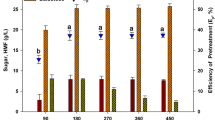
Figure 6 shows the results of detoxification using over-liming of hydrolysate of K. alvarezii. The over-liming treatment of hydrolysates with 1% (w/v) Ca(OH)2 for 30 min showed the highest removal rates of 50 and 70.1% for levulinic acid and HMF, respectively. In addition, this condition removes high amounts of galactose (78.4%) and glucose (86.4%), respectively.
Figure 7 shows the reaction time on the removal of sugars and fermentation inhibitors. The removal of fermentation inhibitors dramatically decreased with an increase in reaction time. In addition, a change in sugar concentration was accompanied by removal rate. Therefore, the over-liming method appears to be inefficient since it removes both sugars and inhibitors. All the detoxification methods used in this study resulted in a reduction in the inhibitor compounds but the over-liming method also resulted in significant sugar removal. The harsh alkaline conditions reduced both inhibitors and the amount of fermentable sugars. Previous studies showed that Ca(OH)2 decreased the level of fermentable sugar more than other alkali such as NaOH. Calcium ions might catalyze alkaline degradation of monosaccharides in the over-liming process [10].
Ethanol fermentation of detoxified hydrolysates
Table 2 shows the results of fermentation after detoxification. All hydrolysates were supplemented with nutrients and contained the same sugar content in order to exclude growth limitation due to nutritional deficiency. The results of indicated that all detoxification methods were capable of improving the yeast performance. The ethanol yield for the detoxified samples at activated charcoal concentrations of 0.5, 1, 3, 5, 10 and 15% ranged between 0.31 and 35% of the theoretical yield, which was higher than that of the raw sample. However, there was still some inhibition in the detoxified hydrolysates since the ethanol yields and productivities were lower than those achieved with reference fermentation containing only pure sugar and nutrients. The low ethanol production can be explained by the remaining HMF in the sample. There was still some inhibition in the detoxified hydrolysates because the toxic compounds were not completely removed by the detoxification treatments.
Conclusion
This work examined the effect of HMF and levulinic acid on ethanol fermentation, and the removal of fermentation inhibitors in the hydrolysate of K. alvarezii. The fermentation inhibitors in the hydrolysate of K. alvarezii were revealed to be hydroxymethylfurfural and levulinic acid. Inhibition of ethanol production by HMF started at 0.05 g/L and ethanol production was strongly inhibited at concentrations greater than 0.5 g/L. In contrast, inhibition by levulinic acid started at 0.5 g/L, strongly increase at 2.5 g/L and complete inhibition was observed at 5 g/L. Overall, levulinic acid was found to be more inhibitory than HMF. In regards to ethanol fermentation, S. cerevisiae was shown to be more productive than P. angophorae. During 24 h of cultivation, cell growth, ethanol production, and ethanol yield reached 6.0 g/L cell mass, 6.8 g/L ethanol, and 0.369 g/g, respectively. Both over-liming and activated charcoal detoxification methods reduced the inhibitor concentrations, but in the over-liming method, significant sugar removal was also observed. The optimum concentration of activated charcoal was determined to be 1% (w/v), whereas the removal of levulinic acid and HMF were 38.8 and 70.37%, respectively. Activated charcoal can remove HMF and levulinic acid over a very short period of time (within 30 min). Fermentation of detoxified hydrolysate produced a higher ethanol yield compared to the raw hydrolysate but this yield was still lower than the control. There was still some inhibition in the detoxified hydrolysates because the toxic compounds were not completely removed by the detoxification methods.
References
Adnan H, Porse H (1987) Culture of Eucheuma cottonii and Eucheuma spinosum in Indonesia. Hydrobiologia 151–152:355–358
Guerrero RD (2001) Farming of carrageenophyte in the Philippine. A success story of red seaweed cultivation. Asia-Pacific Association of Agricultural Research Institutions FAO, Bangkok
Alves LA, Felipe MGA, Silva JB, Silva SS, Prata AMR (1998) Pretreatment of sugar cane bagasse hemicelluloses hydrolyzate for xylitol production by Candida guilliermondii. Appl Biochem Biotechnol 70–72:89–98
Herrera A, Tellez-Luis SJ, Gonzalez-Cabriales JJ, Ramirez JA, Vazquez M (2004) Effect of the hydrochloric acid concentration on the hydrolysis of sorghum straw at atmospheric pressure. J Food Eng 63:103–109
Jeong GT, Park DH (2010) Production of sugars and levulinic acid from marine biomass Gelidium amansii. Appl Biochem Biotechnol 161:41–52
Asli AE, Qatibi AI (2009) Ethanol production from olive cake biomass substrate. Biotechnol Bioprocess Eng 14:118–122
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of dilute-acid liqnocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93:1–10
Larsson S, Reimann A, Nilvebrant NO, Jonsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77–79:91–103
Han M, Moon SK, Kim Y, Kim Y, Chung B, Choi GW (2009) Bioethanol production from ammonia percolated wheat straw. Biotechnol Bioprocess Eng 14:606–611
Alriksson B, Horvarth IS, Sjode A, Nilverant NO (2005) Ammonium hydroxide detoxification of spruce acid hydrolysates. Appl Biochem Biotechnol 121–124:912–922
Alriksson B, Sjöde A, Nilvebrant NO, Jönsson LJ (2006) Optimal conditions for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Appl Biochem Biotechnol 129–132:599–616
Olsson L, Hahn-Hagerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331
Martín C, Galbe M, Wahlbom F, Hahn-Hägerdal B, Jönsson LJ (2002) Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb Technol 31:274–282
Delgenes J, Moletta R, Navarro JM (1996) Effect of lignocelluloses degradation products on ethanol fermentation of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis and Candida shehatae. Enzyme Microb Technol 19:220–225
Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO (2000) Effect of Ca(OH)2 treatment (“overliming”) on the composition and toxicity of bagasse hemicelluloses hydrolysates. Biotechnol Bioeng 69:526–536
Taherzadeh MJ, Gustafsson L, Niklasson C, Liden G (2000) Physiological effect of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53:701–708
Miyafuji H, Danner H, Neureiter M, Thomasser C, Bvochora J, Szolar O, Braun R (2003) Detoxification of wood hydrolysates with wood charcoal for increasing the fermentability of hydrolysates. Enzyme Microb Technol 32:396–400
Acknowledgments
We thank the Brain Busan 21 program for graduate support (MDNM). This research was supported by a grant from Development of Marine-Bioenergy Program Funded by Ministry of Land, Transport and Maritime Affairs of Korean Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meinita, M.D.N., Hong, YK. & Jeong, GT. Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst Eng 35, 93–98 (2012). https://doi.org/10.1007/s00449-011-0608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0608-x