Abstract
Polyhydroxyalkanoates (PHAs) are biodegradable polymers which are considered as an effective alternative for conventional plastics due to their mechanical properties similar to the latter. However, the widespread use of these polymers is still hampered due to their higher cost of production as compared to plastics. The production cost could be overcome by obtaining high yields and productivity. The goal of the present research was to enhance the yield of polyhydroxybutyrate (PHB) with the help of two simple fed-batch cultivation strategies. In the present study, average batch kinetic and substrate limitation/inhibition study data of Alcaligenes latus was used for the development of PHB model which was then adopted for designing various off-line nutrient feeding strategies to enhance PHB accumulation. The predictive ability of the model was validated by experimental implementation of two fed-batch strategies. One such dynamic strategy of fed-batch cultivation under pseudo-steady state with respect to nitrogen and simultaneous carbon feeding strategy resulted in significantly high biomass and PHB concentration of 39.17 g/L and 29.64 g/L, respectively. This feeding strategy demonstrated a high PHB productivity and PHB content of 0.6 g/L h and 75%, respectively, which were remarkably high in comparison to batch cultivation. The mathematical model can also be employed for designing various other nutrient feeding strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyhydroxybutyrate (PHB) is a biodegradable intracellular polymer which is accumulated by bacteria as energy-rich storage material under the nutrient limitation in the presence of excess carbon source [1]. PHB has become a versatile candidate of considerable environmental importance owing to its biodegradable and biocompatible nature along with possibility of functionalization of molecular structure [2]. Although PHB is considered as an effective alternative for conventional plastics, widespread use of this polymer is still hampered due to its higher cost of production as compared to other polymers such as poly-l-lactic acid [3].
The major factors responsible for relatively higher cost of biotechnological PHB production involve high substrate cost, downstream processing cost, low polymer concentration, and productivity of the production processes [4]. The cost of the raw materials accounts for 40–50% of the total production costs wherein carbon source contributes to 70–80% of the total expense [5]. Moreover, low overall polymer yields also affect the economics of PHB production, as a less efficient bioprocess would require more substrate for the production of the same amount of polymer [6]. The low overall productivity contributes to higher capital and operation cost during scale-up.
The production cost can be substantially reduced by design and implementation of efficient bioprocess strategies for improving the overall process kinetics, thereby resulting in higher polymer concentrations and productivities [7, 8]. One of the most essential fermentation requirements for achieving enhanced PHB accumulation during PHB production is maintenance of desirable levels of carbon source, along with limited nitrogen availability in the culture medium during fermentation [9]. Moreover, the idea of dual nutrient limitation for both carbon and nitrogen for tailored synthesis of polyhydroxyalkanotes (PHAs) has also been reported in literature [10]. In some cases, microbial growth inhibition might be caused by high substrate concentrations, e.g., volatile fatty acids and crude glycerol, thus affecting the overall biomass and PHA production rates [6]. Therefore, a meticulous understanding of the PHA production kinetics is extremely essential for designing fermentation strategies involving appropriate maintenance of substrate concentration for enhanced PHA accumulation [7, 11].
The mathematical modeling and simulation could serve as a useful tool for the provision of desirable high carbon and limiting nitrogen concentrations for high PHB concentration and productivity [9, 12, 13]. The biotechnological production of PHAs involves a series of complex process steps including biomass growth, polymer accumulation, and polymer consumption, etc. in which a number of process variables and other influencing parameters are involved [1]. This makes development and optimization of bioprocess for efficient production of PHB one of the main challenging tasks. In this regard, modeling and simulation are very useful tools to completely understand the dynamic process behavior and the underlying mechanisms and to design controlled feeding strategies for high PHB production [2].
Fed-batch cultivation seems to be an ideal approach for obtaining enhanced product accumulation and productivity by maintenance of desirable concentrations of substrate inside the bioreactor. Thus, in the present investigation, a mathematical model-based nutrient feeding approach was adopted for maintaining appropriate concentrations of carbon and nitrogen inside the bioreactor. The model-based fed-batch cultivation appears to be a reliable, fast, and powerful bioprocess engineering tool for maintaining non-limiting and non-inhibitory substrate levels during fermentation. The distinct capability of the model in predicting the different nutrient feeding strategies such as pseudo-steady state with respect to sucrose and nitrogen was thoroughly investigated in the present study. In the present work, Alcaligenes latus have been used for PHB production primarily due to the high PHB yields offered by this strain and the ability to synthesize polymer during growth phase by sucrose [14].
Materials and Methods
Culture and Maintenance
Alcaligenes latus DSM 1124 (now renamed as Azohydromonas australica DSM 1124) was obtained from DSMZ, Germany. The culture was grown on nutrient agar slants at 4 °C and subcultured at regular intervals.
Culture Medium and Growth Conditions
A statistically optimized medium recipe: sucrose 25 g/L, (NH4)2SO4 2.8 g/L, KH2PO4 3.25 g/L, Na2HPO4 3.25 g/L, MgSO4·7H2O 0.2 g/L, and trace element solution (TES): 1.5 mL/L was used for biomass and PHB production [15]. TES consisted of 6 g/L ammonium Fe(III) citrate, 10 g/L CaCl2·2H2O, 0.3 g/L H3BO3, 0.2 g/L CoCl2·6H2O, 0.1 g/L ZnSO4·7H2O, 0.03 g/L MnCl2·4H2O, 0.03 g/L Na2MoO4·2H2O, 0.02 g/L NiSO4·7H2O, and 0.01 g/L CuSO4·5H2O. Phosphates were autoclaved separately, and TES was sterilized by filtration. Medium pH was adjusted aseptically to 7.0 using 2 N NaOH/HCl. The inoculum preparation method was similar to as explained in our previous study [7].
Batch Cultivation of A. latus in Bioreactor
Batch cultivation was carried out in a 7-L stirred tank reactor (STR) (Applikon Dependable Instruments, The Netherlands) using a 4-L statistically optimized media recipe. The reactor was inoculated with 5% (v/v) exponential phase culture from a shake flask. Agitation was carried out by conventional flat blade turbine-type impellers. The temperature was maintained at 33 °C using a chilled water circulator unit. Medium pH was maintained at 7.0 by automatic addition of 2 N NaOH/HCl. Air was sparged aseptically from the base of the bioreactor using perforated L-shaped sparger. The dissolved oxygen (DO) concentration in the bioreactor was measured by an in situ DO probe (Applisens, The Netherlands). The DO levels were kept above 30% of the saturation point by manually tuning the agitation rate and/or aeration rate. Samples were withdrawn at regular intervals of 3 h for the analysis of biomass, PHB sucrose, and nitrogen. Batch kinetic experiments were done in triplicates and average values are reported.
Modeling of PHA Production and Parameter Estimation
A mathematical model for PHB fermentation process was developed using the batch kinetics and independently obtained inhibition data of substrates: carbon and nitrogen [7]. For the model parameter estimation, a system of differential equations was solved using a numerical integration program based on the Runge-Kutta method of the fourth order [16]. The optimization program for the direct search of the minimum of the multivariable function was based on the original algorithm of Rosenbrock, which was later on adopted by Patwardhan and Srivastava and Kaur et al. [13, 17, 18]. The minimization criterion was represented as shown in the following:
where SSWR represents the sum of the square of the weighed residues or objective function; i (varied from 1 to n) and j (varied from 1 to m) represent the number of experimental data points and number of variables, respectively; Wj is the weight of each variable (selected as the maximum value of each variable); and Δij is the difference between the model simulation and corresponding experimental value (y model − y expt). Thus, the optimized values of kinetic parameters (Table 1) were obtained by minimizing the objective function (SSWR).
Model-Based Fed-Batch Cultivation Strategies
In case of pseudo-steady-state fed-batch cultivation with respect to sucrose, a reactor was first operated in batch mode from 0 to 15 h using a statistically optimized medium recipe with an initial sucrose concentration of 25 g/L. At 15 h, when culture was growing exponentially, sucrose feeding (125 g/L) along with proportionate concentrations of other medium components was initiated (as guided by the model). The feed rate in this fed-batch strategy was determined by equating the rate of sucrose consumption (dS 1/dt) of the model to zero and then back calculating the nutrient feed rate. Feeding was continued till 40 h to ensure constant sucrose availability (non-limiting and non-inhibitory concentration) during the previous cultivation period. After that, the batch fermentation was again resumed from 40 to 44 h for utilization of the residual substrates in the reactor.
In another feed strategy, the reactor was first operated as a batch for 22 h with an initial sucrose concentration of 25 g/L. At 22 h of cultivation when the culture was in exponential phase and residual nitrogen concentration had dropped to a limiting value of 0.28 g/L, feeding of nitrogen (at 6.2 g/L) was initiated. This nitrogen-limiting concentration was then maintained constant, i.e., pseudo-steady state with respect to nitrogen for the time period 22 to 48 h as guided by the model. Sucrose (150 g/L) feeding along with proportionate concentration of other medium components was also started at 22 h from different feed bottles at a constant feed rate of 60 mL/h when the concentration of sucrose had dropped below 10.0 g/L to ensure excess availability of sucrose in the bioreactor. After 48 h of fed-batch cultivation, secondary batch fermentation was done for the utilization of the residual substrates inside the reactor. In both cultivations, the samples were collected at regular intervals and analyzed.
Analytical Methods
The optical density of the samples was calculated at 600 nm using a spectrophotometer (OPTIZEN model 3220UV, Mecasys, Korea). Biomass (or cell concentration) was measured by a standard correlation between OD600 nm vs. cell dry weight (CDW). Residual sucrose concentration in fermentation sample was measured by the dinitrosalicylic acid (DNSA) method [19]. Residual ammonia nitrogen was analyzed by the Kjeldahl method [20]. PHB was quantified by gas chromatography (GC 2010 Shimadzu Co., Japan) using benzoic acid as an internal standard [21]. The concentration of PHB samples was calculated from the averaged values of triplicates.
Results and Discussion
Batch Kinetics of PHB Production
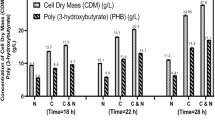
The time course of batch cultivation kinetics of A. latus in the bioreactor is shown in Fig. 1. Batch kinetics of A. latus was studied under controlled environmental conditions with respect to biomass and PHB production. Figure 1 illustrates that after a lag phase of 3 h, the culture started growing exponentially and PHB accumulation also began simultaneously because A. latus produces PHB during the growth phase. At the end of cultivation, a maximum biomass of 8.71 g/L and a PHB concentration of 6.24 g/L were obtained and PHB content of 72% of CDW was accumulated in 36 h. During cultivation, 23.3 g/L sucrose was consumed from its initial concentration of 25 g/L and 1.7 g/L sucrose was left unconsumed in the fermentation broth. PHB fermentation featured a maximum PHB production rate and PHB yield of 0.75 g/h and 0.29 g PHB/g of sucrose, respectively.
Mathematical Model for PHB Fermentation
The batch kinetic data and independently obtained substrate inhibition data of A. latus were used for the development of the mathematical model for PHB production. Various preliminary substrate inhibition studies were carried out to study the effects of different concentrations of nutrients (sucrose, nitrogen, and phosphorous) on the growth of the culture [15]. From these studies, it was concluded that sucrose and nitrogen were the only limiting substrates as varying their concentration beyond a particular range affected the specific growth rate of the culture (data not shown). There was no process limitation by phosphorous as varying its concentration did not affect the growth of the culture. There was no process limitation by other medium constituents as these nutrients were available in excess during the fermentation. The temperature and pH were kept constant at optimized values (temperature 33 °C and pH 7.0) for this strain throughout the fermentation. Various researchers have already optimized these environmental conditions for A. latus DSM 1124 in literature [22, 23]. Therefore, the model was based on the following assumptions: (1) sucrose and nitrogen were the limiting substrates; (2) there was no process limitation by phosphorus and other medium constituents, and these nutrients were available in excess during the fermentation; and (3) the temperature and pH were constant throughout the fermentation.
The mathematical model consisted of the following differential equations:
Biomass production (dX/dt) involves substrate limitation and inhibition by high concentration of substrates, sucrose, and nitrogen as shown in Eq. 1. Y (X + P)/S1 is a lumped parameter representing the yield of both biomass and product with respect to sucrose (S 1). Y X/S2represents the yield of biomass with respect to nitrogen (S 2). K 1 is the growth-associated product (P) formation constant. The model development and statistical validity of the proposed model have been described in detail in an earlier study [7]. Optimized model parameters (Table 1) were then used to solve the differential equations on a computer using the numerical integration program to generate batch kinetic. Figure 1 shows the comparison of the model simulation (smooth lines) with experimental data points, and a good correlation was clearly observed between them.
Model-Based Fed-Batch Cultivation Strategies
The batch model was extrapolated to fed-batch cultivation by applying the mass balances around the bioreactor and incorporating the appropriate dilution terms. The fed-batch model was then adopted for designing fresh nutrient feeding regimes for improvement in PHB accumulation and its productivity. The fed-batch model equations were as follows:
D is the dilution rate and F is the feed rate which involves the flow rate of both sucrose, F 1, and nitrogen, F 2, and V is the working volume of the bioreactor; S 01 and S 02 are the initial concentrations of sucrose and nitrogen, respectively, in the feed bottle. Various off-line simulations (guided by the model) were performed on the computer to identify appropriate nutrient feeding regimes for the addition of the limiting nutrients: sucrose and nitrogen. Two fed-batch cultivation strategies (pseudo-steady-state w.r.t. sucrose and pseudo-steady-state w.r.t. nitrogen) indicating significant improvement in PHB accumulation were selected and experimentally implemented.
Fed-Batch Cultivation Under Pseudo-Steady State with Respect to Sucrose
The typical batch fermentation is associated with the major disadvantage of the limiting nutrient availability during the entire cultivation period particularly at the time when the cells are vigorously growing in the exponential phase. These highly dynamic conditions of batch fermentation eventually result in low PHB concentrations and productivity at the end of the cultivation. Therefore, it is highly desirable to maintain the non-limiting and non-inhibitory concentration of limiting substrate (sucrose) inside the bioreactor for the entire cultivation period in order to achieve high rates of biomass and PHB accumulation. This particular scenario of constant substrate availability during the major period of cultivation would result in high product accumulation as compared to batch fermentation.
Keeping the previous idea in mind, a fed-batch cultivation strategy was designed using the model to ensure constant substrate availability, i.e., pseudo-steady-state w.r.t. sucrose during the entire cultivation. This unique nutrient(s) feeding strategy was designed and simulated off-line on the computer using the model and then experimentally implemented at the time when reasonable cell concentration (vigorously growing cells) was available inside the bioreactor to facilitate PHB accumulation. The fed-batch strategy was initiated as batch and feeding of sucrose at 125 g/L (along with proportionate concentrations of other medium components) were initiated at 15 h when the culture was vigorously growing and residual sucrose concentration in the reactor had dipped to about 17 g/L. Feeding of sucrose was continued till 40 h of cultivation. In this strategy, it was not possible to maintain nitrogen-limiting concentration at a constant level. There was a continuous decrease in nitrogen concentration with time and ultimately reached to very low values where feeding of sucrose needs to be stopped. At 40 h, the model was again simulated for yet another batch cultivation until the residual sucrose concentration in the broth was totally consumed.
By the use of this fed-batch cultivation strategy involving pseudo-steady-state w.r.t. sucrose, it was possible to achieve a maximum biomass concentration of 29.87 g/L and a PHB concentration of 25.05 g/L (as predicted by model) in 44 h. Experimental validation of this nutrient feeding strategy demonstrated an accumulation of maximum biomass and PHB concentrations of 29.16 and 24.38 g/L, respectively, in 44 h, which were very close to model-predicted values. Figure 2 illustrates the time course of observed experimental data during pseudo-steady state and its comparison with the model simulation. As seen from Fig. 2, a good correlation was observed between the experimental data points and model simulations. An overall PHB productivity of 0.55 g/L h was obtained at the end of fermentation, which was considerably higher than 0.17 g/L h achieved during batch cultivation. This strategy demonstrated a 3.2-fold increment in the PHB productivity.
Fed-Batch Cultivation Involving Pseudo-Steady State with Respect to Nitrogen
Literature reports on PHB production have demonstrated that excess availability of carbon source under nitrogen-limiting conditions could significantly enhance the PHB concentration and productivity [9, 24, 25]. It has been also reported in the literature that during nitrogen limitation, NADH oxidase activity of the culture decreases and no anabolic process of the cells occurs, which leads to concomitant increase in intracellular concentrations of NADH. This in turn inhibits the key regulatory enzymes of TCA cycle, citrate synthase, and isocitrate dehydrogenase, resulting in slow down of TCA cycle and accumulation of acetyl-CoA (key precursor of PHB synthesis). The accumulation of acetyl-CoA intracellularly at high levels consequently forces the cells for PHB synthesis [24]. Therefore, the effect of nitrogen limitation on PHB accumulation was studied using a model-based fed-batch cultivation strategy. This unique cultivation scenario of pseudo-steady-state w.r.t. nitrogen-limiting conditions was designed using model by doing various off-line simulations (on the computer) to obtain enhanced PHB accumulation.
Model-based fed-batch cultivation was initiated as a batch, and at 22 h, when biomass in the reactor had reached to a concentration of 5.4 g/L and nitrogen concentration dropped to a limiting value of 0.28 g/L, feeding of nitrogen was initiated. This nitrogen-limiting concentration was then maintained constant, i.e., pseudo-steady-state w.r.t. nitrogen (as guided by the model) by selectively feeding nitrogen (as (NH4)2SO4) at 6.8 g/L. Feeding of sucrose (150 g/L) was also initiated at 22 h at the feed rate of 60 mL/h to remove the sucrose limitation and maintain excess availability of carbon source (sucrose) along with limiting nitrogen concentrations. By the adoption of these nutrient (carbon and nitrogen) feeding strategies, it was possible to achieve a total biomass concentration of 40.12 g/L containing 28.9 g/L PHB in 50 h. Experimental implementation of this strategy exhibited maximum biomass and PHB concentrations of 39.17 and 29.64 g/L, respectively, at the end of cultivation (50 h), and the PHB yield on sucrose was observed to be 0.19 g/g. Figure 3 illustrates the comparison of experimental observations with the model simulation for the fed-batch cultivation under pseudo-steady-state w.r.t. nitrogen. This scenario of cultivation for creating specific environmental conditions, i.e., excess sucrose availability under pseudo-steady state with respect to limiting nitrogen, was rather unique and interesting, which yielded high PHB accumulation as opposed to batch cultivation. This strategy exhibited an improvement in PHB productivity from 0.17 g/L h obtained in batch to 0.6 g/L h in fed-batch, which was a 3.6-fold increment in productivity. PHB content increased very sharply from 51 to 75% after implementation of this strategy.
A Comparative Account of PHB Fermentation
From the previous results, it can be concluded that the mathematical modeling approach not only helps in understanding the dynamic behavior of fermentation process but also serves as a process optimization tool for designing various nutrient feeding strategies. However, up till now, there have been only few reports on mathematical modeling of PHB production by A. latus [7, 26, 27]. A mathematical kinetic model predicting the intracellular polymer accumulation profile and molecular weight distribution of PHB under batch and fed-batch mode was proposed by Penloglou et al. [26]. The proposed metabolic model was found to be robust and could be used as a guiding tool for the design of various process operating profiles for the production of desired PHA grades. But adopting the proposed model for fed-batch cultivation did not showed much improvement either in PHB accumulation or in molecular weight as opposed to batch cultivation. In another study, batch mathematical model was developed for PHB production by A. australica [7]. The developed batch model was then extrapolated to fed batch, and various feeding strategies were designed using the model for improved PHB production. A significantly high PHB concentration of 22.65 g/L with an overall PHB content of 76% CDW was obtained in constant feed-rate fed-batch cultivation.
Several researchers have implemented different feeding strategies during fed-batch cultivations to enhance the PHB concentration and productivity [28,29,30]. The results of different fed-batch cultivations have been summarized and compared with the present study in Table 2. Ienczak et al. reported three feeding strategies for PHB production by high-cell-density cultivation using a mutant strain of Ralstonia eutropha (Cupriavidus necator DSM 545) [30]. One such high-cell-density strategy featured a high PHB concentration of 27.2 g/L with an overall PHB content of 68% using glucose and fructose as carbon source. In another report, Khanna and Srivastava applied alternate feeding strategy of urea and fructose and obtained a maximum PHB concentration and productivity of 18.46 g/L and 0.46 g/L h, respectively [9]. Moreover, overall PHB accumulation was 47% of CDW, which was much lower than the present study. Hence, it is clearly seen that high PHB content (75%) and PHB concentration (29.64 g/L) obtained in the present study by A. latus using model-based fed-batch cultivation strategy were significantly high as compared to literature reports. The developed model not only helped in deciding the appropriate nutrient feeding profiles during the entire cultivation by avoiding the trial-and-error approach of substrate feeding but also demonstrated high intracellular PHB accumulation. The mathematical model can be used as a guiding tool in designing other feeding strategies under fed-batch and continuous mode to further improve the PHB concentration and/or productivity. This will be a topic of our future research.
Conclusion
A batch kinetic mathematical model was developed and successfully used to identify various fresh nutrient feeding strategies. Applicability of the developed mathematical model was validated by performing various dynamic fed-batch cultivations in bioreactor for the enhanced PHB production inside the cells. The pseudo-steady-state fed batch with respect to sucrose and with respect to nitrogen demonstrated accumulation of high PHB concentration of 24.38 and 29.64 g/L, respectively, with an overall PHB content of 75% of DCW. The mathematical model used in the present study is an extremely useful tool for understanding the dynamic process behavior and for designing suitable control feeding approaches for maximum PHB production.
a, exponent indicating type of relationship between S (nitrogen) and μ; K 1, growth associated product formation constant (g/g); K I, substrate inhibition constant for sucrose consumption (g/L); K S1, saturation constant for sucrose consumption (g/L); K S2, saturation constant for nitrogen consumption (g/L); m S1, maintenance coefficient (g sucrose/g cells h); m S2, maintenance coefficient (g nitrogen/g cells h); n 1, constant in equation; q p , specific product formation rate (h−1); q S1, specific sucrose consumption rate (h−1); q S2, specific nitrogen consumption rate (h−1); S 1, sucrose concentration (g/L); S 2, nitrogen concentration (g/L); S m , nitrogen concentration at which complete inhibition occurs (g/L); X, biomass concentration (g/L); Y (X + P)S1, yield of biomass and product on sucrose consumption (g/g); Y X/S2, yield of biomass on nitrogen consumption (g/g); Greek symbol: μ, specific growth rate (h−1); μ max, maximum specific growth rate (h−1)
References
Mozumder, M. S. I., Garcia-Gonzalez, L., De Wever, H., & Volcke, E. I. (2016). Model-based process analysis of heterotrophic-autotrophic poly (3-hydroxybutyrate)(PHB) production. Biochemical Engineering Journal, 114, 202–208.
Novak, M., Koller, M., Braunegg, M., & Horvat, P. (2015). Mathematical modelling as a tool for optimized PHA production. Chemical and Biochemical Engineering Quarterly, 29, 183–220.
Chanprateep, S. (2010). Current trends in biodegradable polyhydroxyalkanoates. Journal of Bioscience and Bioengineering, 110, 621–632.
Hafuka, A., Sakaida, K., Satoh, H., Takahashi, M., Watanabe, Y., & Okabe, S. (2011). Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresource Technology, 102, 3551–3553.
Shen, L., Haufe, J., & Patel, M. K. (2009). Product overview and market projection of emerging bio-based plastics PRO-BIP 2009. Report for European Polysaccharide Network of Excellence (EPNOE) and European Bioplastics, 243.
Kaur, G., & Roy, I. (2015). Strategies for large-scale production of polyhydroxyalkanoates. Chemical and Biochemical Engineering Quarterly, 29, 157–172.
Gahlawat, G., & Srivastava, A. K. (2013). Development of a mathematical model for the growth associated Polyhydroxybutyrate fermentation by Azohydromonas australica and its use for the design of fed-batch cultivation strategies. Bioresource Technology, 137, 98–105.
Dixit, P., Mehta, A., Gahlawat, G., Prasad, G. S., & Choudhury, A. R. (2015). Understanding the effect of interaction among aeration, agitation and impeller positions on mass transfer during pullulan fermentation by Aureobasidium pullulans. RSC Advances, 5(49), 38984–38994.
Khanna, S., & Srivastava, A. K. (2006a). Computer simulated fed-batch cultivation for over production of PHB: a comparison of simultaneous and alternate feeding of carbon and nitrogen. Biochemical Engineering Journal, 27, 197–203.
Zinn, M., Weilenmann, H. U., Hany, R., Schmid, M., & Egli, T. (2003). Tailored synthesis of poly ([R]-3-hydroxybutyrate-co-3-hydroxyvalerate)(PHB/HV) in Ralstonia eutropha DSM 428. Acta Biotechnologica, 23, 309–316.
Mozumder, M. S. I., De Wever, H., Volcke, E. I., & Garcia-Gonzalez, L. (2014). A robust fed-batch feeding strategy independent of the carbon source for optimal polyhydroxybutyrate production. Process Biochemistry, 49, 365–373.
Khanna, S., & Srivastava, A. K. (2006b). Optimization of nutrient feed concentration and addition time for production of poly (β-hydroxybutyrate). Enzyme Microbial Technology, 39(5), 1145–1151.
Patwardhan, P., & Srivastava, A. K. (2008). Fed-batch cultivation of Wautersia eutropha. Bioresource Technology, 99, 1787–1792.
Gahlawat, G., Sengupta, B., & Srivastava, A. K. (2012). Enhanced production of poly(3-hydroxybutyrate) in a novel airlift reactor with in situ cell retention using Azohydromonas australica. Journal of Industrial Microbiology Biotechnology, 39, 1377–1384.
Gahlawat, G., & Srivastava, A. K. (2012). Estimation of fundamental kinetic parameters of polyhydroxybutyrate fermentation process of Azohydromonas australica using statistical approach of media optimization. Applied Biochemistry and Biotechnology, 168, 1051–1064.
Volesky, B., & Votruba, J. (1992). Mathematical model identification. In Modeling and optimization of fermentation process (pp. 38–54). Amsterdam: Elsevier.
Rosenbrock, H. H. (1960). An automatic method of finding the greatest or the least value of a function. Computer Journal, 3, 175–184.
Kaur, G., Srivastava, A. K., & Chand, S. (2012). Mathematical modeling approach for concentration and productivity enhancement of 1,3-propanediol using Clostridium diolis. Biochemical Engineering Journal, 68, 34–41.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Horwitz, W. (1980). Official methods of analysis of the Association of Official Analytical Chemist (thirteenth ed.). Washington, DC: AOAC Methods.
Riis, V., & Mai, W. (1988). Gas chromatographic determination of polyβ-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. Journal of Chromatography A, 445, 285–289.
Grothe, E., Moo-Young, M., & Chisti, Y. (1999). Fermentation optimization for the production of poly(β-hydroxybutyric acid) microbial thermoplastic. Enzyme and Microbial Technology, 25, 132–141.
Grothe, E., & Chisti, Y. (2000). Poly (β-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: behavior of fed-batch cultures. Bioprocess Engineering, 22, 441–449.
Loo, C. Y., & Sudesh, K. (2007). Polyhydroxyalkanoates: bio-based microbial plastics and their properties. Malaysian Polymer Journal, 2, 31–57.
Zafar, M., Kumar, S., Kumar, S., & Dhiman, A. K. (2012). Artificial intelligence based modeling and optimization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production process by using Azohydromonas lata MTCC 2311 from cane molasses supplemented with volatile fatty acids: a genetic algorithm paradigm. Bioresource Technology, 104, 631–641.
Penloglou, G., Chatzidoukas, C., & Kiparissides, C. (2012). Microbial production of polyhydroxybutyrate with tailor-made properties: an integrated modelling approach and experimental validation. Biotechnology Advances, 30, 329–337.
Chatzidoukas, C., Penloglou, G., & Kiparissides, C. (2013). Development of a structured dynamic model for the production of polyhydroxybutyrate (PHB) in Azohydromonas lata cultures. Biochemical Engineering Journal, 71, 2–80.
Yu, P. H., Chua, H., Huang, A. L., & Ho, K. P. (1999). Conversion of industrial food wastes by Alcaligenes latus into polyhydroxyalkanoates. Applied Biochemistry and Biotechnology, 78, 445–454.
Cavalheiro, J. M. B. T., de Almeida, M. C. M. D., Grandfils, C., & da Fonseca, M. M. R. (2009). Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochemistry, 44, 509–515.
Ienczak, J., Quines, L., Melo, A. D., Brandellero, M., Mendes, C., Schmidell, W., & Aragão, G. (2011). High cell density strategy for poly (3-hydroxybutyrate) production by Cupriavidus necator. Brazilian Journal of Chemical Engineering, 28, 585–596.
Sayed, E. I., Azhar, A., Abdelhady, H. M., Abdel Hafez, A. M., & Khodair, T. A. (2009). Batch production of polyhydroxybutyrate (PHB) by Ralstonia eutropha and Alcaligenes latus using bioreactor different culture strategies. Journal of Applied Sciences Research, 5, 556–564.
Penloglou, G., Roussos, A., Chatzidoukas, C., & Kiparissides, C. (2010). A combined metabolic/polymerization kinetic model on the microbial production of poly (3-hydroxybutyrate). New Biotechnology, 27, 358–367.
Acknowledgements
The Senior Research Fellowship (SRF) award by the Department of Biotechnology (DBT), Govt. of India, New Delhi, for the execution of the project is gratefully acknowledged by one of the authors (Ms. Geeta Gahlawat).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gahlawat, G., Srivastava, A.K. Model-Based Nutrient Feeding Strategies for the Increased Production of Polyhydroxybutyrate (PHB) by Alcaligenes latus . Appl Biochem Biotechnol 183, 530–542 (2017). https://doi.org/10.1007/s12010-017-2482-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2482-8