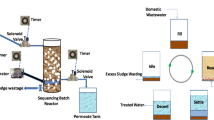
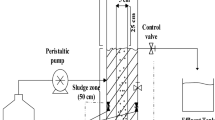
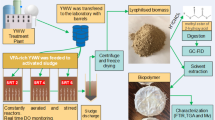
Abstract
The global bioplastics market shows tremendous growth potential in producing sustainable products for diverse applications. The production of polyhydroxybutyrate (PHB) from natural and unutilized (wasted) organic compounds is especially advantageous for achieving simultaneous resource recovery and pollution prevention. This paper investigates the possible avenues to enhance the PHB production from different types of wastewater by virtue of their inherent microorganisms and carbon sources. In contrast to the common notion, waste activated sludge (WAS) is reported to be a highly promising substrate for PHB production; however, it necessitates proper nutrient balancing. It is confirmed that significant recovery of essential nutrients can be obtained by optimizing the substrate-microbe combination, reactor selection and process control techniques. The study further identifies latest technological developments in improving the metabolic pathways for different combinations of carbon and nutrients sources, by varying the operational conditions and identifying suitable microorganisms so that PHB production can be maximized. The study also highlights the scope of possible end-of-life applications for bioplastics in commensuration with achieving sustainable economic feasibility in their processing. Thus, this study aims to present a comprehensive overview on the production, application and reuse options for PHB as a sustainable bioplastic with emphasis on resource recovery strategies.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The world of plastics has created deadly remarks on every face of the planet Earth, even in the deeper beds of the oceans and in the outer space with its endless products capable of staying alive for generations to come. The global plastic production has reached 367 million metric tons in 2020, compared to 200 in 2002 and just 50 in 1976 [1]. Annually, 4–8% of petroleum feedstock is consumed for production of 140 million tonnes of synthetic polymers globally [2]. Plastics are widely consumed in different areas, such as packaging (39.5% of total production), construction supplies (20.1%), automotive machineries (8.6%), electronic machines (5.7%) and agricultural products (3.4%), and the rest includes merchandises like everyday use appliances and sporting kits [2]. It is reported that 26 billion tons of plastic post-consumer waste will be produced by 2050 (simultaneously accounting for 20% of the total oil consumption), half of which will be discarded in the environment, generating a ubiquitous waste management question [3]. Appraising the beauty of creation of synthetic resinous/polymeric substances, however, has ultimately ended up in a global environmental crisis for their safe disposal and recovery, especially on account of their direct ecologic footprints.
The genesis of plastics dates back to the synthesis of organic polymers from natural materials and extraction of petroleum hydrocarbons, credit to their recalcitrant behaviour, keeping them alive for decades and centuries in the environment [4,5,6]. Another catastrophic impact with the end-of-life practices for conventional fossil-based plastics is the release of many harmful residues to the atmosphere (dioxins, hydrogen chloride, sulphur oxides, cadmium, arsenic, zinc and lead) during their bio-/thermal degradation [7]. For example, studies have shown that about 12,000 plastic particles were found per litre of sea ice in the Arctic in 2018 [8]. It is therefore not surprising that approximately 90% of seabirds had plastic particles in their intestines [9]. More disturbingly, however, not only the ocean is damaged by plastic, but also the land and freshwater cannot escape the fate of plastic pollution [10]. Several nations have begun banning the sale of plastic bags, charging customers or levying taxes from stores. China, the largest plastics producer and consumer of the world, also banned imports of some plastics in 2018 [11]. The scale and extent of plastic pollution and the experiences of their inevitable consequences, therefore, demand sustainable production strategies without damaging the economic and related industrial targets adversely. Though sustainability has multiple dimensions of operations in the plastic industry, it is believed that producing alternative materials such as bioplastics using functionally similar materials that are easily bio-degradable can revolutionize the market without compromising on the workability and ease of usage [12].
The world of bioplastics comprises of polymers which are either bio-based (e.g. polyhydroxyalkanoates, PHA) or fossil-based (e.g. polyethyleneterephthalate, PET); more specifically, it indicates those polymers which tend to degrade in shorter life spans (e.g. polycaprolactone, PCL). Though many of the mechanical properties of poly-lactic acid (PLA) are quite similar to that of PET, they are structurally no longer expected to have only pure hydrocarbons as obtained from the fossil feedstock. In other words, the molecular differences in their monomeric structures would be sufficient enough to explain the variability in their physico-chemical characteristics and applications. Technically, these biopolymers are synthesized by numerous types of bacteria as the excess carbon (and energy) storage material within the cell cytoplasm during microbial fermentation processes in response to various nutritional and environmental stress conditions. Among the popular PHA, polyhydroxybutyrate (PHB), a polyester produced by the glucose-fermenting bacteria attracts special attention due to their excellent structural, morphological, thermoplastic and mass transfer properties [13, 14]. They typically contain short-chain chemical structure for their monomers owing to the presence of methyl functional group (-CH3) and an ester linkage group (-COOR) exhibiting superior barrier permeability, thermoplasticity, hydrophobicity, crystallinity and brittleness compared to the synthetic polymers [15, 16]. The market for bioplastics is still naive, capturing only about 1% of the global production in 2017 owing to their increased production cost and lack of social acceptance [17]. Nonetheless, the increasing research attention to the production of bioplastics indicates the emerging trend of replacing conventional plastics in various industries delivering domestic products such as packaging, crockery and cutlery items. Bucci et al. [18] studied the applicability of PHB in food packaging and observed a reasonable life of 60 days before its endogenous decay.
Biosynthesis of PHB from various feedstock materials was attempted by many scientists recently. Many recent studies highlight that the variations in the functionalities of their raw materials (especially carbon source) can influence the biochemical and mechanical characteristics of the PHB products, thereby limiting their commercial-scale production [19,20,21,22,23,24,25,26,27,28]. Identification of suitable bacterial fermentation process is also found to be vital to accentuate the microbial kinetics and PHB accumulation for making bioplastic manufacturing profitable. In addition, there are various techno-economic challenges existing in the promotion of bioplastics in large-scale applications, mainly low yield, high production cost and susceptibility to degradation.
Biotechnologists, over the past few years, have succeeded to acquire a fair deal on simultaneous recovery of nutrients and energy from biochemical systems by manipulating the microbial functioning under various feed-grow conditions [14, 29]. By maintaining proper nutrient loading conditions, the carbonaceous wastewater can be effectively reused as carbon source for PHB production. In this aspect, the mixed activated sludge (MAS) from the wastewater treatment plants (WTP) offers favourable characteristics towards effective recovery of additional resources (bio-energy and bio-products) [30]. It has been reported that the role and activity of each species in such multi-faceted bioreactors depend largely on the organic loading rate (as the nutrient), environmental conditions (temperature, pH, salinity, availability of electron acceptor, etc.) and the hydraulic conditions (residence time, mixing, recirculation, etc.) [31,32,33,34,35].
Though most of the conventional fermentation methodologies such as chemostats (continuous), batch and fed-batch reactors are widely being used in PHB production, their effectiveness towards achieving cost-effective, clean and pure products is not well understood. For example, maintaining distinct phases of nutrient supply and starvation to enhance the biopolymer accumulation is highly limited under constant feed conditions [15]. Since pH and temperature can alter the structure and composition of monomers used for polymerization, the up-scaled production strategies depend highly on the reactor operations. At present, most of the technologies dealing with activated sludge focus primarily on the singular recovery mechanisms while optimizing the treatment. Consequently, there exist conflicts in the efficient utilization of bio-resources (nutrients, biomass and energy) in a synergistic approach. There are multiple research perspectives on the selection and control of process variables, primarily depending on the type of raw materials and the methods of production employed. Hence, a thorough understanding is necessary to entertain biochemical process modification for sustainable bioplastic production.
The present study therefore investigates the latest developments in understanding the mechanisms of efficient PHB production and utilization from various types of wastewater. It also aims to provide an overview of the global trends in simultaneous bioplastics production and nutrient recovery to achieve sustainable solution for cost-effective PHB production as well as sludge handling issues, such as their underutilization and disposal. About a hundred recent research papers on this topic have been selected, of which 85% belongs to the innovative production strategies for PHB from wastewater. A systematic description of the research trends with a few highlighted future prospects is presented in this study.
2 Salient features of bio-processing for PHB production
2.1 Metabolic considerations
PHB can be referred to as the most common intra-cellular polymeric compound present in the cell cytoplasm which is produced under environmental/nutrient stressed conditions (limitations on nitrogen, phosphorous, oxygen or magnesium). The various forms of PHB include poly-3-hydroxybutyrate (P3HB), poly-4-hydroxybutyrate (P4HB), polyhydroxyvalerate (PHV), polyhydroxyhexanoate (PHH), polyhydroxyoctanoate (PHO) and their co-polymers (Fig. 1). The potential use of PHB in food packaging industry depends on its residual resistance against degradation which accounts for the overall physical properties. A summary of the interesting features of PHB is listed in Table 1 to highlight the importance of PHB in industry-ready applications. The variations in the observed physical properties rely primarily on the source, method of extraction and presence of co-substrates as reported in various studies.
The PHBs are known for their readiness to accumulate additional carbon source as well as for their dependable degradability of the same under preferred circumstances by acting as both carbon and energy source (Fig. 2). Acetate and diammonium hydrogen phosphate (DAHP) are found to be the most preferred carbon and nitrogen sources, respectively, for PHB production [50]. Acetyl-CoA, being the primary metabolic substrate in PHB synthesis, can provide suitable central carbon metabolite through various enzymatic reactions. Most of the studies confirmed neutral pH (7.0–7.3) and mesophilic temperature (30–37 °C) as favourable condition for the microbial fermentation for PHB production [21, 51, 52].
A summary of various metabolic pathways for PHB production. CoA and CoA-SH represent coenzymes; A1 represents the specific enzyme acting as catalyst for the reduction and polymerization; ATP represents the adenosine triphosphate; NADH represents nicotinamide adenine dinucleotide with hydrogen (Ref. [15])
In majority of the studies, it is observed that selective microbes under induced nutrient limiting conditions are capable of producing PHB in lab-scale bioreactors as intra-cellular polymeric substances and the extent of their accumulation depends on the carbon source and hydraulic properties of the bioreactor. Most of the scientists are of the opinion that mass-based (either substrate or biomass) ratio can be a better expression for PHB rather than the measured concentration values in order to avoid the limitations of size and scale of the experiments. One reason for this could be the inseparable contribution of PHB towards storage and synthesis is to be commensurate with substrate utilization and oxygen uptake calculations. Another probable reason may be due to the fact that a mass-based (rather, yield-based) expression could be more significant towards evaluating strategies for sustainable PHB production. Khardenavis et al. [50] reported maximum accumulation of PHB (65.84% w/w) for a carbon–nitrogen ratio (C-N) of 50. The production of PHB under anaerobic conditions was found to be about 28.8% of dry biomass (by weight), while a higher generation of PHB (49–50%) was observed under aerobic or anaerobic/aerobic conditions [53]. Consequently, a system combining initially anaerobic conditions and then aerobic conditions would be more beneficial for better PHB yield under excess acetate addition due to the consumption of intra-cellular polymers (carbohydrate and polyphosphate) as energy sources. Hence, from a metabolic point of view, selection of carbon source is the prime factor affecting the enzymatic activities upon the metabolites, leading towards variations in the thermal and physical characteristics of the biopolymers.
2.2 Emerging carbon sources for PHB production
Recent studies proclaim more scientific evidences for the apparent transitions existing in the fermentation pathway to accommodate a wide variety of carbon sources. One unique solution for PHB production from activated sludge using renewable carbon sources is achieved by increasing the production of volatile fatty acids (VFA) through acidogenic fermentation [31]. Depending on the prevailing pH, temperature and feeding pattern, the relationship between VFA and PHA influences the storage capacity of PHA/PHB.
2.2.1 Agro-based wastewater resources
The production of PHB is found to be high (57.98% w/w) from anaerobic wastewater compared to agro-based deproteinized wastewater (milk whey and soyawhey) owing to the high amount of VFA [50]. Earlier, Huey [54] reported PHB production using cafeteria wastewater as the substrate. It is observed that high PHB productivity (67% w/w) can be achieved from mixed culture using wastewater as substrate [55]. Synthesis of PHB using jatropha oil by Cupriavidus necator H16 was attempted by Batcha et al. [49]. In another similar study, the parboiled rice mill effluent was investigated for its replacement as a cheap carbon source for PHB production using Acinetobacter junii BP 25 in a two-stage batch experiment [56]. Recent studies highlight the direct correlation of carbon source from wastewater on the PHB yield in terms of highest and lowest CODs using pure cultures [57].
2.2.2 Industrial wastewater resource
Wastewater from sugar industry provides another possible source of PHB production due to the presence of nitrogen in the molasses. Dalsasso et al. [58] demonstrated that dilution of molasses with vinasse makes a good combination for enhanced PHB production with high yield and low cost. When the organic matter present in the spentwash was subjected to PHB production (while varying chemical oxygen demand (COD):total Kjeldahl nitrogen (TKN) ratio as 30:1 to 60:1), most of the nutrients in the spentwash were immobilized resulting in reduced microbial activity, decreased COD removal and low PHB synthesis [59]. However, under dual limiting conditions (both carbon and nitrogen with a low C-N ratio), high biomass concentration resulted in increased PHB accumulation and high COD removal under simultaneous nitrogen removal mechanism.
2.2.3 Industrial products
Chandrika et al. [56] reported the possibility of utilizing glycerol as carbon source for industrial level production of PHA due to its high productivity ((2.38 ± 0.23 g/L). However, this also indicated an associated high growth of biomass (2.73 ± 0.08 g/L) with high PHA content (87.17%). In a recent study, Werlang et al. [29] observed PHB production from a combination of glucose and glycerol as carbon source, by utilizing hydrolysate of the Arthrospira platensis biomass. Cavaille et al. [60] suggested butyrate as a better carbon source for mixed culture instead of acetate resulting in increased substrate utilization rate, PHB yield and production rate. Dobroth et al. [61] observed the potential of crude glycerol as a carbon source using mixed microbial consortia (MMC) and determined that the enriched MMC produced exclusively polyhydroxybutyrate (PHB) utilizing the methanol fraction.
3 PHB production enhancement scenarios
An important modification from the conventional process dynamic models of activated sludge process (focusing on biomass growth) is the possibility of multiple removal mechanisms for the organic compounds (usually expressed as COD) such as sorption, accumulation and storage. Numerous studies have been carried out with the objective of optimizing the various parameters involved in the PHB production process from WAS. The accumulation of PHB in the biomass cells has been successfully achieved mainly by optimizing the nutritional conditions, aeration modes, carbon source concentrations and pH levels [62]. A detailed comparison of various enrichment conditions in batch experiments is compared and presented in Table 2.
3.1 Reactor operation conditions
Based on the limitations in continuous reactors to achieve growth control by spontaneous feed control, a sequencing batch reactor (SBR) operating under fed-batch mode or feast-famine mode is usually recommended for controlling the biomass growth and PHB production [93]. Considering the frequency of operation, a short cycle time is most recommended for SBR to achieve high PHB production [51]. High concentration of influent nitrate was found to have detrimental effect of PHB accumulation during the anoxic operation of SBR by Ciggin et al. [74]. Wang et al. [66] reported higher production of PHB (7% more) using two-stage batch process compared with fed-batch fermentation of sucrose with Alcaligenes latus ATCC 29,714. Limitation on nitrogen source was effected by optimizing the upstream process time. It is also possible to conduct the studies in three-stage system where the first-stage reactor will convert organic matter to VFA through acidogenesis, and the second-stage reactor will enrich the acid-producing microorganisms while the third stage is unique for PHB accumulation.
One remarkable implication of the repeated feast-famine cycle is the respective accumulation and deprivation of PHB in the SBR setup. It is to be noted that greater operational cycle length can lead to the disruption of stored PHB as the internal carbon source during the famine phase [76]. Under conditions of ammonium starvation, the SBR operated in feast-famine mode and produced high PHB yield (89%) after 7.6 h [80]. While comparing the conventional fed-batch reactors with two-stage reactors for fermentation reactions, higher biomass yield can be expected in fed-batch mode due to the presence of extended log phase. However, two-stage mode enabled better process control, economic operation and higher PHB yield with minimum requirement of carbon source [66, 70].
3.2 Modification of physical variables
A central composite rotary design (CCRD) was proposed by Pandian et al. [84] to optimize the medium for PHB production in terms of concentrations of dairy waste, rice bran, sea water and pH. The same approach was used by Tripathi et al. [94] to optimize pH, temperature and agitation speed for enhanced PHB production in batch cultivation by Alcaligenes sp. using dry molasses as the substrate. They have observed a yield of 76.8% (mass based) under the conditions of pH as 6.54, temperature as 34.5 °C and agitation speed as 3.13 Hz. The process optimization by one factor at a time by Chandrika et al. [56] resulted in high yield of homopolymer PHB (2.64 ± 018 g/L with 94.28% PHB content) and a copolymer, polyhydroxybutyrate-co-hydroxyvalerate (P3(HB-co-HV)) (85.93% content) from Acinetobacter junii BP 25 using two-stage batch cultivation mode. Another promising application of the optimal design of an integrated microalgae-based bio-refinery using mixed integer nonlinear programming was addressed by Prieto et al. [95] for the production of bio-diesel with PHB as one of the main by-products. The operation of a SBR with wastewater feed was optimized by a uniform design approach coupled with grey relational analysis considering influent characteristics such as pH, COD, nitrogen and phosphorous as the critical parameters [83].
Carucci et al. [63] demonstrated the significance of oxygen uptake rate (OUR) as an indication of PHB storage during the utilization of readily decomposable organic matter (11.4 mg/g VSS h for filtered wastewater against 2.4 mg/g VSS h for acetate media) which accounts for about 20–22% of the total oxygen requirement. They reported that storage of PHB from other substrates is also to be considered apart from acetate, which still gives the major contribution. High PHB production was reported by Primasari et al. [64] in the presence of excess carbon source, but without aeration. Controlling pre-aeration to enhance PHB accumulation was achieved by Liu et al. [96] with short and moderate pre-aeration volumes.
3.3 Induced co-substrates and nutrient limitations
The PHB production from different substrates exhibited different metabolic pathways, at least until the production of acetyl-CoA while entering the Krebs cycle. While fatty acids and alcohols show direct pathway for acetyl-CoA, substrates like glutamic acid differ in their metabolic pathway [81]. Limiting nitrogen has resulted in increased PHB storage and reduced direct growth of biomass. However, the accumulation of nitrite as an intermediate compound is found to be significant in estimating the dynamic growth response from the microbes [74]. Also, the limitation of phosphorous can lead to reduced protein synthesis, leading towards higher PHA accumulation using mixed activated sludge and wastewater as substrate [86]. Reddy and Mohan [75] reported high PHA storage at high substrate load (40.3% CDW), low nitrogen (45.1% CDW) and low phosphorous (54.2% CDW) conditions.
Experiencing substantial nutrient limitation (nitrogen and phosphorous) was observed to be beneficial for the microbial transformations of carbon source (most commonly, sodium acetate) to PHB under excess conditions, while some sodium acetate was consumed for biomass growth [62]. A strong phosphorous limiting condition (and even phosphorous starvation) was found to activate the PHA-accumulating organisms to decrease their catalytic biomass growth and direct majority of the carbon source towards PHA storage [60]. Even though high PHB content was obtained from such studies, the overall production of PHA was found to be low due to the inherent growth limitations on the activated sludge culture.
3.4 Extraction and recovery of PHB
Solvent extraction is the most commonly used method to separate PHB from the bulk biomass. It is advantageous due to the high extraction efficiency, absence of bacterial endotoxin and reduced structural deterioration of the PHB polymers. However, it is highly uneconomical at large-scale production with high amount of toxic by-products such as chlorinated solvents and cyclic carbonates. Pandian et al. [84] reported that surfactant-chelate digestion could be a promising solution for efficient and sustainable extraction of PHB molecules at a mass ratio of 0.0075 and 0.01 with respect to dry biomass. Other techniques like digestion, solubilization and solvent-free extraction are reported to be effective only on a limited type of feed organics. Since the efficient extraction of PHB defines the immediate scope for industrial scale production, more innovative strategies have to be evolved to optimize the PHB production.
4 Recent advances in sustainable PHB production strategies
4.1 Strategies to achieve simultaneous nutrient/energy recovery with PHB production
Enhancing the production of PHB based on activated sludge from wastewater primarily depends on optimizing the process control, realizing the metabolic pathways and storage mechanisms and improving the mechanical properties of produced PHB based on advanced characterization techniques [62]. Based on the understanding of the preferred metabolic pathways, PHB production is followed by the acetyl-CoA production along with the reducing power of NADH molecules while the biomass synthesis prefers acetyl-CoA and ATP molecules (sometimes NADPH also) [93]. It is to be noted that higher nitrogen conditions can facilitate the biomass cells to undergo tricarboxylic acid (TCA) cycle metabolic pathway for energy production, thereby reducing the availability of acetyl-CoA towards PHB synthesis. Thus, the key factor in maintaining the conditions favourable for PHB production lies in the distribution of acetyl-CoA between these two pathways based on the availability of NADH as well as ATP, respectively.
Production of PHB with simultaneous removal of phosphorous from wastewater depends on the selectivity of microbes and phosphorous release-carbon uptake ratio [78, 97]. When glucose is the prime carbon source under the abundance of glycogen accumulating bacteria, Embden-Meyerhof-Parnas (EMP) pathway to produce acetyl-CoA was replaced by succinate-propionate pathway to produce propionyl-CoA. This is found to have detrimental effect on removal of phosphorous and as well as PHB production [86]. In a similar study, the decrease in phosphorous content of active biomass resulted in a reduced PHB yield (and content) with an increase in the biomass yield [52]. In general, limitations on nitrogen and phosphorous were found to be beneficial for PHB accumulation in aerobic conditions, while limiting phosphorous sources enhanced PHB accumulation in anaerobic conditions [91].
As seen before, high carbon content can be bypassed largely towards cell growth inhibiting the microbial synthesis of PHB, or it can cause cell lysis due to the overflow accumulated PHB leading towards cell death, thereby reducing the PHB yield. Based on the batch experiments, Mulders et al. [52] observed that the volumetric productivity (g PHB/L/h) of PHB is higher in phosphorous-limited systems compared to the nitrogen-limited systems due to the trivial C:N:P ratios in the feed substrate. Mulders et al. [52] reported high cellular content of PHB (> 75% w/w) for higher carbon-nutrient ratios (C:N or C:P). This also infers the sensitivity of the biopolymer accumulation mechanisms as well as the requirement of using a nutrient-limited wastewater regardless of the microorganism. Hence, it is important to ensure the proper nutrient balance in the reactor as expressed by various ratios specific to the environmental conditions.
On another aspect with terminal electron acceptor, PHB production from both aerobic and anaerobic conditions experiences systemic drawbacks. Under strictly aerobic conditions, only a fraction of the carbon source is converted to PHB so as to meet the requirements for other purposes. Furthermore, the polyphosphate content inside the biomass may degrade PHB as the readily available intra-cellular energy source, thereby decreasing the PHB content in the biomass and increasing the recovery cost. In the case on anaerobic conditions, the presence of PHB in biomass is too low to be recovered sustainably. Hence, a better option for simultaneous nutrient removal and resource recovery (phosphorous and PHB) could be a sequential anaerobic–aerobic treatment [53, 91]. This is confirmed by Liu et al. [96] indicating the supremacy of anaerobic/aerobic process over the feast-famine cycles for PHB accumulation in laboratory-scale batch experiments. A similar approach for enhanced biological phosphorous removal (EBPR) in batch experiments under anaerobic, aerobic and anaerobic/aerobic conditions also resulted in substantial PHB production [53].
4.2 Considerations in PHB production kinetics and microbial characterization
The optimum period for fermentation and the yield of PHB depends on the nature of nutrient sources (e.g. carbon and nitrogen) as well the type of microbial strains [69, 98]. The selection of microorganisms is important because of their ability to utilize inexpensive carbon source, growth rate, biopolymer production rate and possible extent of biopolymer accumulation in their cells (Table 3). Enrichment of selective methanotrophic-heterotrophic bacteria from sewage sludge can potentially accumulate more PHB [77]. The presence of nitrogen sources such as peptone and yeast extract resulted in increasing the cell growth of Bacillus subtilis EPAH18 while reducing PHB accumulation in the cells [90]. This illustrates the necessity of maintaining nitrogen limiting conditions to sufficient PHB production with reduced cell growth. They found that highest PHB production was achieved at the end of the exponential growth phase where lactose as a carbon source was consumed to 25% of its initial value, inferring the reduction in biomass accumulation. Bernat and Wojnowska‐Baryla [99] observed degradation of PHB during denitrification (for nitrogen removal) instead of polyphosphate accumulation by P. denitrificans without any cell growth. They also reported that addition of a suitable carbon source can readily accelerate the denitrification rate in the activated sludge model.
On a similar note, the substrate utilization rate under nitrogen-limited condition was not showing any increase despite biomass growth indicating the exhaustion of exponential phase [52]. This is in accordance with the interpretation of activated sludge model-3 (ASM3) showing that rapid aerobic degradation of organic matter will be limited by the PHB accumulation due to the prevailing denitrification condition [93]. This also suggested the existence of simultaneous nitrification–denitrification (SND) condition under limited oxygen supply which is favourable for PHB production. Thus, the reducing power of organic carbon to PHB by the heterotrophic PHB-storing bacteria confirms simultaneous nitrogen removal and PHB storage under oxygen-limited conditions. This is also explained in terms of the varying oxidation states during SND under feast-famine conditions [100]. While acetate can act as the electron donor, the SND during the feast phase was limited by the ammonium oxidation. However, during the famine phase, SND was limited by the depletion of acetate and ammonium oxidation was inhibited by the heterotrophic species. Thus, SND essentially involves two contrasting conditions: aerobic nitration (in the absence of organics) and anoxic denitritation (in the presence of organics as electron donors). The process control on reaction time can favour more PHB accumulation without direct oxidation especially during the aerobic feast conditions. The above process has been presented using a schematic diagram (Fig. 3).
Configurations for the combined selection of PHA storing biomass and nitrogen removal from sludge reject water by applying the aerobic/anoxic feast/famine regime and nitrogen removal via nitrite in a single stage reactor (redrawn from [13])
Higher PHB storage at the start of anoxic stage was observed with a strong oxygen supply in aeration, thus favouring endogenous denitritation [100]. In addition to the utility as internal carbon source for SND during the aeration phase, the accumulated PHB serves as electron donor for denitritation in the absence of external substrate (especially particular organics). With respect to the difference in the mode of operation, two-stage fermentation by A. latus (ATCC 29,714) using sucrose as the carbon source and with nitrogen limitation (induced after aerobic phase, between 16 and 26 h of operation) was found to be optimal for enhancing PHB production [66].
Attempts to vary the organic acid substrates ultimately result in selecting different metabolic pathways during the synthesis and accumulation of PHB. Some bacterial species (A. eutrophus, A. Latus and mutant strain of Azotobacter vinelandii) are capable of accumulating PHA during their growth without experiencing any nutrient stress. Rebah et al. [88] reported that rhizobial strains have higher PHB productivity (27–40% w/w) in standard medium compared to the wastewater sludge (3.7% w/w). The recombinant E. coli was reported to have significant PHB productivity (40% w/w) using fermentation acids [67]. Mulders et al. [52] observed that a combination of microorganisms based on their preference for PHB production can result in improved accumulation over longer period even with a lower concentration. In a similar study, Primasari and Wei [20] reported that longer acclimatization period could take up more organic compounds, thereby increasing the PHB accumulation capacity. If the P. acidivorans culture is allowed to grow on the leachate from the organic fraction of municipal solid waste, substantial accumulation of PHB (> 75% content) can be obtained only for higher carbon-nutrient ratios (COD: P > 511 and COD: N > 26) [52]. The high temperature lysates during the anaerobic fermentation of sludge through thermal cracking can result in increased production of acetic acid to increase the PHB production [30].
Utilization of cyanobacterial biomass for PHB production is emerging as a sustainable option since it can easily culture on wastewater, but for one limiting condition that exists on the threshold inorganic carbon sources. Unlike other carbon sources, high concentration of dissolved inorganic carbon promotes glycogen accumulation while decreasing PHB production during the dark phase [21]. Hence, small amount of dissolved inorganic carbon is favourable for the biosynthesis of carbon storage polymers.
4.3 Bioplastics versus bio-energy—priority analysis of PHB utilization
While comparing the operational strategies for the production of bioplastics versus extraction of bio-energy, it is important to realize that PHB production can be achieved without any supplementary enrichment stage due to the limited nutrients for biomass growth and accumulation [60]. From the point of view of the overall production cost of PHB, this approach can eliminate the requirements of additional energy and chemical reagents. While bio-energy production remains an essential priority for anaerobic wastewater treatment systems, it is possible to achieve similar productivity for PHB only by maintaining high biomass concentration. Therefore, bio-solids in such bioreactors are characterized with high growth rate, yield and density [19]. This indicates the possibility of achieving simultaneous production of bioplastics and bio-energy, which is, however, dependent on the prevailing environment established in wastewater/sludge treatment facilities.
In addition to the supply control on carbon source, recent studies have mentioned a few operational controls on other substrates towards achieving simultaneous production of bio-energy and bioplastics. In a recent study by Dinesh et al. [92], simultaneous production of hydrogen and PHB was achieved from acid-treated rice straw and rice husk. The induced environmental stress caused by oxygen-limited conditions for Bacillus species resulted in a decrease in pH owing to the acidification (through pyruvate formate-lyase and formate dehydrogenase systems). The resulting formate and acetyl-CoA were directed to the PHB production based on the prevailing nutrient stress conditions. Some of the purple non-sulphur reducing bacteria (PNSB) are also capable of hydrolysing propionate to acetate, leading to the formation of acetyl-CoA which serves as the prime precursor in the PHB synthesis [72]. In addition, some of the PNSB strains such as Rhodopseudomonas palustris RG31 and WP3-5 are capable of producing hydrogen gas simultaneously through the extended metabolic pathway.
The degradation of PHB results in accumulation of non-toxic R-3-hydroxybutyric acid which finds application in biomedical field, especially as drug carrier to blood [84]. Miao et al. [100] observed that the degradation kinetics of PHB resembles first-order kinetics across all SBRs. During degradation, the internal PHB hydrolysis rate becomes the rate-limiting step compared to the oxygen utilization rate.
4.4 Biodegradability and end-of-use options for PHB
The biodegradability of various bioplastics under simulated landfill conditions was investigated by many scientists earlier [101,102,103,104]. By employing anaerobic digested sludge, they found that natural aliphatic polyester such as poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/HV; 92/8, w/w) degraded within 20 days of cultivation, while synthetic aliphatic polyesters such as poly-lactic acid, poly(butylene succinate) and poly(butylene succinate-co-ethylene succinate) did not degrade at all in 100 days [105]. Finelli et al. [106] reported that blending of PHB with ethyl cellulose in activated sludge and in enzymatic solution could degrade the PHB completely.
It is inevitable that in the absence of external carbon source, the pre-accumulated PHB molecules inside the microbes could be employed for cell synthesis and simultaneous denitrification [107]. It is commonly observed in SND batches with associated oxygen uptake and nitrous oxide emission [108]. In general, it was observed that the degradation of PHB in an SBR is independent of the type of electron acceptor and solids retention time [109]. One common reason for decrease in PHB accumulation (or degradation of PHB) can be attributed to the change in stage of electron acceptor, especially when high aeration is provided [110]. It is also observed that when the dominant composition shifted from PHB to PHV, the amount of generated hydrogen decreased from 51.2 to 41.1 mL/g VSS even under the same PHA level (around 130 mg/g VSS) indicating the PHB decomposition [73]. Brzezinka et al. [111] isolated typical activated sludge bacteria of Aeromonas and Rhodococcus genus to degrade PHB and their co-polymers. They observed that the respiratory activity of microorganisms is different for a variety of biopolymers depending significantly on the type of polymer. The highest oxygen consumption was noted in the presence of PCL (280 mgO2/L after 7 days of incubation) while the lowest was in PLA (220 mgO2/L after 7 days of incubation).
4.5 Economic considerations of PHB utilization from wastewater
It is strongly anticipated that the cost of production of PHB can be curtailed by employing mixed cultures and cheap substrates derived from waste organic matter. This will reduce the cost of sterilization and sterile fermentation during the mass production of PHB. As reported by da Costa et al. [112], the use of mixed cultures from wastewater for fermentation followed by downstream treatment with alkali could reduce the cost by 28%, global warming potential by 44% and non-renewable energy usage by 32%. Apart from the cost of substrate, the next important cost factor is the efficient extraction and recovery of produced polymer. The total cost of production of PHB as a bioplastic depends on the availability of carbon and nutrient sources (substrates), performance of microorganisms (growth, yield, productivity), facilitation of operating conditions such as pre-treatment and conditioning of feed materials (e.g. waste activated sludge, industrial effluent, other organic wastewater), conditions of fermentation (pH, temperature, aeration, hydraulic and biochemical process control, etc.) and extraction (separation and purification). Based on the cost of production of PHB from the available substrates, it can be inferred that waste-based substrates can be invariably economical and sustainable (Table 4).
5 Challenges and future scope
-
In short, the major challenges in sustainable PHB production from wastewater include high cost of production, short of reliable and flexible technology, limited market supply of bioplastics, low consumer awareness, lack of unified bioplastics labelling method, the end-of-life management of bioplastics and the induced bio-toxicity. As of now, the real-time applications of PHB are limited due to the lack of cost-effectiveness in the production process in spite of its high potentiality. This poses a severe obstacle in the growth and scale-up of PHB production at the industrial level. Carbon source, one of the major players in the production of PHB, contributes to about 50% of the total cost of production. Thus, more focus is needed in producing low-cost substrates to produce PHB in mass quantity.
-
One major challenge in using wastewater originating from various organic industries is the lack of growth controlling nutrients which will directly affect the production and accumulation of PHB. The PHB accumulation capacity of specific strains, however, is always high in standard medium when compared to the wastewater sludge. This inherent issue could be solved to a large extent by providing sufficient enrichment at the growing stage.
-
It is widely reported that feasible productivity of PHB from mixed activated sludge culture with a variety of organic nutrients may be much less compared to the outputs from pure cultures growing in isolated standard medium due to the complex nature of the wastewater. While attempting to utilize the emerging carbon and nutrient sources for PHB production, it is more likely that the target species may not be able to digest some of the substrates in their raw form which necessitates additional pre-treatment steps to convert them to easily degradable organics. Naranjo et al. [114] reported that thermal, acidic or enzymatic pre-treatments are necessary to convert cellulose and hemicelluloses from banana peel to simple sugars.
-
Since wastewater sludge (be in aerobic or anaerobic) preferably promotes cell growth, PHA accumulation is comparatively less in all reported studies. Moreover, the stored PHB also undergoes breakdown in response to the exhaustion of limiting nutrients in the sludge. Hence, proper sludge pre-treatment becomes a necessity to enhance the bio-availability of carbon as well as PHB production.
-
Usage of anaerobic granular sludge as seed for preparing aerobic granular sludge has invoked research attention to improve the mechanical and morphological properties of the PHB produced [79]. This also suggests utilization of aerobic co-composting as an alternative method for producing granular sludge capable of producing PHB [33, 115].
-
One peculiar case is the possibility of simultaneous production of PHB and extracellular polymeric substances (EPS) under abundance of glucose and ammonium sulphate by selective species such as Ralstonia eutropha ATCC 17,699 [69]. Based on the stoichiometric and food-microorganism conditions, it is important to establish proper carbon-nutrient ratios (C:N:P) for wastewaters having varying carbon sources in order to control the processes of biomass growth and PHA production [71].
-
It is important to enhance the preservation characteristics of PHB especially in the absence of external carbon source during wastewater degradation. Theoretically, it is important to control the PHB preservation by varying proportion of PHB derivation from acetate, rather than directly improving PHB synthesis rate. This can also achieve better control on excess biomass production, thereby enhancing the PHB content and productivity.
-
The utilization of leachate from the organic fraction of municipal solid waste for PHB production seems still challenging owing to the complexities associated with enrichment of suitable microbes. One practical solution could be to introduce the leachate to a selected species (P. acidivorans) established on a standard medium.
-
Apart from the wise selection of feedstock, genetic modifications and blending of productive strains are emerging as the biotechnological solution for enhancing PHB productivity [12]. In addition, blending of manufactured nano-materials (MNM) in waste activated sludge can be a good option for simultaneous PHB production and enhanced biological phosphorous removal under anoxic conditions [116].
-
One challenge with the structural features of produced PHB is that crystalline structure of PHB homopolymer may lead to some mechanical behaviour which may not render it compatible to various production processes [117].
-
Another aspect which needs attention is the microbial strain used for the production of PHB. Mixed microbial cultures can help in reducing the cost of production [12]. Thus, studies can be carried in these aspects so that scale-up of PHB processes can be easily understood. Moreover, research in improving the microbial strain employing genetic engineering and molecular tools can lead to efficient carbon utilization and enhanced PHB production. Hence, innovative research from biotechnology can aid in obtaining high cell biomass yield with high PHB output.
6 Conclusion
The present study reviewed remarkable research advancements in the field of production, application and end-of-life applications of PHB in the recent past. We realize that the real-time applications of PHB are limited due to the lack of cost-effectiveness in the production process in spite of its high potentiality. The futuristic production systems for PHB suffer technically from the fundamental fact that it is produced in a survival mode as a secondary metabolite rather than in proportion to the active biomass growth rate. The study strongly recommends proper sludge pre-treatment, which becomes a necessity to enhance the bio-availability of carbon from waste activated sludge as well as PHB production. Hence, special attention is required to ensure the PHB production in a scale suitable for industrial applications. It is strongly anticipated that the cost of production of PHB can be curtailed by employing mixed cultures and cheap substrates derived from waste organic matter. The research findings highlighted existing challenges and provided some potential research areas to further the industrial production of bioplastics in order to pose as a prime alternative to the conventional plastics.
Abbreviations
- ADSF:
-
Aerobic dynamic substrate feeding
- ASM3:
-
Activated sludge model-3
- CCRD:
-
Central composite rotary design
- C-N:
-
Carbon-nitrogen ratio
- COD:
-
Chemical oxygen demand
- DAHP:
-
Diammonium hydrogen phosphate
- DCW:
-
Dry cell weight
- EBPR:
-
Enhanced biological phosphorous removal
- EMP:
-
Embden-Meyerhof-Parnas
- EPS:
-
Extracellular polymeric substances
- FF:
-
Feast-famine
- MAS:
-
Mixed activated sludge
- MLSS:
-
Mixed liquor suspended solids
- MMC:
-
Mixed microbial consortia
- MNM:
-
Manufactured nano-materials
- P3HB:
-
Poly-3-hydroxybutyrate
- P4HB:
-
Poly-4-hydroxybutyrate
- PCL:
-
Polycaprolactone
- PET:
-
Polyethyleneterephthalate
- PHA:
-
Polyhydroxyalkanoates
- PHB:
-
Polyhydroxybutyrate
- PHH:
-
Polyhydroxyhexanoate
- PHO:
-
Polyhydroxyoctanoate
- PHV:
-
Polyhydroxyvalerate
- PNSB:
-
Purple non-sulphur reducing bacteria
- SBR:
-
Sequencing batch reactor
- SND:
-
Simultaneous nitrification–denitrification
- SRT:
-
Solids retention time
- TCA:
-
Tricarboxylic acid
- TKN:
-
Total Kjeldahl nitrogen
- VFA:
-
Volatile fatty acids
- VSS:
-
Volatile suspended solids
- WAS:
-
Wasted activated sludge
- WTP:
-
Wastewater treatment plant
References
Link 1: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/. Accessed 29 Nov 2020
Hopewell J, Dvorak R, Kosior E (2009) Plastics recycling: challenges and opportunities. Phil Tran R Soc B 364:2115–2126
Guglielmi G (2017) In the next 30 years, we’ll make four times more plastic waste than we ever have. Science. https://www.sciencemag.org/news/2017/07/next-30-years-we-ll-make-fourtimes-more-plastic-waste-we-ever-have. Accessed 20 April 2021
Volova TG (2004) Polyhydroxyalkanoates-plastic materials of the 21st century: production, properties, applications. Nova Science Publishers, Hauppauge, p 282
DiGregorio BE (2009) Biobased performance bioplastic: Mirel. Chem Biol 16(1):1–2
Keshavarz T, Roy I (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Cur Opin Microbiol 13(3):321–326
Harding KG, Dennis JS, von Blottnitz H, Harrison ST (2007) Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically based poly-beta-hydroxybutyric acid using life cycle analysis. J Biotechnol 130(1):57–66
Kanhai LDK, Gårdfeldt K, Lyashevska O, Hassellöv M, Thompson RC, O’Connor I (2018) Microplastics in sub-surface waters of the Arctic Central Basin. Mar Pollut Bull 130:8–18
Wilcox C, Sebille EV, Hardesty BD (2015) Threat of plastic pollution to seabirds is global, pervasive and increasing. Proc Nat Acad Sci 112(38):1–6
Rochman MC (2018) Microplastics research – from sink to source. Science 360(6384):28–29
Zhu J, Wang C (2020) Biodegradable plastics: green hope or greenwashing? Mar Pollut Bull 161:111774
Sirohi R, Pandey JP, Tarafdar A, Sindhu R, Parameswaran B, Pandey A (2020) Applications of poly-3-hydroxybutyrate based composite in advanced applications of polysaccharides and their composites. Mater Res Found 68:45–59
Frison N, Katsou E, Malamis S, Oehmen A, Fatone F (2015) Development of a novel process integrating the treatment of sludge reject water and the production of polyhydroxyalkanoates (PHAs). Environ Sci Tech 49(18):10877–10885
Sirohi R, Pandey JP, Gaur VK, Gnansounou E, Sindhu R (2020) Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour Technol 311:123536
McAdam B, Brennan Fournet M, McDonald P, Mojicevic M (2020) Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 12(12):2908
Saratale RG, Cho SK, Saratale GD, Kadam AA, Ghodake GS, Kumar M, Bharagava RN, Kumar G, Kim DS, Mulla SI, Shin HS (2021) A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour Technol 9:124685
Kakadellis S, Harris ZM. Don’t scrap the waste: the need for broader system boundaries in bioplastic food packaging life-cycle assessment – a critical review. J Clean Prod 274:122831
Bucci DZ, Tavares LBB, Sell I (2007) Biodegradation and physical evaluation of PHB packaging. Poly Test 26(7):908–915
Fergala A, AlSayed A, Eldyasti A (2018) Factors affecting the selection of PHB accumulating methanotrophs from waste activated sludge while utilizing ammonium as their nitrogen source. J Chem Technol Biotechnol 93(5):1359–1369
Primasari B, Wei RK (2019) Simultaneous pollutant removal and PHB accumulation in simple anaerobic treatment of oily wastewater. MATEC Web Conf 276:06031
Rueda E, García-Galán MJ, Díez-Montero R, Vila J, Grifoll M, García J (2020) Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour Technol 295:122233
Sirohi R, Gaur VK, Pandey AK, Sim SJ, Kumar S (2021) Harnessing fruit waste for poly-3-hydroxybutyrate production: a review. Bioresour Technol 326:124734
Sirohi R, Pandey JP, Tarafdar A, Agarwal A, Chaudhuri SK, Sindhu R (2021) An environmentally sustainable green process for the utilisation of damaged wheat grains for poly-3-hydroxybutyrate production. Environ Tech Innov 21:101271
Reshmy R, Thomas D, Philip E, Paul SA, Madhavan A, Sindhu R, Sirohi R, Varjani S, Pugazhendhi A, Pandey A, Binod P (2021) Bioplastic production from renewable lignocellosic feedstocks: a review. Rev Environ Sci Biotech 20:167–187
Saratale GD, Bhosale R, Shobana S, Banu RJ, Pugazhendhi A, Mahmoud E, Sirohi R, Bhatia SK, Atabani AE, Mulone V, Yoon JJ, Shin HS, Kumar G (2020) A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour Technol 314:123800
Chaudhari Y, Pathak B, Fulekar MH (2012) PHA-production, application and its bioremediation in environment -I. Res J Environ Sci 1:46–52
Yousuf RG (2018) Novel polyhydroxybutyrate (PHB) production using a waste date seed feedstock. Doctoral Thesis. The University of Manchester, United Kingdom
Sirohi R, Pandey JP, Tarafdar A, Agarwal A, Chaudhuri SK, Sindhu R (2021) An environmentally sustainable green process for the utilization of damaged wheat grains for poly-3-hydroxybutyrate production. Environ Technol Innov 21:101271
Werlang EB, Moraes LB, Muller MVG, Julich J, Corbellini VA, de Farias Neves F, de Souza D, Benitez LB, de Souza Schneider RDC (2020) Polyhydroxybutyrate (PHB) production via bioconversion using bacillus pumilus in liquid phase cultivation of the biomass of Arthrospira platensis hydrolysate as a carbon source. Waste Biom Valor: 1–11
Liu F, Li J, Zhang XL (2019) Bioplastic production from wastewater sludge and application. In IOP Conf Series: Earth Environ Sci 344 (1): 012071
Jayakrishnan U, Deka D, Das G (2020) Influence of inoculum variation and nutrient availability on polyhydroxybutyrate production from activated sludge. Int J Biol Macromol 163:2032–2047
Balaganesh P, Vasudevan M, Shahir S, Suneethkumar SM, Natarajan N (2020) Evaluation of sugarcane and soil quality amended by sludge derived compost and chemical fertilizer. Nature Environ Poll Technol 19(4):1737–1741
Balaganesh P, Vasudevan M, Natarajan N (2020) Insights into the application of co-composting for soil nutrient stability – a review. IOP Conf Series: Mater Sci Eng 955:012093
Vishnupavya LU, Danyaa MB, Vishvapriya K, DeepikaSree SK, Vasudevan M, Geethamani R (2020) Energy recovery efficiency of low cost microbial fuel cell using domestic wastewater. IOP Conf Series: Mater Sci Eng 955:012099
Balaganesh P, Vasudevan M, Natarajan N, Kumar SMS (2020) Improving soil fertility and nutrient dynamics with leachate attributes from sewage sludge by impoundment and co-composting. Clean Soil Air Water 48(12):2000125
Machado MLC, Pereira NC, Miranda LF, Terence MC, Pradella JGC (2010) Estudo das propriedades mecânicas e térmicas do polímero poli-3-hidroxibutirato (PHB) e de compósitos PHB/pó de madeira. Polímeros:Ciência e Tecnologia 20(1):65–71
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Poly Sci 25(10):1503–1555
Casarin SA, Agnelli JAM, Malmonge SM, Rosário F (2013) Blendas PHB/copoliésteres biodegradáveis: biodegradação em solo. Polímeros 23(1):115–122
Rodrigues JAFR, Parra DF, Lugão AB (2005) Crystallization on films of phb/peg blends. J Ther Anal Calor 79(2):379–381
Sharma R, Ray AR (2006) Polyhydroxybutyrate, its copolymers and blends. J Macromol Sci Part C 35(2):327–359
Ayorinde FO, Saeed KA, Price E, Morrow A, Collins WE, McInnis F, Pollack SK, Eribo BE (1998) Production of poly(_-hydroxybutyrate) from saponified Vernonia galamensis oil by Alcaligenes eutrophus. J Indus Microbiol Biotechnol 21(1):46–50
Srubar WV III, Wright CZ, Tsui A, Frank CW (2012) Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Poly Degrad Stab 97(10):1922–1929
Mousavioun P, George G, Doherty W (2012) Environmental degradation of lignin/poly(hydroxybutyrate) blends. Poly Degrad Stab 97(7):1114–1122
Santos AF, Polese L, Crespi MS, Ribeiro CA (2009) Kinetic model of poly(3-hydroxybutyrate) thermal degradation from experimental non isothermal data. J Ther Anal Calor 96(1):287–291
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Ann Rev Microbiol 56(1):403–432
Alejandra R-C, Margarita C-M, Soledad M-CM (2012) Enzymatic degradation of poly(3-hydroxybutyrate) by a commercial lipase. Poly Degrad Stab 97(11):2473–2476
Barham PJ, Keller A, Otun EL, Holmes PA (1984) Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J Mater Sci 19(9):2781–2794
Ho M-H, Li S-Y, Ciou C-Y, Wu T-M (2014) The morphology and degradation behavior of electrospun poly(3-hydroxybutyrate)/magnetite and poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/magnetite composites. J Appl Polym Sci 131(22):1–9
Batcha AFM, Reddy Prasad DM, Khan RM, Abdullah H (2014) Biosynthesis of poly (3-hydroxybutyrate) (PHB) by Cupriavidus necator H16 from jatropha oil as carbon source. Bioproc Biosyst Eng 37:943–951
Khardenavis A, Guha PK, Kumar MS, Mudliar SN, Chakrabarti T (2005) Activated sludge is a potential source for production of biodegradable plastics from wastewater. EnvironTechnol 26(5):545–552
Ozdemir S, Akman D, Cirik K, Cinar O (2014) Effect of cycle time on polyhydroxybutyrate (PHB) production in aerobic mixed cultures. Appl Biochem Biotechnol 172:2390–2399
Mulders M, Tamis J, Stouten GR, Kleerebezem R (2020) Simultaneous growth and poly(3-hydroxybutyrate) (PHB) accumulation in a Plasticicumulans acidivorans dominated enrichment culture. J Biotechnol X 8:100027
Rodgers M, Wu G (2010) Production of polyhydroxybutyrate by activated sludge performing enhanced biological phosphorus removal. Bioresour Technol 101(3):1049–1053
Huey SCS (2006) Polyhydroxybutyrate (PHB) production from cafeteria wastes under anoxic and aerobic conditions in sequencing batch reactor. Undergraduate Thesis, Universiti Teknologi Malaysia, Malaysia
Khardenavis AA, Suresh Kumar M, Mudliar SN, Chakrabarti T (2007) Biotechnological conversion of agro-industrial wastewaters into biodegradable plastic, poly β-hydroxybutyrate. BioresourTechnol 98:3579–3584
Chandrika PS, Sabarinathan D, Anburajan P, Preethi K (2017) Bioprocess optimisation of PHB homopolymer and copolymer P3(HB-co-HV) by Acinetobacter junii BP25 utilizing rice mill effluent as sustainable substrate. Environ Technol. https://doi.org/10.1080/09593330.2017.1330902
Reddy MV, Mawatari Y, Onodera R, Nakamura Y, Yajima Y, Chang Y-C (2019) Bacterial conversion of waste into polyhydroxybutyrate (PHB): a new approach of bio-circular economy for treating waste and energy generation. Bioresour Technol Rep 7:100246
Dalsasso RR, Pavan FA, Bordignon SE, de Aragao GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Proc Biochem 85:12–18
Khardenavis AA, Vaidya AN, Suresh Kumar M, Chakrabarti T (2009) Utilisation of molasses spentwash for production of bioplastics by waste activated sludge. Waste Manag 29:2558–2565
Cavaillé L, Grousseau E, Pocquet M, Lepeuple AS, Uribelarrea JL, Hernandez-Raquet G, Paul E (2013) Polyhydroxybutyrate production by direct use of waste activated sludge in phosphorus-limited fed-batch culture. Bioresour Technol 149:301–309
Dobroth ZT, Hu S, Coats ER, McDonald AG (2011) Polyhydroxybutyrate synthesis on biodiesel wastewater using mixed microbial consortia. Bioresour Technol 102:3352–3359
Liu Z, Wang Y, He N, Huang J, Zhu K, Shao W, Wang H, Yuan W, Li Q (2011) Optimization of polyhydroxybutyrate (PHB) production by excess activated sludge and microbial community analysis. J Hazard Mater 185(1):8–16
Carucci A, Dionisi D, Majone M, Rolle E, Smurra P (2001) Aerobic storage by activated sludge on real wastewater. Water Res 35(16):3833–3844
Primasari B, Ibrahim S, Suffian M, Annuar M, Xung L, Remmie I (2011) Aerobic treatment of oily wastewater: effect of aeration and sludge concentration to pollutant reduction and PHB accumulation. World Acad Sci Eng Technol 54:914–918
Yu M (2014) Sustainable wastewater systems: impact of operational strategies and carbon sources on poly-β-hydroxybutyrate (PHB) accumulation and nutrient removal in sequencing batch reactor. M.S. Thesis, Iowa State University
Wang B, Sharma-Shivappa RR, Olson JW, Khan SA (2012) Upstream process optimisation of polyhydroxybutyrate (PHB) by Alcaligenes latus using two-stage batch and fed-batch fermentation strategies. Bioproc Biosyst Eng 35(9):1591–1602
Eshtaya MK, Rahman NAA, Hassan MA (2013) Bioconversion of restaurant waste into Polyhydroxybutyrate (PHB) by recombinant E.Coli through anaerobic digestion. Int J Environ Waste Manag 11(1): 27–37
Khan MR, Prasad RDM, Abdullah H, Batcha AFM (2013) Kinetic analysis on cell growth and biosynthesis of poly(3-hydroxybutyrate) (PHB) in Cupriavidus necator H16. Int J Biosci Biochem Bioinfor 3(5):516–519
Wang J, Yu H-Q (2007) Biosynthesis of polyhyroxybutyrate(PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Appl Microbiol Biotechnol 75:871–878
Wang J, Yu HQ (2006) Cultivation of polyhydroxybutyrate-rich aerobic granular sludge in a sequencing batch reactor. Water Sci Technol 6(6):81–87
Changli L, Min Z, Jianhui G, Chenchen L, Yang D, Wenhui X (2010) Concentration of organic matter effect on activated sludge PHB accumulation. Int Conf Chem Biol Environ Eng IPCBEE 1:57–59
Wu SC, Liou SZ, Lee CM (2012) Correlation between bio-hydrogen production and 256 polyhydroxybutyrate (PHB) synthesis by Rhodopseudomonas palustris WP3–5. Bioresour 257 Technol. 113:44–50.
Wang J, Li W-W, Yue Z-B, Yu H-Q (2014) Cultivation of aerobic granules for polyhydroxybutyrate production from wastewater. Biores Technol 159:442–445
Ciggin AS, Karahan O, Orhon D (2009) Effect of high nitrate concentration on PHN storage in sequencing batch reactor under anoxic conditions. Bioresour Technol 100:1376–1382
Reddy MV, Mohan SV (2012) Effect of substrate load and nutrients concentration on the polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour Technol 114:573–582
Jiang Y, Marang L, Kleerebezem R, Muyzer G, van Loosdrecht MCM (2011) Effect of temperature and cycle length on microbial competition in PHB-producing sequencing batch reactor. ISME J 5:896–907
Zhang T, Wang X, Zhou J, Zhang Y (2017) Enrichments of methanotrophic-heterotrophic cultures with high poly-β-hydroxybutyrate (PHB) accumulation capacities. J Environ Sci 65:133–143
Jena J, Kumar R, Dixit A, Pandey S, Das T (2015) Evaluation of simultaneous nutrient and COD removal with polyhydroxybutyrate (PHB) accumulation using mixed microbial consortia under anoxic condition and their bioinformatics analysis. PLoS ONE 10(2):e0116230
Fang F, Liu X-W, Xu J, Yu H-Q, Li Y-M (2009) Formation of aerobic granules and their PHB production at various substrate and ammonium concentrations. Bioresour Technol 100:59–63
Johnson K, Kleerebezem R, van Loosdrecht MCM (2010) Influence of ammonium on the accumulation of polyhydroxybutyrate (PHB) in aerobic open mixed cultures. J Biotechnol 147:73–79
Dionisi D, Majone M, Miccheli A, Puccetti C, Sinisi C (2004) Glutamic acid removal and PHB storage in the activated sludge process under dynamic conditions. Biotechnol Bioeng 86:842–851
Sakthiselvan P, Madhumathi R (2018) Kinetic evaluation on cell growth and biosynthesis of polyhydroxybutyrate (PHB) by Bacillus safensis EBT 1 from sugarcane bagasse. Eng Agric Environ Food 11(3). https://doi.org/10.1016/j.eaef.2018.03.003.
Li D, Fang Q, Yi D (2016) Maximizing the accumulation of poly-β-hydroxybutyrate (PHB) in low-carbon urban sewage. Desalin Water Treat 57:25927–25938
Pandian SR, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S (2010) Optimisation and fed-batch production of PHB utilising dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour Technol 101:705–711
Ceyhan N, Ozdemir G (2011) Poly-hydroxybutyrate (PHB) production from domestic wastewater using Enterobacter aerogenes 12Bi strain. Afr J Microbiol Res 5(6):690–702
Yuan Q, Sparling R, Oleszkiewicz J (2015) Polyhydroxybutyrate production from municipal wastewater activated sludge with different carbon sources. Air Soil Water Res 8: ASWR-S27218
Dalsasso RR, Pavan FA, Bordignon SE, de Aragao GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem 85:12–18
Rebah FB, Prevost D, Tyagi RD, Belbahri L (2009) Poly-β-hydroxybutyrate production by fast-growing rhizobia cultivated in sludge and in industrial wastewater. Appl Biochem Biotechnol 158:155–163
Suresh Kumar M, Mudliar SN, Reddy KMK, Chakrabarti T (2004) Production of biodegradable plastics from activated sludge generated from a food processing industrial wastewater treatment plant. Bioresour Technol 95:327–330
Peña-Jurado E, Pérez-Vega S, Pérez-Reyes I, Gutiérrez-Méndez N, Vazquez-Castillo J, Salmerón I (2019) Production of poly (3-hydroxybutyrate) from a dairy industry wastewater using Bacillus subtilis EPAH18: Bioprocess development and simulation. Biochem Eng J 151:107324
Xiaoyan L, Ziking J, Yongyu Q, Daizong C, Xiaguang C, Min Z (2017) Production of poly-β-hydroxybutyrate by activated sludge in sequencing batch reactor under aerobic conditions. J Wuhan Univ Technol Mater Sci 32:733–738
Dinesh GH, Nguyen DD, Ravindran B, Chang SW, Vo D-VN, Bach Q-V, Tran NH, Basu MJ, Mohanrasu K, Murugan RS, Swetha TA, Sivapraksh G, Selvaraj A, Arun A (2020) Simultaneous biohydrogen (H2) and bioplastic (poly-β-hydroxybutyrate-PHB) productions under dark, photo, and subsequent dark and photo fermentation utilizing various wastes. Int J Hydrog Energ 45(10):5840–5853
Third AK, Burnett N, Ruwisch RC (2003) Simultaneous nitrification and denitrification using stored substrate (PHB) as the electron donor in an SBR. Biotechnol Bioeng 83(6):706–720
Tripathi AD, Srivastava SK, Singh RP (2013) Statistical optimisation of physical process variables for bioplastic (PHB) production by Alcaligenes sp. Biomass Bioenerg 55:243–250
Prieto CVG, Ramos FD, Estrada V, Villar MA, Diaz S (2017) Optimisation of an integrated algae-based biorefinery for the production of biodiesel, astaxanthin and PHB. Energy 139:1159–1172
Liu C, Liu D, Qi Y, Zhang Y, Liu X, Zhao M (2016) The effect of anaerobic-aerobic and feast-famine cultivation pattern on bacterial diversity during poly-β-hydroxybutyrate production from domestic sewage sludge. Environ Sci Poll Res 23:12966–12975
Chen H-b, Yang Q, Li X-m, Wang Y, Luo K, Zeng G-m (2013) Post-anoxic denitrification via nitrite driven by PHB in feast-famine sequencing batch reactor. Chemosphere 92:1349–1355
Bharti SN, Swetha G (2016) Need for bioplastics and role of biopolymer PHB: A short review. J Petrol Environ Biotechnol 7(2):1000272
Bernat K, Baryla IW (2008) The effect of different nitrogen sources on denitrification with PHB under aerobic condition. Environ Technol 29:81–89
Miao L, Wang S, Zhu R, Cao T, Peng Y (2015) The effect of oxygen supply on nitrogen removal via nitrite using stored substrate (PHB) as the electron donor in SBRs. Biochem Eng J 104:130–137
Martins-Franchetti SM, De Campos A, Marconato JC (2012) The influence of soil and landfill leachate microorganisms in the degradation of PVC/PCL films cast from DMF. Polimeros 22:220–227
Adamcova D, Radziemska M, Fronczyk J, Zloch J, Vaverkova MD (2017) Research on biodegradability of degradable/biodegradable plastic material in various types of environments. Sci Rev Eng Environ Sci 26(1): 3–14
Xochitl Q-P, del Consuelo MH-B, del Consuelo MM-S, Maria E-VR, Alethia V-M (2021) Degradation of plastics in simulated landfill conditions. Polymers 13:1–13
Vaverkova M, Kotovicova J, Adamcova D (2011) Testing the biodegradability and biodegradation rates of degradable/biodegradable plastics within simulated environment. Polska Akademia Nauk, Oddzial w Krakowie 12: 93-101
Shin PY, Kim MH, Kim JM (1997) Biodegradability and degradable plastics exposed to anaerobic digested sludge and simulated landfill conditions. J Environ Poly Degrad 5(1):33–39
Finelli L, Scandola M, Sadocco P (1998) Biodegradation of blends of bacterial poly(3-hydroxybutyrate) with ethyl cellulose in activated sludge and in enzymatic solution. Macromol Chem Phy 199:695–703
Qin L, Liu Y, Tay J-H (2005) Denitrification on poly-β-hydroxybutyrate in microbial granular sludge sequencing batch reactor. Water Res 39:1503–1510
Jia W, Zhang J, Xie H, Yan Y, Wang J, Zhao Y, Xu X (2012) Effect of PHB and oxygen uptake rate on nitrous oxide emission during simultaneous nitrification denitrification process. Bioresour Technol 113:232–238
Beun JJ, Verhoef EV, van Loosdrecht MCM, Heijnen JJ (2000) Stoichiometry and kinetics of poly-β-hydroxybutyrate metabolism under denitrifying conditions in activated sludge cultures. Biotechnol Bioeng 68(5):496–507
Huda SMS, Satoh H, Mino T (2013) Anaerobic digestion of polyhydroxybutyrate accumulated in excess activated sludge. J Water Environ Technol 11(5):429–438
Brzezinka MS, Walczak M, Kalwasinska A, Richert A, Swiatczak J, Deja-Sikora E, Burkowska-But A (2020) Biofilm formation during biodegradation of polylactide, poly (3,4 hydroxybutyrate) and poly(ɛ-caprolactone) in activated sludge. BioMacromol. https://doi.org/10.1016/j.ijbiomac.2020.05.107
da Costa PJ, Santos SMP, Duarte AC, Rocha-Santos T (2016) Nanoplastics in the environemtn – Sources, fates and effects. Sci Tot Environ 566–567:15–26
Koutinas AA, Garcia IL, Kopsahelis N, Papanikolaou S, Webb C, Villar MA, López JA (2013) Production of fermentation feedstock from Jerusalem artichoke tubers and its potential for Polyhydroxybutyrate synthesis. Waste Biom Valor 4(2):359–370
Naranjo JM, Cardona CA, Higuita JC (2014) Use of residual banana for polyhydroxybutyrate (PHB) production: case of study in an integrated biorefinery. Waste Manag 34(12):2634–2640
Vasudevan M, Karthika K, Gowthaman S, Karthick K, Balaganesh P, Suneeeth Kumar SM, Natarajan N (2019) Aerobic in-vessel co-composting of dewatered sewage sludge with mixed municipal wastes under subhumid and semiarid atmospheric conditions. Energy Sources A Recover Utilization Environ Effects: 1–12
Priester J, Van De Werfhorst LC, Ge Y, Adeleye A, Tomar S, Tom ML, Piceno YM, Andersen G, Holden PA (2014) Effects of TiO2 and Ag nanoparticles on polyhydroxybutyrate biosynthesis by activated sludge bacteria. Environ Sci Technol 48(24):14712–14720
Carofiglio VE, Stufano P, Cancelli N, De Benedictis VM, Centrone D, De Benedetto E, Cataldo A, Sannino A, Demitri C (2017) Novel PHB/Olive mill wastewater residue composite based film: thermal, mechanical and degradation properties. J Environ Chem Eng 5(6):6001–6007
Acknowledgements
The authors acknowledge the technical and official support rendered by their parent institutes for conducting the present study.
Author information
Authors and Affiliations
Contributions
NN contributed in the collection of literature and in preparation of the tables and figures and MV in writing and reviewing the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Identification of modified metabolic pathways in polyhydroxybutyrate synthesis
• Promotion of establishing F/M relation for various wastewater and mixed cultures
• Comparison of optimized experimental conditions for polyhydroxybutyrate production
• Critical overview of polyhydroxybutyrate production cost and suggestions for circular economy
• Selection of sustainable options for simultaneous benefits from polyhydroxybutyrate biosynthesis
Rights and permissions
About this article
Cite this article
Vasudevan, M., Natarajan, N. Towards achieving sustainable bioplastics production and nutrient recovery from wastewater—a comprehensive overview on polyhydroxybutyrate. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02399-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-02399-z